Metabolite Profiling of Triterpene Glycosides of the Far Eastern Sea Cucumber Eupentacta fraudatrix and Their Distribution in Various Body Components Using LC-ESI QTOF-MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Profiling and Structural Identification of the Triterpene Glycosides from E. fraudatrix
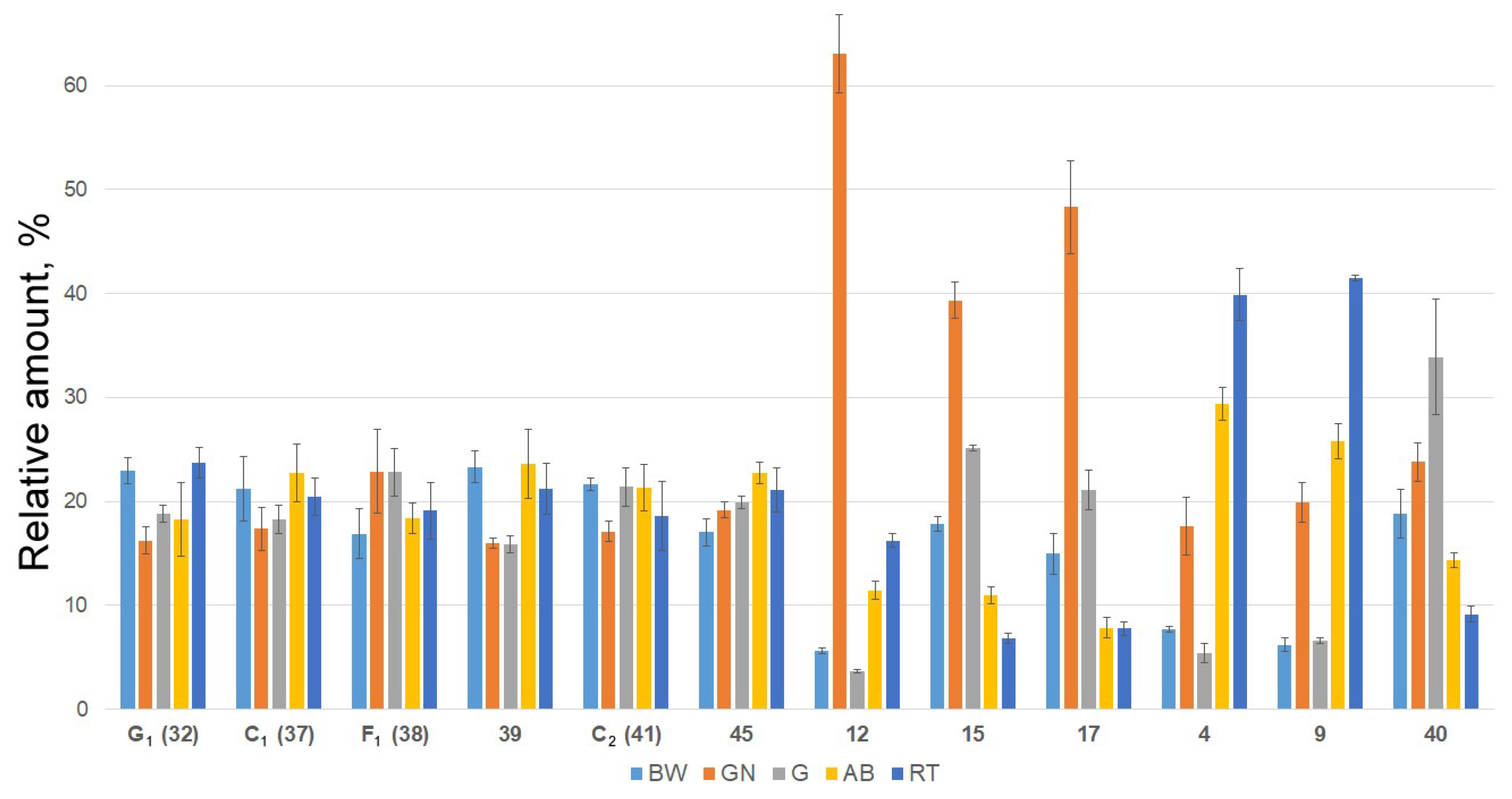
2.2. Distribution of Detected Glycosides in the Different Body Components
3. Materials and Methods
3.1. Chemicals
3.2. Animal Material
3.3. Sample Preparation and Solid-Phase Extraction (SPE)
3.4. LC-MS Analysis
3.5. Quantitative Analysis of Detected Triterpene Glycosides in Various Body Component
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucumbers (Holothurioidea, Echinodermata), biological activities and functions. In Studies in Natural Product Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2008; Volume 35, pp. 135–196. [Google Scholar]
- Careaga, V.P.; Maier, M.S. Cytotoxic triterpene glycosides from sea cucumbers. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 515–528. [Google Scholar]
- Chludil, H.D.; Murray, A.P.; Seldes, A.M.; Maier, M.S. Biologically active triterpene glycosides from sea cucumbers (Holothuroidea, Echinodermata). In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2003; Volume 28, pp. 587–615. [Google Scholar]
- Kim, S.K.; Himaya, S.W.A. Triterpene glycosides from sea cucumbers and their biological activities. Adv. Food Nutr. Res. 2012, 63, 297–319. [Google Scholar]
- Maier, M.S.; Roccatagliata, A.J.; Kuriss, A.; Chludil, H.; Seldes, A.M.; Pujol, C.A.; Damonte, E.B. Two new cytotoxic and virucidal trisulfated triterpene glycosides from the antarctic sea cucumber Staurocucumis liouvillei. J. Nat. Prod. 2001, 64, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Pislyagin, E.A.; Menchinskaya, E.S.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Immunomodulatory and anticancer activity of sea cucumber triterpene glycosides. In Studies in Natural Products Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2014; Volume 41, pp. 75–94. [Google Scholar]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, S.; Flammang, P.; Meriaux, C.; Bonnel, D.; Salzet, M.; Fournier, I.; Wisztorski, M. Localization of secondary metabolites in marine invertebrates: Contribution of MALDI MSI for the study of saponins in Cuvierian tubules of H. forskali. PLoS ONE 2010, 5, e13923. