Sponge-Inspired Dibromohemibastadin Prevents and Disrupts Bacterial Biofilms without Toxicity
Abstract
:1. Introduction
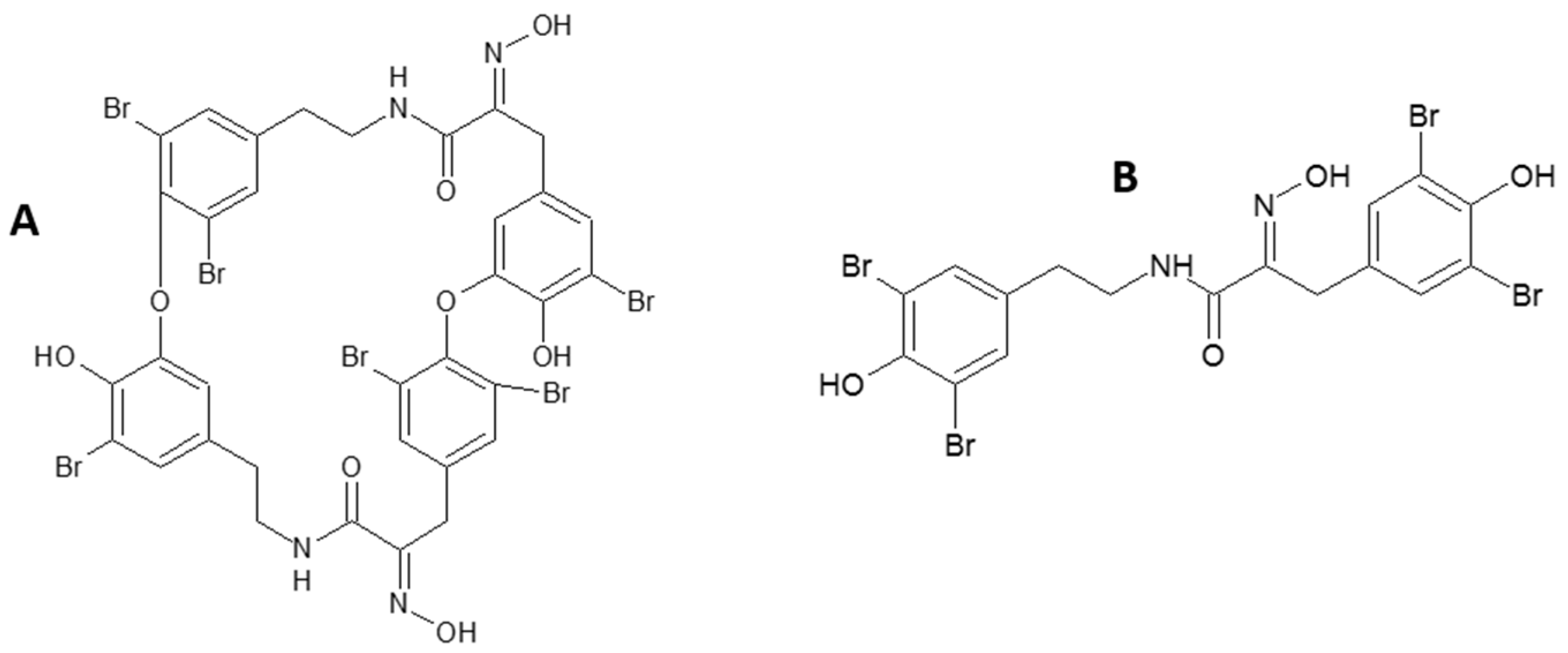
