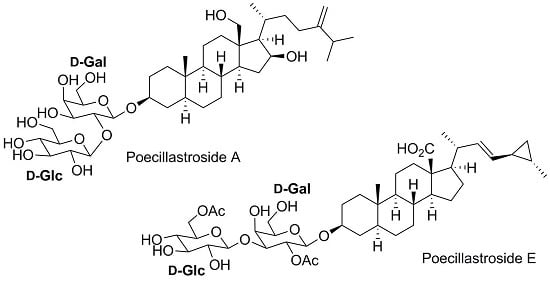
Poecillastrosides, Steroidal Saponins from the Mediterranean Deep-Sea Sponge Poecillastra compressa (Bowerbank, 1866)
Abstract
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Determination of the Absolute Configuration of the Pyranoses
3.5. Evaluation of the Biological Activities
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from sea cucumbers (holothuroids): Chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J. Nat. Toxins 1999, 8, 235–248. [Google Scholar] [PubMed]
- Makarieva, T.N.; Stonik, V.A.; Kapustina, I.I.; Boguslavsky, V.M.; Dmitrenoik, A.S.; Kalinin, V.I.; Cordeiro, M.L.; Djerassi, C. Biosynthetic studies of marine lipids. 42. Biosynthesis of steroid and triterpenoid metabolites in the sea cucumber Eupentacta fraudatrix. Steroids 1993, 58, 508–517. [Google Scholar] [CrossRef]
- Burnell, D.J.; Apsimon, J.W. Chapter 6—Echinoderm Saponins. In Marine Natural Products; Scheuer, P.J., Ed.; Academic Press: Waltham, MA, USA, 1983; pp. 287–389. [Google Scholar]
- Qi, S.; Zhang, S.; Huang, J.; Xiao, Z.; Wu, J.; Li, Q. Complete 1H and 13C NMR assignments of four new steroidal glycosides from a gorgonian coral Junceella juncea. Magn. Reson. Chem. 2005, 43, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Dai, C.-F.; Duh, C.-Y. Cytotoxic Pregnane Steroids from the Formosan Soft Coral Stereonephthya crystalliana. J. Nat. Prod. 2006, 69, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Kobayashi, M.; Okamoto, Y.; Yoshikawa, M.; Hamamoto, Y. Structures of Sarasinosides A1′, B1′, and C1′; New Norlanostane-Triterpenoid Oligoglycosides from the Palauan Marine Sponge Asteropus sarasinosum. Chem. Pharm. Bull. 1987, 35, 5036–5039. [Google Scholar] [CrossRef] [PubMed]
- Espada, A.; Jiménez, C.; Rodríguez, J.; Crews, P.; Riguera, R. Sarasinosides D–G: Four new triterpenoid saponins from the sponge Asteropus sarasinosum. Tetrahedron 1992, 48, 8685–8696. [Google Scholar] [CrossRef]
- Lee, H.-S.; Seo, Y.; Cho, K.W.; Rho, J.-R.; Shin, J.; Paul, V.J. New triterpenoid saponins from the sponge Melophlus isis. J. Nat. Prod. 2000, 63, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.-F.; Edrada, R.A.; Ebel, R.; Nimtz, M.; Wray, V.; Proksch, P. Norlanostane triterpenoidal saponins from the marine sponge Melophlus sarassinorum. J. Nat. Prod. 2005, 68, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jeon, J.-E.; Lee, Y.-J.; Lee, H.-S.; Sim, C.J.; Oh, K.-B.; Shin, J. Nortriterpene Glycosides of the Sarasinoside Class from the Sponge Lipastrotethya sp. J. Nat. Prod. 2012, 75, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.S.; Kalinovskii, A.I.; Stonik, V.A.; Evtushenko, E.V.; Elyakov, G.B. Structure of ulososide A, a new triterpenoid glycoside from the Ulosa sp. sponge. Russ. Chem. Bull. 1994, 43, 1265–1269. [Google Scholar] [CrossRef]
- Antonov, A.S.; Kalinovsky, A.I.; Stonik, V.A. Ulososide B, a new unusual norlanostane-triterpene glycoside and its genuine aglycone from the Madagascar sponge Ulosa sp. Tetrahedron Lett. 1998, 39, 3807–3808. [Google Scholar] [CrossRef]
- Colorado, J.; Muñoz, D.; Marquez, D.; Marquez, M.; Lopez, J.; Thomas, O.P.; Martinez, A. Ulososides and Urabosides—Triterpenoid Saponins from the Caribbean Marine Sponge Ectyoplasia ferox. Molecules 2013, 18, 2598–2610. [Google Scholar] [CrossRef] [PubMed]