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, S.; Caulier, G.; Todesco, M.; Gerbaux, P.; Fournier, I.; Wisztorski, M.; Flammang, P. The triterpene glycosides of Holothuria forskali: Usefulness and efficiency as a chemical defense mechanism against predatory fish. J. Exp. Biol. 2011, 214, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Anisimov, M.M. The influence of holotoxin A1 to the Ca2+-transport and meiotic maturation of oocytes from the sea cucumber Stichopus japonicus. J. Evol. Biochem. Physiol. 1990, 26, 9–13. [Google Scholar]
- Kalinin, V.I.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides of sea cucumbers (Holothuroidea, Echinodermata) as taxonomic markers. Nat. Prod. Commun. 2015, 10, 21–26. [Google Scholar] [PubMed]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Qualitative and quantitative saponin contents in five sea cucumbers from the Indian Ocean. Mar. Drugs 2010, 8, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Bondoc, K.G.V.; Lee, H.; Cruz, L.J.; Lebrilla, C.B.; Juinio-Meсez, M.A. Chemical fingerprinting and phylogenetic mapping of saponin congeners from three tropical holothurian sea cucumbers. Comp. Biochem. Physiol. B 2013, 166, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Elucidation of molecular diversity and body distribution of saponins in the sea cucumber Holothuria forskali (Echinodermata) by mass spectrometry. Comp. Biochem. Physiol. B 2009, 152, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Dolmatov, I.Y.; Ivantey, V.A. Histogenesis of longitudinal muscle bands in holothurians. Russ. J. Dev. Biol. 1993, 24, 67–72. [Google Scholar]
- Dolmatov, I.Y.; Yushin, V.V. Larval development of Eupentacta fraudatrix (Holothuroidea, Dendrochirota). Asian Mar. Biol. 1993, 10, 123–132. [Google Scholar]
- Dolmatov, I.Y. Regeneration of the aquapharyngeal complex in the holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota). In Monographs in Developmental Biology; Taban, C.H., Boilly, B., Eds.; Karger: Basel, Switzerland, 1992; Volume 23, pp. 40–50. [Google Scholar]
- Mashanov, V.S.; Dolmatov, I.Y.; Heinzeller, T. Transdifferentiation in holothurian gut regeneration. Biol. Bull. 2005, 209, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Lamash, N.E.; Dolmatov, I.Y. Proteases from the regenerating gut of the holothurian Eupentacta fraudatrix. PLoS ONE 2013, 8, e58433. [Google Scholar] [CrossRef] [PubMed]