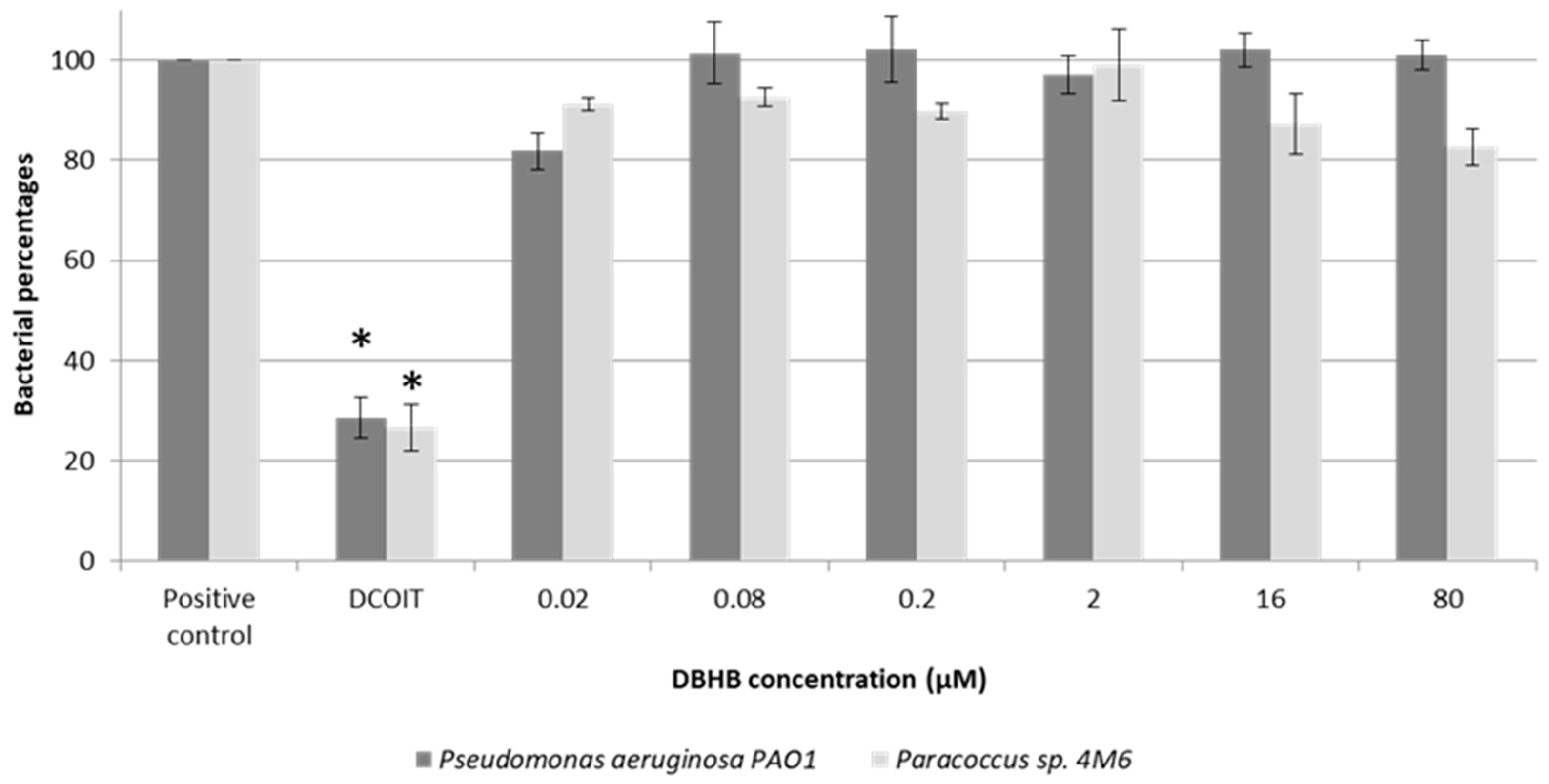
2. Results
2.1. Anti-Bacterial Activity
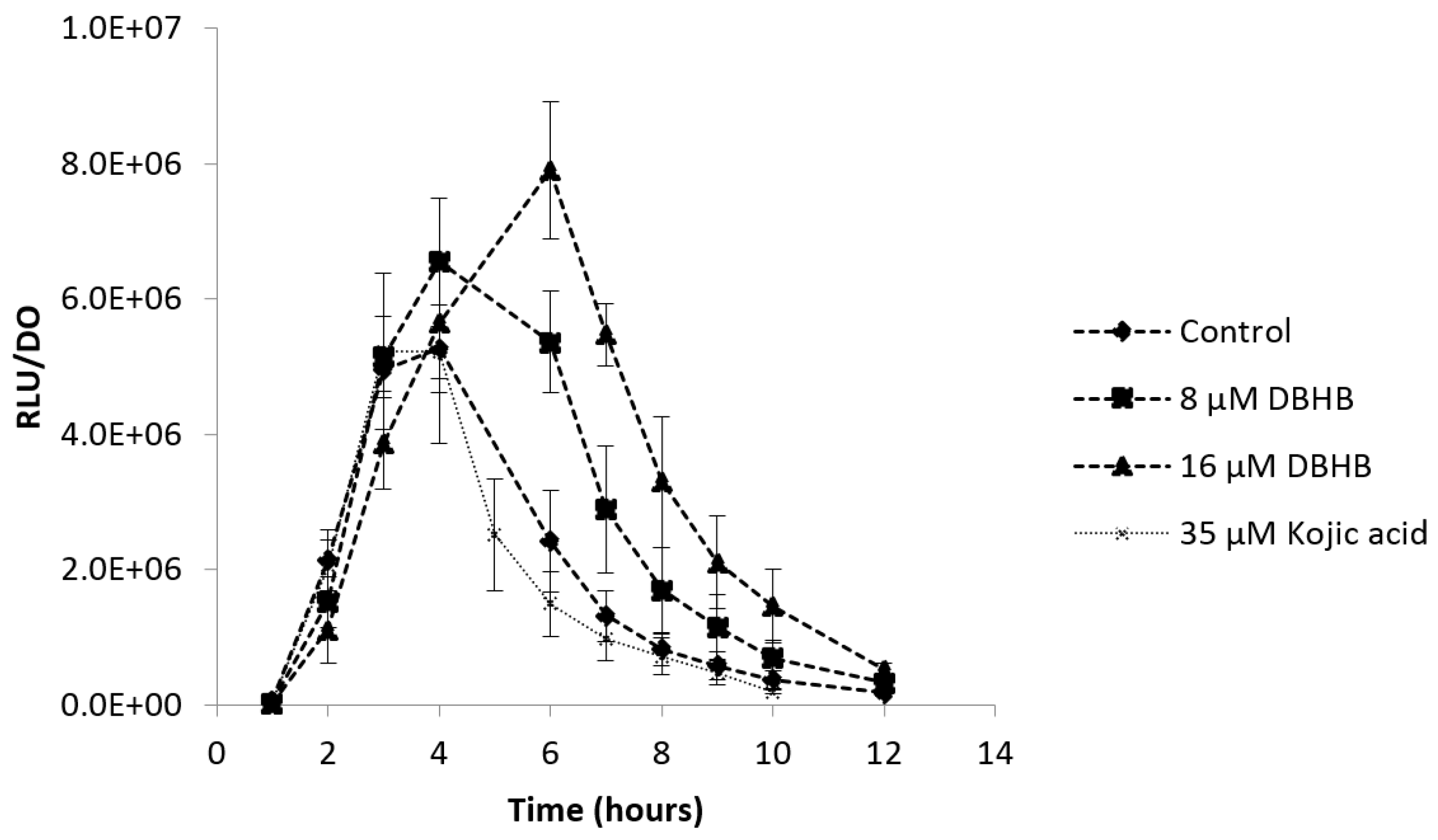
2.2. Impact of DBHB on AHL Production
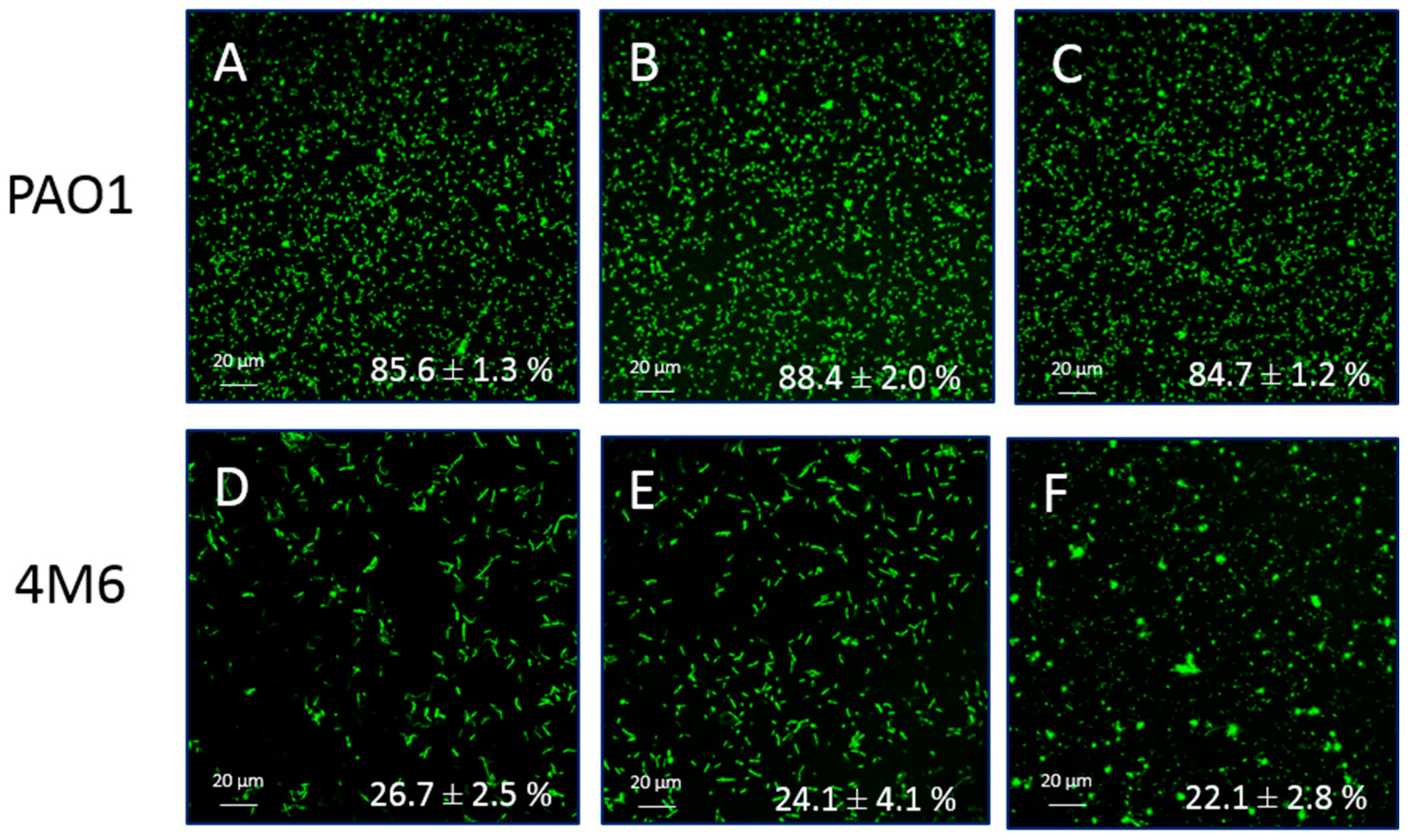