- Cachet, N.; Regalado, E.L.; Genta-Jouve, G.; Mehiri, M.; Amade, P.; Thomas, O.P. Steroidal glycosides from the marine sponge Pandaros acanthifolium. Steroids 2009, 74, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Regalado, E.L.; Tasdemir, D.; Kaiser, M.; Cachet, N.; Amade, P.; Thomas, O.P. Antiprotozoal Steroidal Saponins from the Marine Sponge Pandaros acanthifolium. J. Nat. Prod. 2010, 73, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Regalado, E.L.; Jimenez-Romero, C.; Genta-Jouve, G.; Tasdemir, D.; Amade, P.; Nogueiras, C.; Thomas, O.P. Acanthifoliosides, minor steroidal saponins from the Caribbean sponge Pandaros acanthifolium. Tetrahedron 2011, 67, 1011–1018. [Google Scholar] [CrossRef]
- Regalado, E.L.; Turk, T.; Tasdemir, D.; Gorjanc, M.; Kaiser, M.; Thomas, O.P.; Fernandez, R.; Amade, P. Cytotoxic and haemolytic steroidal glycosides from the Caribbean sponge Pandaros acanthifolium. Steroids 2011, 76, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Choi, B.W.; Lee, B.H.; Hwang, K.-H.; Lee, U.C.; Jeong, D.S.; Lee, N.H. Wondosterols A-C, three steroidal glycosides from a Korean marine two-sponge association. Tetrahedron 1999, 55, 13171–13178. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Paloma, L.G.; Minale, L.; Riccio, R.; Debitus, C. Structure chacterization by two-dimensional NMR spectroscopy, of two marine triterpene oligoglycosides from a pacific sponge of the genus Erylus. Tetrahedron 1992, 48, 491–498. [Google Scholar] [CrossRef]
- Gulavita, N.K.; Wright, A.E.; Kelly-Borges, M.; Longley, R.E.; Yarwood, D.; Sills, M.A. Eryloside E from an Atlantic sponge Erylus goffrilleri. Tetrahedron Lett. 1994, 35, 4299–4302. [Google Scholar] [CrossRef]
- Stead, P.; Hiscox, S.; Robinson, P.S.; Pike, N.B.; Sidebottom, P.J.; Roberts, A.D.; Taylor, N.L.; Wright, A.E.; Pomponi, S.A.; Langley, D. Eryloside F, a novel penasterol disaccharide possessing potent thrombin receptor antagonist activity. Bioorg. Med. Chem. Lett. 2000, 10, 661–664. [Google Scholar] [CrossRef]
- Shin, J.; Lee, H.-S.; Woo, L.; Rho, J.-R.; Seo, Y.; Cho, K.W.; Sim, C.J. New triterpenoid saponins from the sponge Erylus nobilis. J. Nat. Prod. 2001, 64, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.S.; Kalinovsky, A.I.; Stonik, V.A.; Afiyatullov, S.S.; Aminin, D.L.; Dmitrenok, P.S.; Mollo, E.; Cimino, G. Isolation and Structures of Erylosides from the Carribean Sponge Erylus formosus. J. Nat. Prod. 2007, 70, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, M.; Crews, P. A triterpene tetrasaccharide, formoside, from the Caribbean Choristida sponge Erylus formosus. Tetrahedron Lett. 1994, 35, 7501–7504. [Google Scholar] [CrossRef]
- Takada, K.; Nakao, Y.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. Nobiloside, a New Neuraminidase Inhibitory Triterpenoidal Saponin from the Marine Sponge Erylus nobilis. J. Nat. Prod. 2002, 65, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.; Al-Trabeen, K.; Badran, M.; Wray, V.; Edrada, R.; Proksch, P.; Ebel, R. New steroidal saponins from the sponge Erylus lendenfeldi. ARKIVOC 2004, 37, 17–27. [Google Scholar]
- Sandler, J.S.; Forsburg, S.L.; Faulkner, D.J. Bioactive steroidal glycosides from the marine sponge Erylus lendenfeldi. Tetrahedron 2005, 61, 1199–1206. [Google Scholar] [CrossRef]
- Okada, Y.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. Sokodosides, Steroid Glycosides with an Isopropyl Side Chain, from the Marine Sponge Erylus placenta. J. Org. Chem. 2006, 71, 4884–4888. [Google Scholar] [CrossRef] [PubMed]
- Gabant, M.; Schmitz-Afonso, I.; Gallard, J.-F.; Menou, J.-L.; Laurent, D.; Debitus, C.; Al-Mourabit, A. Sulfated Steroids: Ptilosteroids A–C and Ptilosaponosides A and B from the Solomon Islands Marine Sponge Ptilocaulis spiculifer. J. Nat. Prod. 2009, 72, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Kalinovsky, A.I.; Antonov, A.S.; Afiyatullov, S.S.; Dmitrenok, P.S.; Evtuschenko, E.V.; Stonik, V.A. Mycaloside A, a new steroid oligoglycoside with an unprecedented structure from the Caribbean sponge Mycale laxissima. Tetrahedron Lett. 2002, 43, 523–525. [Google Scholar] [CrossRef]