- Zaika, O.A.; Dolmatova, L.S. Cooperative apoptosis of coelomocytes of the holothurian Eupentacta fraudatrix and its modulation by dexamethasone. Adv. Biosci. Biotechnol. 2013, 4, 908–917. [Google Scholar] [CrossRef]
- Dolmatova, L.S.; Ulanova, O.A.; Dolmatov, I.Y. Comparative study of the effect of dexamethasone and new holothurians’ extract on the level of the cytokine similar substances in certain immune cells types in the holothurian Eupentacta fraudatrix. Pac. Med. J. 2014, 34–38. [Google Scholar]
- Dolmatova, L.S.; Ulanova, O.A. Dexamethasone treatment in vitro resulted in different responses of two fractions of phagocytes of the holothurian Eupentacta fraudatrix. Russ. J. Mar. Biol. 2015, 41, 503–506. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides A1, A3, A4, A5, A6, A12 and A15, seven new minor non-sulfated tetraosides and unprecedented 25-keto, 27-norholostane aglycone. Nat. Prod. Commun. 2012, 7, 517–525. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and cytotoxic action of cucumariosides A2, A7, A9, A10, A11, A13 and A14, seven new minor non-sulfated tetraosides and an aglycone with an uncommon 18-hydroxy group. Nat. Prod. Commun. 2012, 7, 845–852. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjashenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Stonik, V.A. 3β-O-Glycosylated 16β-acetoxy-9β-H-lanosta-7,24-diene-3β,18,20β-triol, an intermediate metabolite from the sea cucumber Eupentacta fraudatrix and its biosynthetic significance. Biochem. Syst. Ecol. 2012, 44, 53–60. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological activity of cucumariosides B1 and B2, two new minor non-sulfated unprecedented triosides. Nat. Prod. Commun. 2012, 7, 1157–1162. [Google Scholar] [PubMed]
- Afiyatullov, S.S.; Kalinovskii, A.I.; Stonik, V.A. Structure of cucumariosides C1 and C2—2 New triterpene glycosides from the Eupentacta fraudatrix holothurian. Khim. Prir. Soedin. 1987, 6, 831–837. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Tishchenko, L.Ya.; Stonik, V.A.; Kalinovskii, A.I.; Elyakov, G.B. The structure of cucumarioside G1—A new triterpene glycoside from the sea cucumber Cucumaria fraudatrix. Khim. Prir. Soedin. 1985, 2, 244–248. [Google Scholar]