- Antonov, A.S.; Afiyatullov, S.S.; Kalinovsky, A.I.; Ponomarenko, L.P.; Dmitrenok, P.S.; Aminin, D.L.; Agafonova, I.G.; Stonik, V.A. Mycalosides B–I, Eight New Spermostatic Steroid Oligoglycosides from the Sponge Mycale laxissima. J. Nat. Prod. 2003, 66, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Campagnuolo, C.; Fattorusso, E.; Taglialatela-Scafati, O. Feroxosides A–B, two norlanostane tetraglycosides from the Caribbean sponge Ectyoplasia ferox. Tetrahedron 2001, 57, 4049–4055. [Google Scholar] [CrossRef]
- Glensk, M.; Wray, V.; Nimtz, M.; Schöpke, T. Silenosides A–C, Triterpenoid Saponins from Silene vulgaris. J. Nat. Prod. 1999, 62, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hong, J.; Lee, C.-O.; Im, K.S.; Choi, J.S.; Jung, J.H. Cytotoxic Sterols and Saponins from the Starfish Certonardoa semiregularis. J. Nat. Prod. 2004, 67, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jang, H.; Hong, J.; Lee, C.-O.; Bae, S.-J.; Shin, S.; Jung, J.H. New cytotoxic sulfated saponins from the starfish Certonardoa semiregularis. Arch. Pharm. Res. 2005, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Ivanchina, N.V.; Huong, T.T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Fedorov, S.N.; Dyshlovoy, S.A.; Long, P.Q.; Stonik, V.A. Two new asterosaponins, archasterosides A and B, from the Vietnamese starfish Archaster typicus and their anticancer properties. Bioorg. Med. Chem. Lett. 2010, 20, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Gustafson, K.R.; Crouch, R.C.; Groweiss, A.; Pannell, L.K.; Van, Q.N.; Boyd, M.R. Application of High-Field NMR and Cryogenic Probe Technologies in the Structural Elucidation of Poecillastrin A, a New Antitumor Macrolide Lactam from the Sponge Poecillastra Species. Org. Lett. 2002, 4, 3293–3296. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Choi, B.W.; Rashid, M.A.; Gamble, W.R.; Cardellina, J.H.; Van, Q.N.; Lloyd, J.R.; McMahon, J.B.; Gustafson, K.R. Structural Assignment of Poecillastrins B and C, Macrolide Lactams from the Deep-Water Caribbean Sponge Poecillastra Species. J. Nat. Prod. 2007, 70, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Natori, T.; Kataoka, Y.; Kato, S.; Kawai, H.; Fusetani, N. Poecillanosine, a new free radical scavenger from the marine sponge Poecillastra spec. aff. tenuilaminaris. Tetrahedron Lett. 1997, 38, 8349–8350. [Google Scholar] [CrossRef]
- Killday, K.B.; Longley, R.; McCarthy, P.J.; Pomponi, S.A.; Wright, A.E.; Neale, R.F.; Sills, M.A. Sesquiterpene-Derived Metabolites from the Deep Water Marine Sponge Poecillastra sollasi. J. Nat. Prod. 1993, 56, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Stonik, V.A.; D’Yachuk, O.G.; Dmitrenok, A.S. Annasterol sulfate, a novel marine sulfated steroid, inhibitor of glucanase activity from the deep water sponge Poecillastra laminaris. Tetrahedron Lett. 1995, 36, 129–132. [Google Scholar] [CrossRef]
- Sun, H.H.; Gross, S.S.; Gunasekera, M.; Koehn, F.E. Weinbersterol disulfates A and B, antiviral steroid sulfates from the sponge Petrosia weinbergi. Tetrahedron 1991, 47, 1185–1190. [Google Scholar] [CrossRef]
- Tvaroska, I.; Taravel, F.R. Carbon-Proton Coupling Constants In The Conformational Analysis of Sugar Molecules. In Advances in Carbohydrate Chemistry and Biochemistry; Derek, H., Ed.; Academic Press: Waltham, MA, USA, 1995; Volume 51, pp. 15–61. [Google Scholar]
- Stenutz, R. Coupling Constants of Pyranoses. Available online: http://www.stenutz.eu/sop/a704.html (accessed on 19 February 2017).