- Kalinin, V.I.; Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A.; Milgrom, Yu.M.; Rashkes, Ya.V. Cucumarioside G4—A new triterpene glycoside from the holothurian Eupentacta fraudatrix. Khim. Prir. Soedin. 1992, 6, 600–603. [Google Scholar]
- Kalinin, V.I.; Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A. Cucumarioside G3—A minor triterpene glycoside from the holothurian Eupentacta fraudatrix. Khim. Prir. Soedin. 1992, 6, 635–636. [Google Scholar] [CrossRef]
- Avilov, S.A.; Kalinin, V.I.; Makarieva, T.N.; Stonik, V.A.; Kalinovsky, A.I. Structure of cucumarioside G2, a novel nonholostane glycoside from the sea cucumber Eupentacta fraudatrix. J. Nat. Prod. 1994, 57, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Kalinovskii, A.I.; Afiyatullov, S.S. Triterpene glycosides of the holothurin Eupentacta pseudoquinquisemita. Khim. Prir. Soedin. 1988, 2, 221–225. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I. Structure of cucumariosides H5, H6, H7 and H8, triterpene glycosides from the sea cucumber Eupentacta fraudatrix and unprecedented aglycone with 16,22-epoxy-group. Nat. Prod. Commun. 2011, 6, 1075–1082. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I. Structures and cytotoxic properties of cucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat. Prod. Res. 2012, 26, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Popov, R.S.; Avilov, S.A.; Silchenko, A.S.; Kalinovsky, A.I.; Dmitrenok, P.S.; Grebnev, B.B.; Ivanchina, N.V.; Kalinin, V.I. Cucumariosides F1 and F2, two new triterpene glycosides from the sea cucumber Eupentacta fraudatrix and their LC-ESI MS/MS identification in the starfish Patiria pectinifera, a predator of the sea cucumber. Biochem. Syst. Ecol. 2014, 57, 191–197. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Aminin, D.L.; Kalinin, V.I. Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Nat. Prod. Res. 2013, 27, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides I1, I3, I4, three new minor disulfated pentaosides. Nat. Prod. Commun. 2013, 8, 1053–1058. [Google Scholar] [PubMed]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Antonov, A.S.; Kalinovsky, A.I.; Afiyatullov, S.S.; Leshchenko, E.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I.; Stonik, V.A. Erylosides F8, V1–V3, and W–W2—New triterpene oligoglycosides from the Carribean sponge Erylus goffrilleri. Carbohydr. Res. 2017, 449, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Kalinovskii, A.I.; Stonik, V.A. The structure of psolusoside A—The major triterpene glycoside of the sea cucumber Psolus fabricii. Khim. Prir. Soedin. 1985, 2, 212–217. [Google Scholar]