- Wang, Y.-H.; Avula, B.; Fu, X.; Wang, M.; Khan, I.A. Simultaneous Determination of the Absolute Configuration of Twelve Monosaccharide Enantiomers from Natural Products in a Single Injection by a UPLC-UV/MS Method. Planta Med. 2012, 78, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Paudel, L.; Adams, R.W.; Király, P.; Aguilar, J.A.; Foroozandeh, M.; Cliff, M.J.; Nilsson, M.; Sándor, P.; Waltho, J.P.; Morris, G.A. Simultaneously Enhancing Spectral Resolution and Sensitivity in Heteronuclear Correlation NMR Spectroscopy. Angew. Chem. Int. Ed. 2013, 52, 11616–11619. [Google Scholar] [CrossRef] [PubMed]
- Bonini, C.; Kinnel, R.B.; Li, M.; Scheuer, P.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 38. Isolation, structure elucidation, and partial synthesis of papakusterol, a new biosynthetically unusual marine sterol with a cyclopropyl-containing side chain. Tetrahedron Lett. 1983, 24, 277–280. [Google Scholar] [CrossRef]
- Catalan, C.A.N.; Lakshmi, V.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 39. 24ξ,25ξ-24,26-Cyclocholest-5-en-3β-ol, a novel cyclopropyl sterol. Steroids 1982, 40, 455–463. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Kimura, M.; Terasawa, T.; Khalifa, F.A.M.; Ikekawa, N. Stereocontrolled synthesis and determination of the C-24 and C-25 stereochemistry of glaucasterol. Tetrahedron Lett. 1984, 25, 1805–1808. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mitsuhashi, H. Marine sterols. XII. Glaucasterol, a novel C27 sterol with a unique side chain, from the soft coral Sarcophyton glaucum. Steroids 1982, 40, 665–672. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Nist, B.J. The Nuclear Magnetic Resonance Spectra of Cyclopropane Derivatives. J. Am. Chem. Soc. 1963, 85, 2788–2790. [Google Scholar] [CrossRef]
- Audoin, C.; Bonhomme, D.; Ivanisevic, J.; Cruz, M.; Cautain, B.; Monteiro, M.; Reyes, F.; Rios, L.; Perez, T.; Thomas, O.P. Balibalosides, an Original Family of Glucosylated Sesterterpenes Produced by the Mediterranean Sponge Oscarella balibaloi. Mar. Drugs 2013, 11, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Braña, A.F.; Sarmiento-Vizcaíno, A.; Pérez-Victoria, I.; Otero, L.; Fernández, J.; Palacios, J.J.; Martín, J.; de la Cruz, M.; Díaz, C.; Vicente, F.; et al. Branimycins B and C, Antibiotics Produced by the Abyssal Actinobacterium Pseudonocardia carboxydivorans M-227. J. Nat. Prod. 2017, 80, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Cautain, B.; de Pedro, N.; Schulz, C.; Pascual, J.; Sousa, T.d.S.; Martin, J.; Pérez-Victoria, I.; Asensio, F.; González, I.; Bills, G.F.; et al. Identification of the Lipodepsipeptide MDN-0066, a Novel Inhibitor of VHL/HIF Pathway Produced by a New Pseudomonas Species. PLoS ONE 2015, 10, e0125221. [Google Scholar] [CrossRef] [PubMed]
- Wessjohann, L.A.; Brandt, W.; Thiemann, T. Biosynthesis and Metabolism of Cyclopropane Rings in Natural Compounds. Chem. Rev. 2003, 103, 1625–1648. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Cranick, S.; Pomponi, S.A. New Sterol Ester from a Deep Water Marine Sponge, Xestospongia sp. J. Nat. Prod. 1991, 54, 1119–1122. [Google Scholar] [CrossRef]
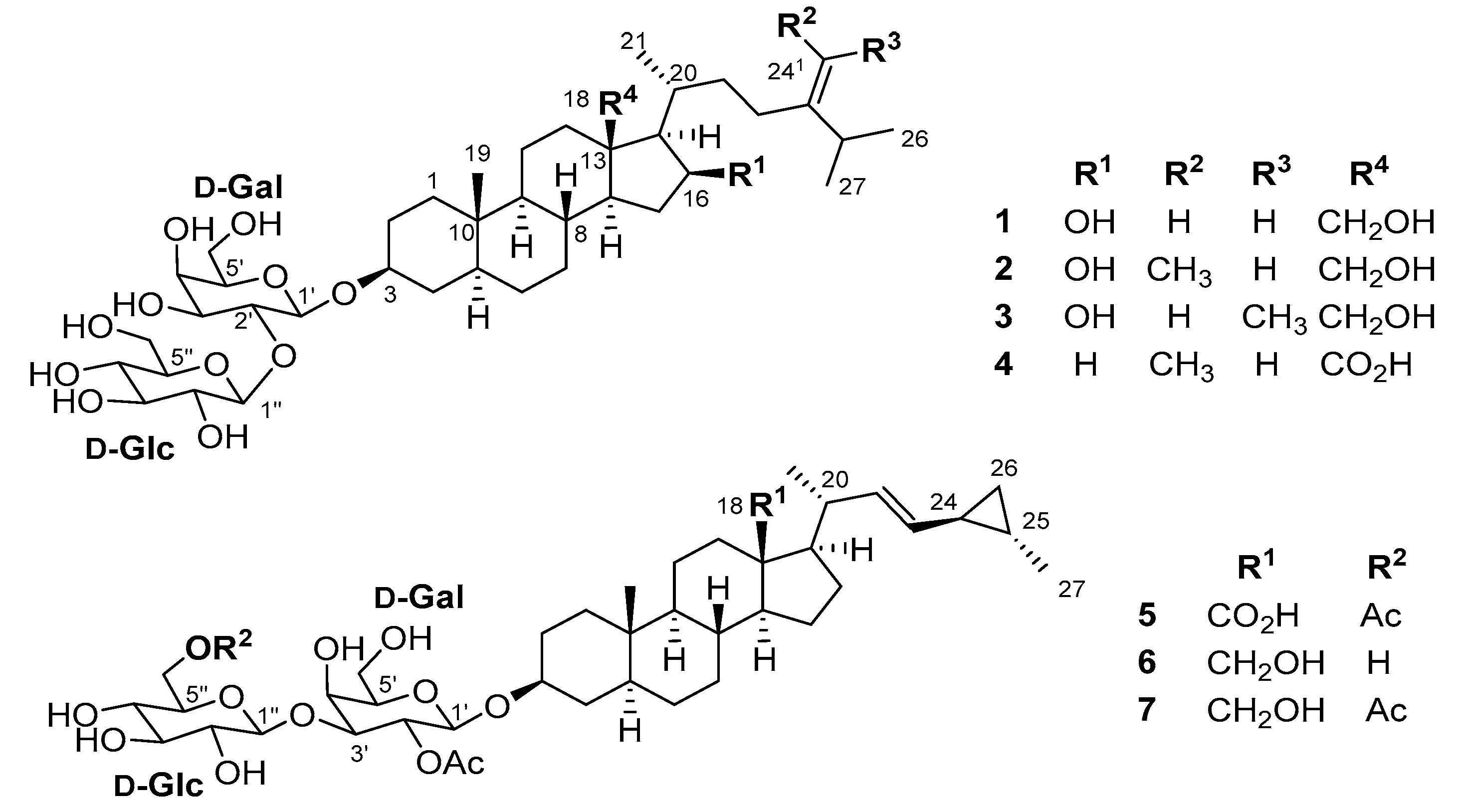
| No. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | |
| 1 | 1.70, m | 38.1 | 1.69, m | 38.1 | 1.69, m | 38.1 | 1.69, m | 38.2 |
| 0.98, m | 0.98, m | 0.98, m | 0.98, m | |||||
| 2 | 1.90, m | 30.5 | 1.90, m | 30.5 | 1.90, m | 30.5 | 1.92, m | 30.5 |
| 1.50, m | 1.50, m | 1.50, m | 1.48, m | |||||
| 3 | 3.72, m | 80.2 | 3.72, m | 80.2 | 3.72, m | 80.2 | 3.72, m | 80.3 |
| 4 | 1.71, m | 35.5 | 1.71, m | 35.6 | 1.71, m | 35.5 | 1.70, m | 35.6 |
| 1.34, m | 1.34, m | 1.34, m | 1.32, m | |||||
| 5 | 1.12, m | 46.2 | 1.12, m | 46.2 | 1.12, m | 46.2 | 1.12, m | 46.1 |
| 6 | 1.34, m | 29.9 | 1.34, m | 29.9 | 1.34, m | 29.8 | 1.32, m | 29.9 |
| 1.32, m | 1.31, m | 1.31, m | 1.29, m | |||||
| 7 | 1.73, m | 33.3 | 1.74, m | 33.3 | 1.75, m | 33.3 | 1.74, m | 33.1 |
| 0.94, m | 0.94, m | 0.95, m | 0.92, m | |||||
| 8 | 1.67, m | 36.1 | 1.67, m | 36.1 | 1.67, m | 36.1 | 1.38, m | 38.5 |
| 9 | 0.75, m | 56.2 | 0.74, m | 56.2 | 0.74, m | 56.2 | 0.72, m | 55.9 |
| 10 | 36.8 | 36.9 | 36.8 | 36.8 | ||||
| 11 | 1.51, m | 22.8 | 1.52, m | 22.8 | 1.52, m | 22.8 | 1.63, m | 24.4 |
| 1.31, m | 1.32, m | 1.32, m | 1.34, m | |||||
| 12 | 2.01, m | 38.9 | 2.01, m | 38.8 | 2.01, m | 38.8 | 2.64, m | 38.2 |
| 1.11, m | 1.10, m | 1.10, m | 1.09, m | |||||
| 13 | 48.1 | 48.1 | 48.1 | 55.8 | ||||
| 14 | 1.10, m | 55.1 | 1.10, m | 55.1 | 1.10, m | 55.1 | 1.39, m | 58.4 |
| 15 | 2.17, m | 38.5 | 2.16, m | 38.6 | 2.16, m | 38.6 | 1.81, m | 26.5 |
| 1.34, m | 1.33, m | 1.33, m | 1.19, m | |||||