- Kalinin, V.I.; Kalinovskii, A.I.; Stonik, V.A.; Dmitrenok, P.S.; Elkin, Y.N. Structure of psolusoside B—A nonholostane triterpene glycoside of the holothurian genus Psolus. Khim. Prir. Soedin. 1989, 3, 361–368. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Chingizova, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dautova, T.N. Nine new triterpene glycosides, magnumosides A1–A4, B1, B2, C1, C2 and C4, from the Vietnamese sea cucumber Neothyonidium (=Massinium) magnum: Structures and activities against tumor cells independently and in synergy with radioactive irradiation. Mar. Drugs 2017, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Heftman, E.; Mosettig, E. Biochemistry of Steroids; Reinhold Publishing Corporation: New York, NY, USA, 1960; p. 231. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I.; Jayasandhya, P.; Rajan, G.C.; Padmakumar, K.P. Structures and biological activities of typicosides A1, A2, B1, C1 and C2, triterpene glycosides from the sea cucumbers Actinocucumis typica. Nat. Prod. Commun. 2013, 8, 301–310. [Google Scholar] [PubMed]
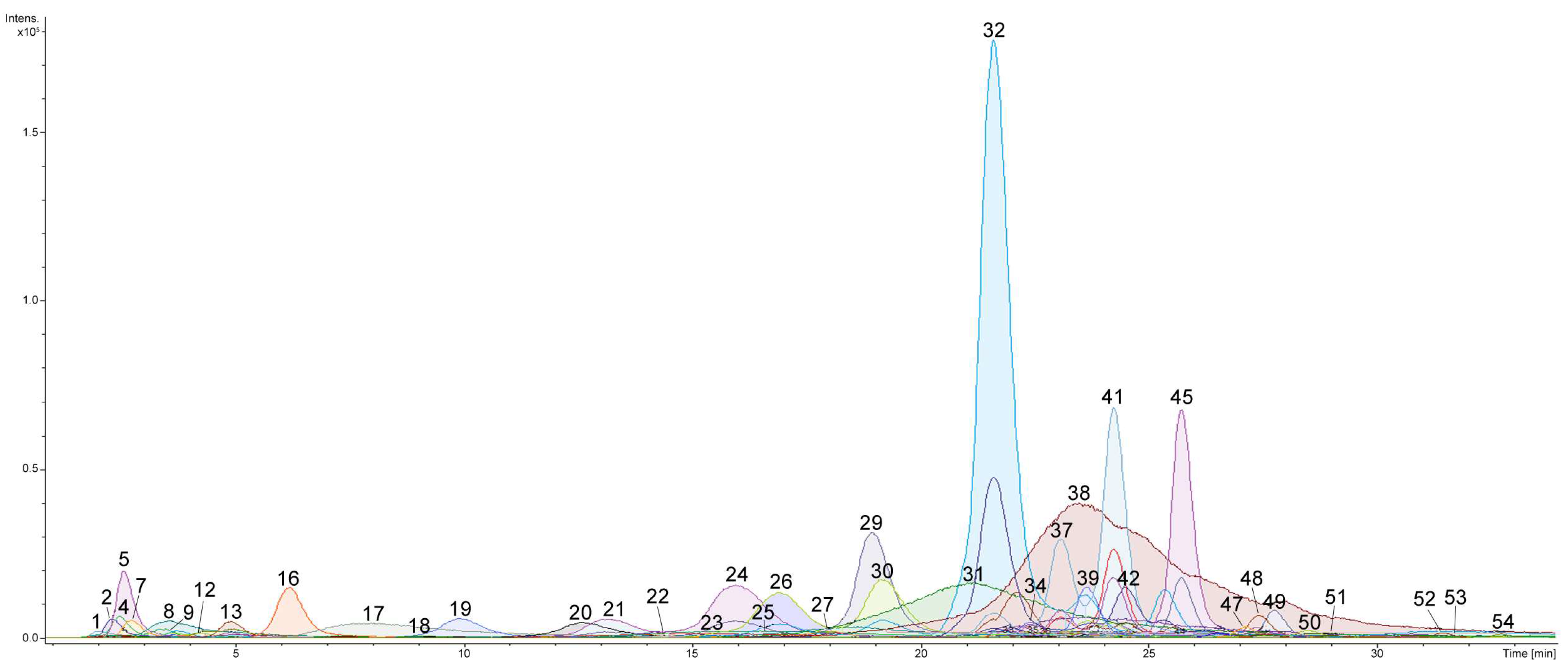
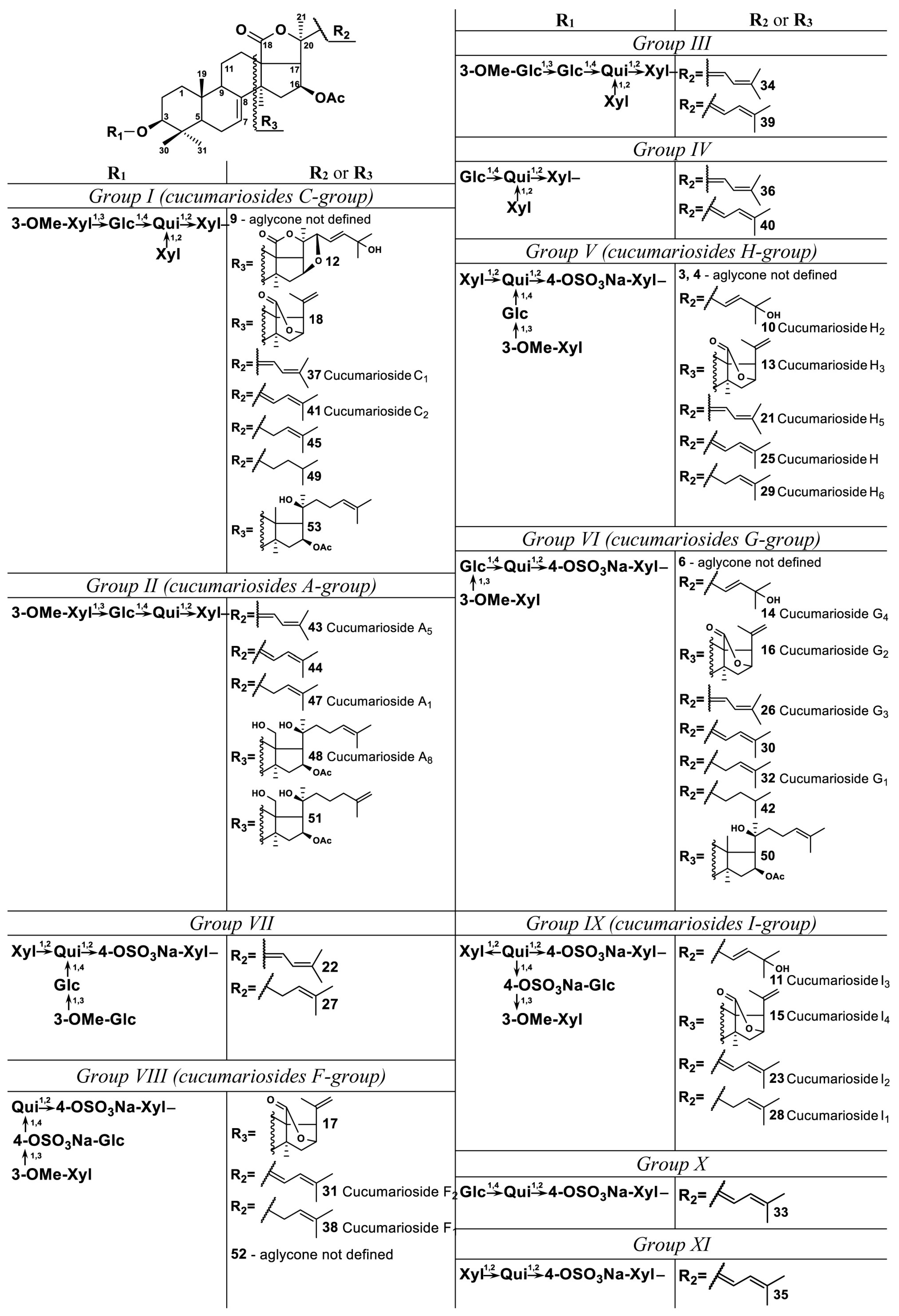
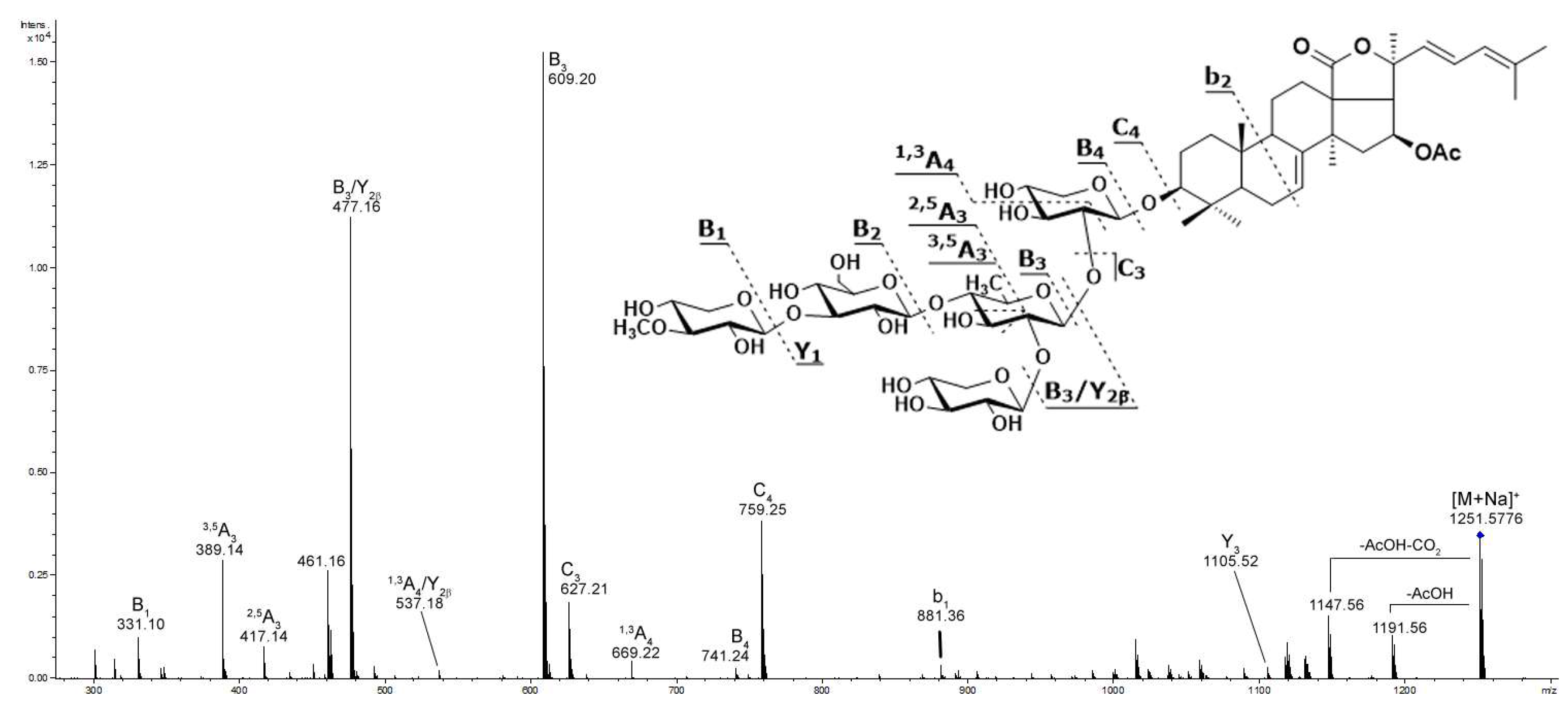
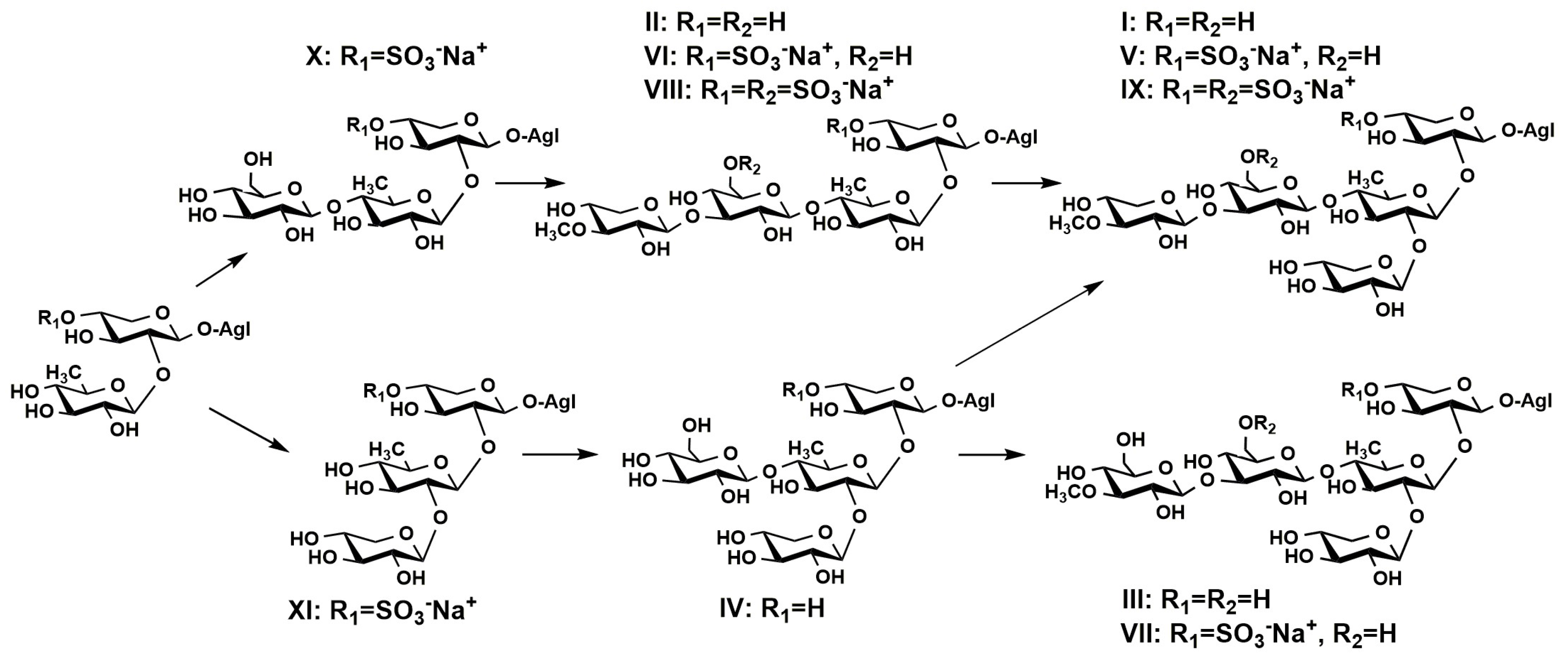
| No. a | Rt (min) | Elemental Composition b | Measured m/z | Molecular Ion Type | Calculated m/z | Δ (ppm) | Content of Detected Compounds in Different Organs (μg/g) c | Identification (ChemSpider ID) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | GN | G | AB | RT | ||||||||
| 1 | 2.0 | C53H83O28SNa | 1199.4788 | [M − Na]− | 1199.4797 | 0.8 | 1.28 | 2.07 | 0.74 | 4.41 | 5.06 | |
| 2 | 2.3 | C58H91O31SNa | 1315.5265 | [M − Na]− | 1315.5271 | 0.4 | 2.06 | 3.89 | 2.02 | 10.76 | 12.19 | |
| 3 | 2.4 | C60H93O31SNa | 1341.5430 | [M − Na]− | 1341.5427 | −0.2 | 0.58 | 3.02 | 0.51 | 2.15 | 1.98 | |
| 4 | 2.5 | C57H85O30SNa | 1281.4854 | [M − Na]− | 1281.4852 | −0.2 | 3.12 | 7.12 | 2.21 | 11.88 | 16.15 | |
| 5 | 2.6 | C53H83O27SNa | 1183.4848 | [M − Na]− | 1183.4848 | 0.0 | 8.51 | 12.85 | 6.27 | 33.07 | 39.82 | |
| 6 | 2.7 | C55H85O27SNa | 1209.4998 | [M − Na]− | 1209.5004 | 0.5 | 0.89 | 4.65 | 0.61 | 2.50 | 2.50 | |
| 7 | 2.8 | C52H77O26SNa | 1149.4426 | [M − Na]− | 1149.4429 | 0.3 | 2.30 | 7.72 | 2.00 | 10.60 | 12.71 | |