| 16 | 4.26, td (7.7, 3.7) | 72.8 | 4.26, td (7.9, 3.7) | 72.8 | 4.26, td (7.9, 3.7) | 72.8 | 1.80, m | 24.4 |
| 0.89, m | ||||||||
| 17 | 1.19, m | 62.3 | 1.19, m | 62.3 | 1.19, m | 62.3 | 1.48, m | 57.4 |
| 18 | 3.95, d (11.6) | 62.6 | 3.95, d (11.6) | 62.6 | 3.95, d (11.6) | 62.4 | 180.1 | |
| 3.59, d (11.6) | 3.60, d (11.6) | 3.60, d (11.6) | ||||||
| 19 | 0.88, s | 12.8 | 0.88, s | 12.8 | 0.88, s | 12.9 | 0.76, s | 12.8 |
| 20 | 1.94, m | 31.6 | 1.93, m | 32.2 | 1.93, m | 32.0 | 1.49, m | 38.8 |
| 21 | 1.02, d (6.8) | 19.0 | 1.07, d (6.7) | 19.1 | 1.02, d (6.7) | 19.1 | 1.09, d (6.3) | 19.1 |
| 22 | 1.87, m | 35.5 | 1.73, m | 35.5 | 1.83, m | 36.8 | 1.45, m | 36.0 |
| 1.21, m | 1.18, m | 1.18, m | 1.14, m | |||||
| 23 | 2.15, m | 32.4 | 2.13, m | 26.8 | 2.04, m | 29.1 | 2.07, m | 29.9 |
| 1.98, m | 1.94, m | 1.83, m | 1.90, m | |||||
| 24 | 158.0 | 148.2 | 146.9 | 147.9 | ||||
| 241 | 4.71, br s 4.70, br s | 106.7 | 5.19, q (6.7) | 116.6 | 5.17, q (6.7) | 117.7 | 5.18, q (6.7) | 116.8 |
| 242 | 1.59, d (6.3) | 13.4 | 1.58, d (6.3) | 12.8 | 1.56, d (6.7) | 13.4 | ||
| 25 | 2.29, h (6.5) | 34.8 | 2.24, m | 36.0 | 2.85, m | 29.8 | 2.19, m | 35.6 |
| 26 | 1.03, d (6.8) | 22.5 | 0.99, d (6.8) | 22.7 | 0.99, d (6.8) | 21.4 | 0.98, d (6.8) | 22.7 |
| 27 | 1.03, d (6.8) | 22.3 | 0.99, d (6.8) | 22.6 | 0.99, d (6.8) | 21.4 | 0.98, d (6.8) | 22.6 |
| 1′ | 4.49, d (7.6) | 101.8 | 4.49, d (7.6) | 101.8 | 4.49, d (7.6) | 101.8 | 4.48, d (7.5) | 101.8 |
| 2′ | 3.70, m | 80.8 | 3.69, t (10.2) | 80.8 | 3.69, t (10.2) | 80.8 | 3.70, t (10.2) | 80.8 |
| 3′ | 3.65, dd (9.6, 3.3) | 74.8 | 3.65, dd (9.6, 3.3) | 74.8 | 3.65, dd (9.6, 3.3) | 74.8 | 3.64, dd (9.5, 3.3) | 74.8 |
| 4′ | 3.84, d (3.2) | 70.0 | 3.84, d (3.2) | 70.0 | 3.84, d (3.2) | 70.0 | 3.84, d (3.1) | 70.0 |
| 5′ | 3.50, t (6.1) | 76.4 | 3.50, t (6.1) | 76.4 | 3.50, t (6.1) | 76.4 | 3.49, t (6.2) | 76.4 |
| 6′ | 3.73, m | 62.7 | 3.73, m | 62.7 | 3.73, m | 62.7 | 3.73, m | 62.7 |
| 3.71, m | 3.71, m | 3.71, m | 3.71, m | |||||
| 1″ | 4.56, d (7.9) | 105.2 | 4.56, d (7.9) | 105.2 | 4.56, d (7.9) | 105.2 | 4.56, d (7.9) | 105.2 |
| 2″ | 3.25, dd (9.1, 7.9) | 75.8 | 3.25, dd (9.1, 7.9) | 75.8 | 3.25, dd (9.1, 7.9) | 75.8 | 3.25, dd (9.0, 7.8) | 75.8 |
| 3″ | 3.37, t (8.8) | 77.7 | 3.37, t (8.8) | 77.7 | 3.37, t (8.8) | 77.7 | 3.37, t (8.9) | 77.7 |
| 4″ | 3.33, t (9.3) | 71.4 | 3.33, t (9.3) | 71.4 | 3.33, t (9.3) | 71.4 | 3.33, t (9.4) | 71.4 |