| 8 | 3.5 | C53H82O30S2Na2 | 631.2170 | [M − 2Na]2− | 631.2172 | 0.3 | 7.23 | 13.08 | 5.68 | 26.16 | 27.96 | |
| 9 | 3.8 | C57H86O27 | 1201.5275 | [M − H]− | 1201.5284 | 0.7 | 19.90 | 63.76 | 21.23 | 82.84 | 132.97 | |
| 10 | 4.0 | C60H93O30SNa | 1325.5472 | [M − Na]− | 1325.5478 | 0.4 | 2.05 | 3.45 | 2.13 | 3.96 | 3.21 | Cucumarioside H2 * (ID29215132) |
| 11 | 4.3 | C60H92O33S2Na2 | 702.2493 | [M − 2Na]2− | 702.2487 | −0.9 | 5.40 | 5.53 | 5.24 | 4.21 | 3.49 | Cucumarioside I3 * (ID30771157) |
| 12 | 4.3 | C58H90O26 | 1201.5634 | [M − H]− | 1201.5648 | 1.1 | 5.98 | 66.99 | 3.88 | 12.14 | 17.24 | |
| 13 | 5.0 | C53H81O27SNa | 1181.4692 | [M − Na]− | 1181.4691 | 0.0 | 67.31 | 65.70 | 56.11 | 38.44 | 22.47 | Cucumarioside H3 * (ID29215133) |
| 14 | 5.0 | C55H85O26SNa | 1193.5051 | [M − Na]− | 1193.5055 | 0.4 | 45.93 | 45.05 | 51.93 | 44.31 | 42.30 | Cucumarioside G4 ** (ID29216498) |
| 15 | 5.0 | C53H80O30S2Na2 | 630.2098 | [M − 2Na]2− | 630.2093 | −0.7 | 31.18 | 68.95 | 44.00 | 19.20 | 11.98 | Cucumarioside I4 * (ID30771158) |
| 16 | 6.2 | C48H73O23SNa | 1049.4268 | [M − Na]− | 1049.4269 | 0.1 | 182.20 | 258.47 | 199.67 | 104.16 | 78.53 | Cucumarioside G2 ** (ID16737749) |
| 17 | 7.7 | C48H72O26S2Na2 | 564.1882 | [M − 2Na]2− | 564.1882 | 0.0 | 164.93 | 533.31 | 233.14 | 86.44 | 85.75 | |
| 18 | 9.1 | C53H82O24 | 1101.5126 | [M − H]− | 1101.5123 | −0.2 | 95.81 | 79.93 | 94.56 | 92.03 | 77.07 | |
| 19 | 9.9 | C62H95O31SNa | 1367.5580 | [M − Na]− | 1367.5584 | 0.3 | 131.88 | 158.75 | 151.50 | 88.37 | 59.04 | |
| 20 | 12.4 | C57H87O27SNa | 1235.5156 | [M − Na]− | 1235.5161 | 0.4 | 87.55 | 123.77 | 115.97 | 76.27 | 67.76 | |
| 21 | 13.0 | C60H91O29SNa | 1307.5369 | [M − Na]− | 1307.5372 | 0.2 | 102.46 | 101.10 | 101.29 | 75.06 | 56.43 | Cucumarioside H5 * (ID29212282) |
| 22 | 14.4 | C61H93O30SNa | 1337.5472 | [M − Na]− | 1337.5478 | 0.4 | 39.15 | 33.91 | 27.47 | 30.81 | 26.60 | |
| 23 | 14.5 | C60H90O32S2Na2 | 693.2437 | [M − 2Na]2− | 693.2434 | −0.5 | 132.94 | 144.25 | 135.53 | 117.55 | 79.00 | Cucumarioside I2 * |
| 24 | 15.6 | C57H86O30S2Na2 | 657.2330 | [M − 2Na]2− | 657.2328 | −0.3 | 164.12 | 260.64 | 225.03 | 129.11 | 119.00 | |
| 25 | 15.7 | C60H91O29SNa | 1307.5370 | [M − Na]− | 1307.5372 | 0.2 | 355.92 | 300.10 | 325.16 | 257.61 | 205.87 | Cucumarioside H ** |