| 5″ | 3.29, m | 78.4 | 3.29, m | 78.4 | 3.29, m | 78.4 | 3.28, m | 78.4 |
| 6″ | 3.84, dd (11.2, 2.3) | 62.4 | 3.84, dd (11.1, 2.3) | 62.4 | 3.84, dd (11.1, 2.3) | 62.4 | 3.84, dd (13.5, 2.8) | 62.4 |
| 3.71, m | 3.71, m | 3.71, m | 3.71, m | |||||
| No. | 5 | 6 | 7 | |||
|---|---|---|---|---|---|---|
| δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | |
| 1 | 1.70, m | 38.0 | 1.72, m | 38.2 | 1.72, m | 38.2 |
| 0.97, m | 0.97, m | 0.98, m | ||||
| 2 | 1.85, m | 30.4 | 1.86, m | 30.7 | 1.87, m | 30.8 |
| 1.44, m | 1.46, m | 1.46, m | ||||
| 3 | 3.62, m | 79.9 | 3.63, m | 80.0 | 3.62, m | 80.0 |
| 4 | 1.58, m | 35.8 | 1.58, m | 35.9 | 1.58, m | 36.0 |
| 1.17, m | 1.17, m | 1.19, m | ||||
| 5 | 1.12, m | 46.0 | 1.09, m | 46.1 | 1.10, m | 46.1 |
| 6 | 1.32, m | 30.3 | 1.32, m | 29.9 | 1.31, m | 30.4 |
| 1.29, m | 1.29, m | 1.27, m | ||||
| 7 | 1.76, m | 33.1 | 1.68, m | 33.5 | 1.67, m | 33.5 |
| 0.94, m | 0.87, m | 0.87, m | ||||
| 8 | 1.53, m | 38.8 | 1.43, m | 37.1 | 1.43, m | 37.0 |
| 9 | 0.73, m | 55.9 | 0.68, m | 56.0 | 0.68, m | 56.0 |
| 10 | 36.7 | 36.8 | 36.8 | |||
| 11 | 1.63, m | 24.4 | 1.53, m | 22.3 | 1.53, m | 22.3 |
| 1.31, m | 1.36, m | 1.34, m | ||||
| 12 | 2.63, m | 38.4 | 2.44, d (12.8) | 35.9 | 2.44, dt (12.7, 3.4) | 35.9 |
| 1.10, m | 0.94, m | 0.94, m | ||||
| 13 | 55.6 | 47.9 | 47.9 | |||
| 14 | 1.38, m | 58.4 | 1.11, m | 57.6 | 1.12, m | 57.6 |
| 15 | 1.75, m | 30.8 | 1.70, m | 29.9 | 1.71, m | 29.9 |
| 1.30, m | 1.30, m | 1.29, m | ||||
| 16 | 1.78, m | 25.8 | 1.54, m | 25.0 | 1.54, m | 24.9 |
| 1.53, m | 0.98, m | 0.98, m | ||||
| 17 | 1.46, m | 57.3 | 1.15, m | 58.2 | 1.16, m | 58.1 |
| 18 | 180.1 | 3.65, d (11.5) | 60.2 | 3.65, d (11.1) | 60.4 | |
| 3.45, d (11.6) | 3.45, d (11.7) | |||||
| 19 | 0.73, s | 12.7 | 0.83, s | 12.7 | 0.83, s | 12.7 |
| 20 | 1.92, m | 42.4 | 2.26, m | 41.7 | 2.26, m | 41.7 |
| 21 | 1.07, d (6.3) | 21.2 | 1.07, d (5.9) | 22.1 | 1.07, d (6.4) | 22.1 |
| 22 | 5.21, dd (15.1, 8.5) | 134.6 | 5.22, dd (14.8, 9.0) | 136.0 | 5.22, dd (15.2, 8.9) | 136.0 |
| 23 | 4.90, m | 132.4 | 4.94, dd (14.8, 8.1) | 131.6 | 4.94, dd (15.2, 8.3) | 131.6 |
| 24 | 0.96, m | 23.4 | 0.93, m | 23.4 | 0.93, m | 23.4 |
| 25 | 0.62, m | 15.5 | 0.62, m | 15.5 | 0.62, m | 15.5 |
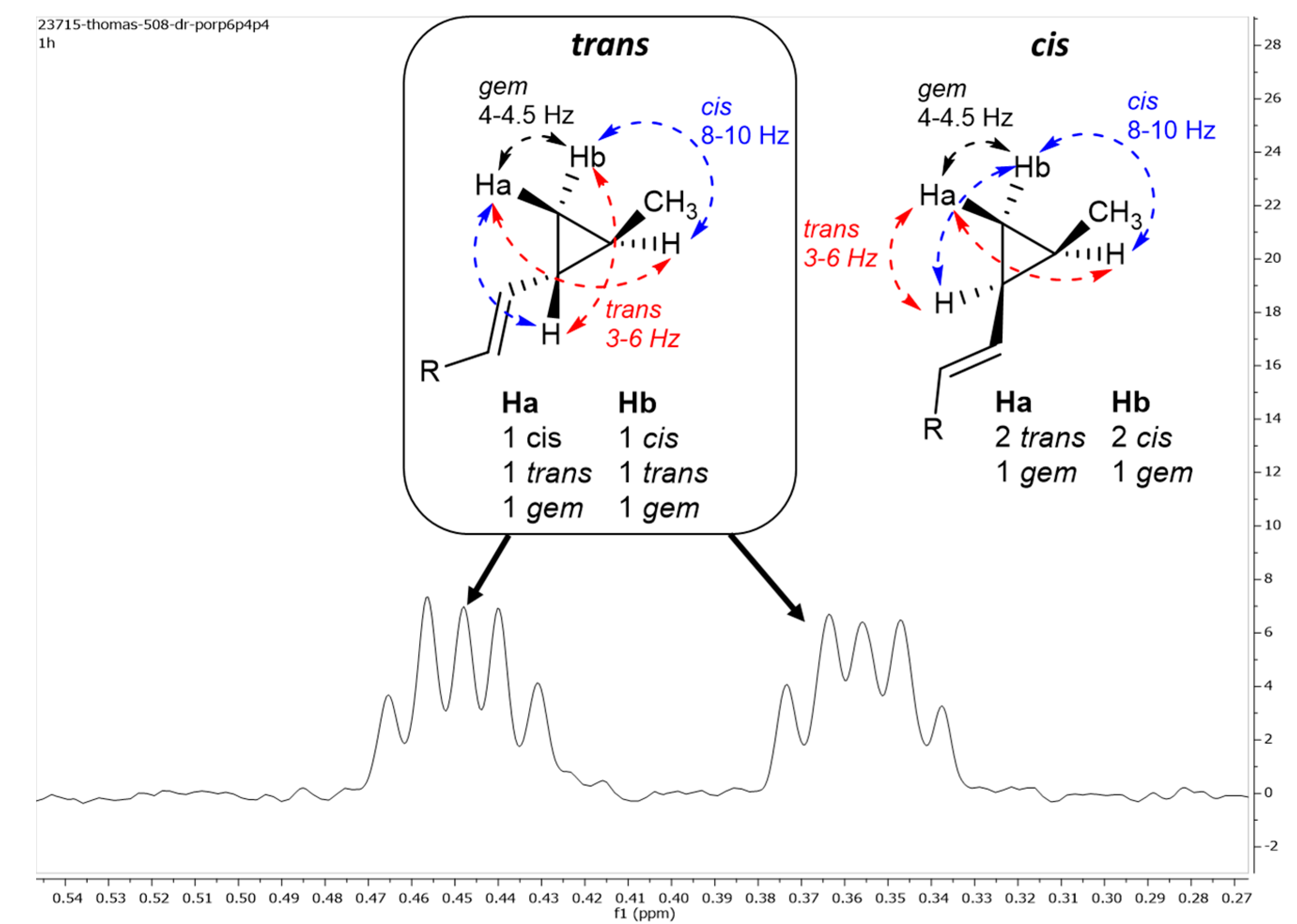
| 26 | 0.44, td (9.0, 4.5) | 15.2 | 0.45, m | 15.2 | 0.45, m | 15.2 |
| 0.36, dt (9.0, 4.5) | 0.36, m | 0.35, m | ||||
| 27 | 1.03, d (5.9) | 18.8 | 1.03, d (5.8) | 18.9 | 1.03, d (5.9) | 18.9 |
| 1′ | 4.56, d (8.0) | 101.1 | 4.55, d (7.9) | 101.2 | 4.56, d (8.0) | 101.2 |
| 2′ | 5.11, dd (8.4, 8.1) | 72.5 | 5.12, dd (9.0, 7.7) | 72.6 | 5.11, dd (10.1, 8.0) | 72.4 |
| 2′-Ac | 2.06, s | 21.2 | 2.06, s | 21.2 | 2.06, s | 21.2 |
| 172.2 | 171.2 | 172.2 | ||||
| 3′ | 3.76, dd (10.2, 3.3) | 82.4 | 3.80, dd (10.0, 2.8) | 82.2 | 3.76, dd (10.1, 3.2) | 82.4 |
| 4′ | 4.07, d (3.2) | 70.2 | 4.11, d (3.1) | 70.2 | 4.07, d (3.4) | 70.2 |
| 5′ | 3.55, t (6.1) | 76.4 | 3.56, t (6.2) | 76.4 | 3.55, t (6.4) | 76.4 |
| 6′ | 3.74, m | 62.3 | 3.74, m | 62.2 | 3.74, m | 62.1 |
| 3.73, m | 3.72, m | 3.72, m | ||||
| 1″ | 4.39, d (7.6) | 106.0 | 4.38, d (7.9) | 106.0 | 4.38, d (8.0) | 106.0 |
| 2″ | 3.21, t (8.3) | 74.6 | 3.19, t (8.3) | 74.8 | 3.21, t (8.3) | 74.7 |
| 3″ | 3.32, t (10.1) | 77.7 | 3.35, m | 77.9 | 3.33, m | 77.9 |
| 4″ | 3.28, t (9.6) | 71.6 | 3.28, m | 71.3 | 3.29, m | 71.5 |
| 5″ | 3.46, m | 75.3 | 3.64, m | 80.0 | 3.46, m | 75.3 |
| 6″ | 4.38, d (11.9) | 64.7 | 3.84, m | 62.5 | 4.38, dd (11.9, 2.7) | 64.7 |
| 4.20, dd (11.9, 6.1) | 3.67, m | 4.20, dd (11.9, 6.2) | ||||
| 6″-Ac | 2.06, s | 20.8 | 2.06, s | 20.8 | ||
| 172.8 | 172.8 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabro, K.; Kalahroodi, E.L.; Rodrigues, D.; Díaz, C.; Cruz, M.d.l.; Cautain, B.; Laville, R.; Reyes, F.; Pérez, T.; Soussi, B.; et al. Poecillastrosides, Steroidal Saponins from the Mediterranean Deep-Sea Sponge Poecillastra compressa (Bowerbank, 1866). Mar. Drugs 2017, 15, 199. https://doi.org/10.3390/md15070199
Calabro K, Kalahroodi EL, Rodrigues D, Díaz C, Cruz Mdl, Cautain B, Laville R, Reyes F, Pérez T, Soussi B, et al. Poecillastrosides, Steroidal Saponins from the Mediterranean Deep-Sea Sponge Poecillastra compressa (Bowerbank, 1866). Marine Drugs. 2017; 15(7):199. https://doi.org/10.3390/md15070199
Chicago/Turabian StyleCalabro, Kevin, Elaheh Lotfi Kalahroodi, Daniel Rodrigues, Caridad Díaz, Mercedes de la Cruz, Bastien Cautain, Rémi Laville, Fernando Reyes, Thierry Pérez, Bassam Soussi, and et al. 2017. "Poecillastrosides, Steroidal Saponins from the Mediterranean Deep-Sea Sponge Poecillastra compressa (Bowerbank, 1866)" Marine Drugs 15, no. 7: 199. https://doi.org/10.3390/md15070199