| 26 | 16.8 | C55H83O25SNa | 1175.4946 | [M − Na]− | 1175.4950 | 0.3 | 157.60 | 196.62 | 189.16 | 161.61 | 191.52 | Cucumarioside G3 ** (ID29213085) |
| 27 | 17.8 | C61H95O30SNa | 1339.5629 | [M − Na]− | 1339.5634 | 0.4 | 24.08 | 25.54 | 25.95 | 22.53 | 24.23 | |
| 28 | 18.1 | C60H92O32S2Na2 | 694.2510 | [M − 2Na]2− | 694.2512 | 0.3 | 188.33 | 207.58 | 222.25 | 202.43 | 149.95 | Cucumarioside I1 * (ID30771156) |
| 29 | 19.0 | C60H93O29SNa | 1309.5524 | [M − Na]− | 1309.5529 | 0.4 | 349.25 | 387.00 | 349.37 | 310.11 | 243.40 | Cucumarioside H6 * (ID29212283) |
| 30 | 19.1 | C55H83O25SNa | 1175.4946 | [M − Na]− | 1175.4950 | 0.3 | 196.05 | 245.41 | 225.43 | 159.63 | 183.33 | |
| 31 | 20.8 | C55H82O28S2Na2 | 627.2223 | [M − 2Na]2− | 627.2223 | −0.1 | 650.73 | 1465.87 | 1013.44 | 492.83 | 518.87 | Cucumarioside F2 * (ID34981778) |
| 32 | 21.5 | C55H85O25SNa | 1177.5101 | [M − Na]− | 1177.5106 | 0.4 | 1399.21 | 990.35 | 1149.58 | 1114.02 | 1446.29 | Cucumarioside G1 ** (ID29212825) |
| 33 | 21.8 | C49H73O21SNa | 1029.4364 | [M − Na]− | 1029.4371 | 0.6 | 3.49 | 4.46 | 4.22 | 3.38 | 2.41 | |
| 34 | 22.4 | C61H94O27 | 1257.5905 | [M − H]− | 1257.5910 | 0.4 | 353.39 | 277.23 | 270.44 | 373.37 | 400.82 | |
| 35 | 22.4 | C48H71O20SNa | 999.4257 | [M − Na]− | 999.4265 | 0.8 | 4.00 | 4.03 | 4.08 | 3.80 | 3.78 | |
| 36 | 22.5 | C54H82O22 | 1081.5224 | [M − H]− | 1081.5225 | 0.1 | 20.68 | 29.78 | 40.22 | 19.97 | 12.52 | |
| 37 | 23.1 | C60H92O26 | 1227.5799 | [M − H]− | 1227.5804 | 0.4 | 2304.76 | 1883.92 | 1982.79 | 2465.98 | 2218.90 | Cucumarioside С1 ** |
| 38 | 23.2 | C55H84O28S2Na2 | 628.2301 | [M − 2Na]2− | 628.2301 | 0.0 | 1397.27 | 1896.52 | 1889.40 | 1520.29 | 1581.81 | Cucumarioside F1 * (ID34981780) |
| 39 | 23.6 | C61H94O27 | 1257.5907 | [M − H]− | 1257.5910 | 0.2 | 1232.75 | 844.94 | 836.78 | 1248.59 | 1121.92 | |
| 40 | 23.8 | C54H82O22 | 1081.5221 | [M − H]− | 1081.5225 | 0.4 | 78.61 | 99.33 | 141.63 | 60.09 | 38.25 | |
| 41 | 24.2 | C60H92O26 | 1227.5796 | [M − H]− | 1227.5804 | 0.7 | 5042.17 | 3983.86 | 4978.07 | 4965.12 | 4323.97 | Cucumarioside С2 ** |
| 42 | 24.5 | C55H87O25SNa | 1179.5253 | [M − Na]− | 1179.5263 | 0.8 | 107.49 | 63.87 | 80.66 | 86.34 | 113.97 | |
| 43 | 24.6 | C55H84O22 | 1095.5380 | [M − H]− | 1095.5381 | 0.1 | 30.86 | 30.40 | 35.66 | 40.90 | 55.20 | Cucumarioside A5 ** (ID29214535) |
| 44 | 25.6 | C55H84O22 | 1095.5379 | [M − H]− | 1095.5381 | 0.2 | 12.26 | 15.38 | 19.73 | 17.26 | 25.04 | |
| 45 | 25.7 | C60H94O26 | 1229.5956 | [M − H]− | 1229.5961 | 0.4 | 3169.77 | 3565.18 | 3696.87 | 4227.02 | 3920.64 | |
| 46 | 26.6 | C47H75O18SNa | 959.4674 | [M − Na]− | 959.4680 | 0.6 | 4.31 | 5.66 | 5.78 | 4.76 | 6.27 | |
| 47 | 27.1 | C55H86O22 | 1097.5534 | [M − H]− | 1097.5538 | 0.4 | 128.12 | 114.38 | 137.12 | 144.01 | 155.36 | Cucumarioside A1 * (ID29214532) |
| 48 | 27.5 | C55H90O22 | 1101.5851 | [M − H]− | 1101.5851 | 0.0 | 38.22 | 39.78 | 38.21 | 45.55 | 54.26 | Cucumarioside A8 * (ID29215118) |
| 49 | 27.7 | C60H96O26 | 1231.6110 | [M − H]− | 1231.6117 | 0.6 | 328.18 | 276.91 | 355.16 | 406.50 | 399.65 | |
| 50 | 28.5 | C55H89O24SNa | 1165.5467 | [M − Na]− | 1165.5470 | 0.3 | 7.70 | 6.64 | 7.32 | 8.26 | 9.17 | |
| 51 | 28.9 | C55H90O22 | 1101.5843 | [M − H]− | 1101.5851 | 0.7 | 19.39 | 17.88 | 17.46 | 18.56 | 21.60 | |
| 52 | 31.3 | C55H84O27S2Na2 | 620.2328 | [M − 2Na]2− | 620.2326 | −0.3 | 52.92 | 53.12 | 56.30 | 58.02 | 62.22 | |
| 53 | 31.4 | C60H98O25 | 1217.6314 | [M − H]− | 1217.6324 | 0.9 | 34.13 | 32.21 | 31.91 | 35.58 | 44.22 | |
| 54 | 32.6 | C56H92O23 | 1131.5951 | [M − H]− | 1131.5957 | 0.5 | 19.77 | 18.64 | 21.13 | 23.62 | 19.24 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, R.S.; Ivanchina, N.V.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Dolmatov, I.Y.; Stonik, V.A.; Dmitrenok, P.S. Metabolite Profiling of Triterpene Glycosides of the Far Eastern Sea Cucumber Eupentacta fraudatrix and Their Distribution in Various Body Components Using LC-ESI QTOF-MS. Mar. Drugs 2017, 15, 302. https://doi.org/10.3390/md15100302
Popov RS, Ivanchina NV, Silchenko AS, Avilov SA, Kalinin VI, Dolmatov IY, Stonik VA, Dmitrenok PS. Metabolite Profiling of Triterpene Glycosides of the Far Eastern Sea Cucumber Eupentacta fraudatrix and Their Distribution in Various Body Components Using LC-ESI QTOF-MS. Marine Drugs. 2017; 15(10):302. https://doi.org/10.3390/md15100302
Chicago/Turabian StylePopov, Roman S., Natalia V. Ivanchina, Alexandra S. Silchenko, Sergey A. Avilov, Vladimir I. Kalinin, Igor Yu. Dolmatov, Valentin A. Stonik, and Pavel S. Dmitrenok. 2017. "Metabolite Profiling of Triterpene Glycosides of the Far Eastern Sea Cucumber Eupentacta fraudatrix and Their Distribution in Various Body Components Using LC-ESI QTOF-MS" Marine Drugs 15, no. 10: 302. https://doi.org/10.3390/md15100302