2.3. Anti-Adhesion Activity
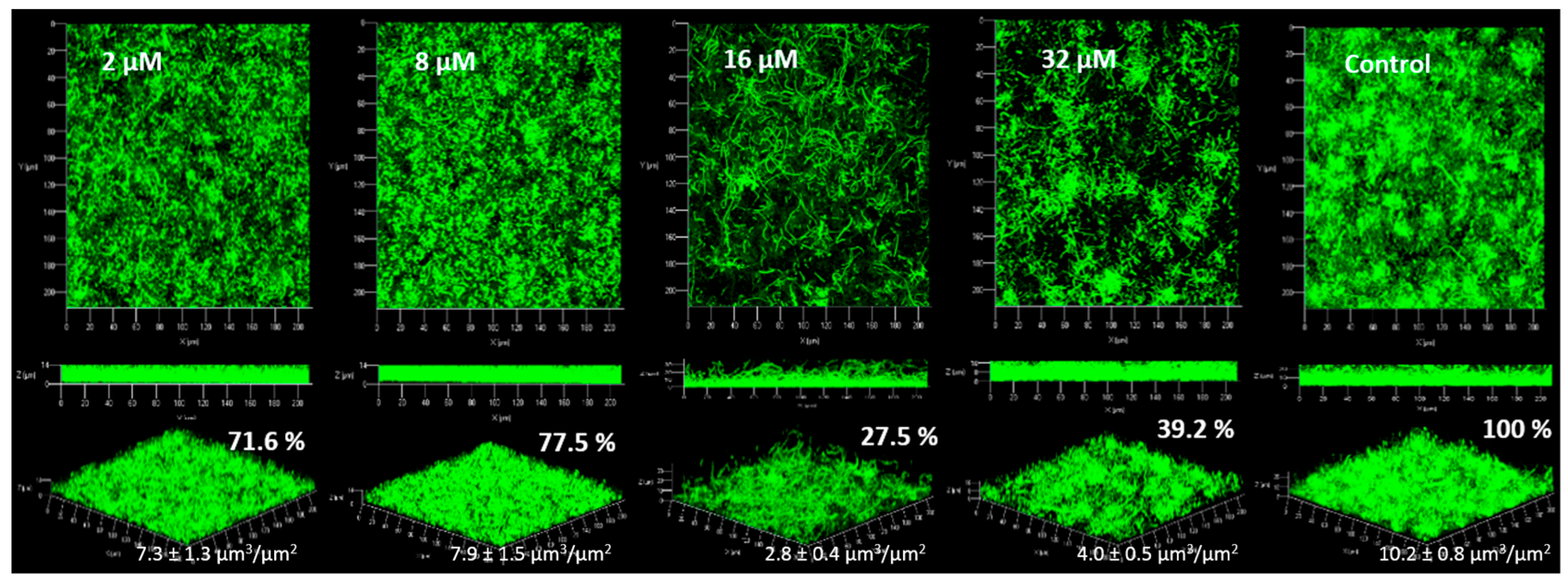
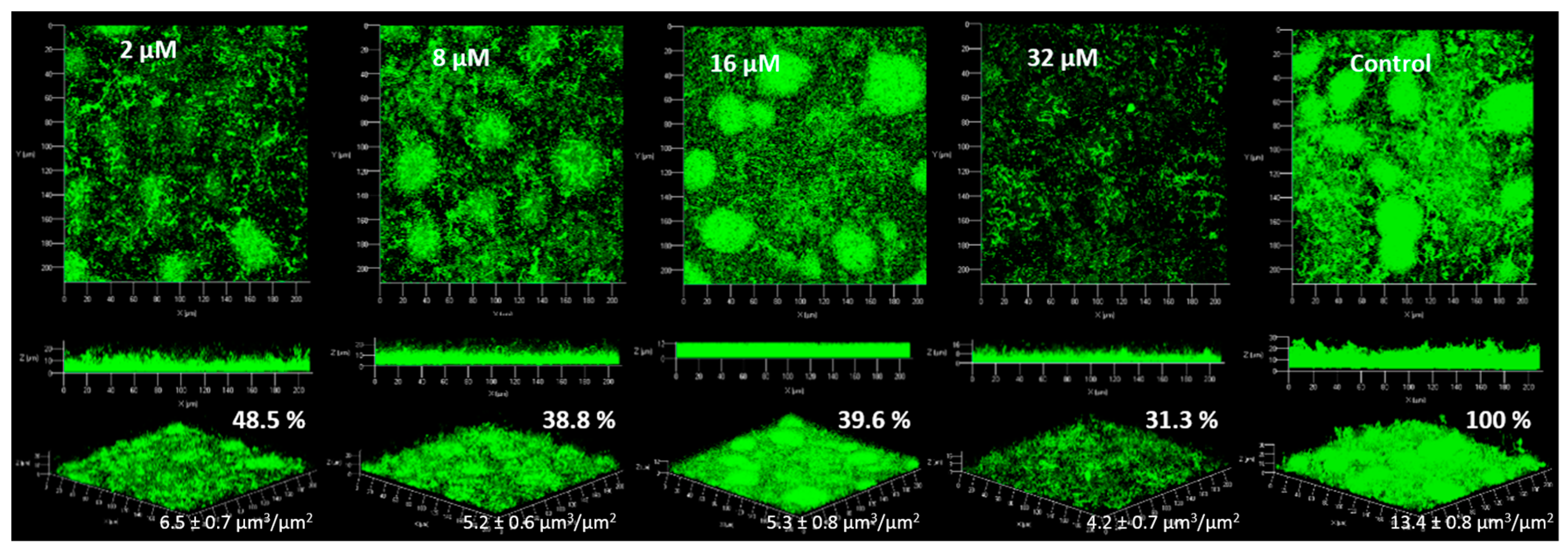
2.4. Anti-Biofilm Activity
2.4.1. Biofilm Formation
2.4.2. Biofilm Degradation
2.5. Anti-Quorum Sensing Activity
3. Discussion
3.1. Anti-Bacterial Activity
3.2. Impact of DBHB on AHL Production
3.3. Anti-Adhesion Activity
3.4. Anti-Biofilm Activity
3.5. Anti-Quorum Sensing Activity
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Impact of DBHB on AHL Production
4.3. Anti-Bacterial Activity
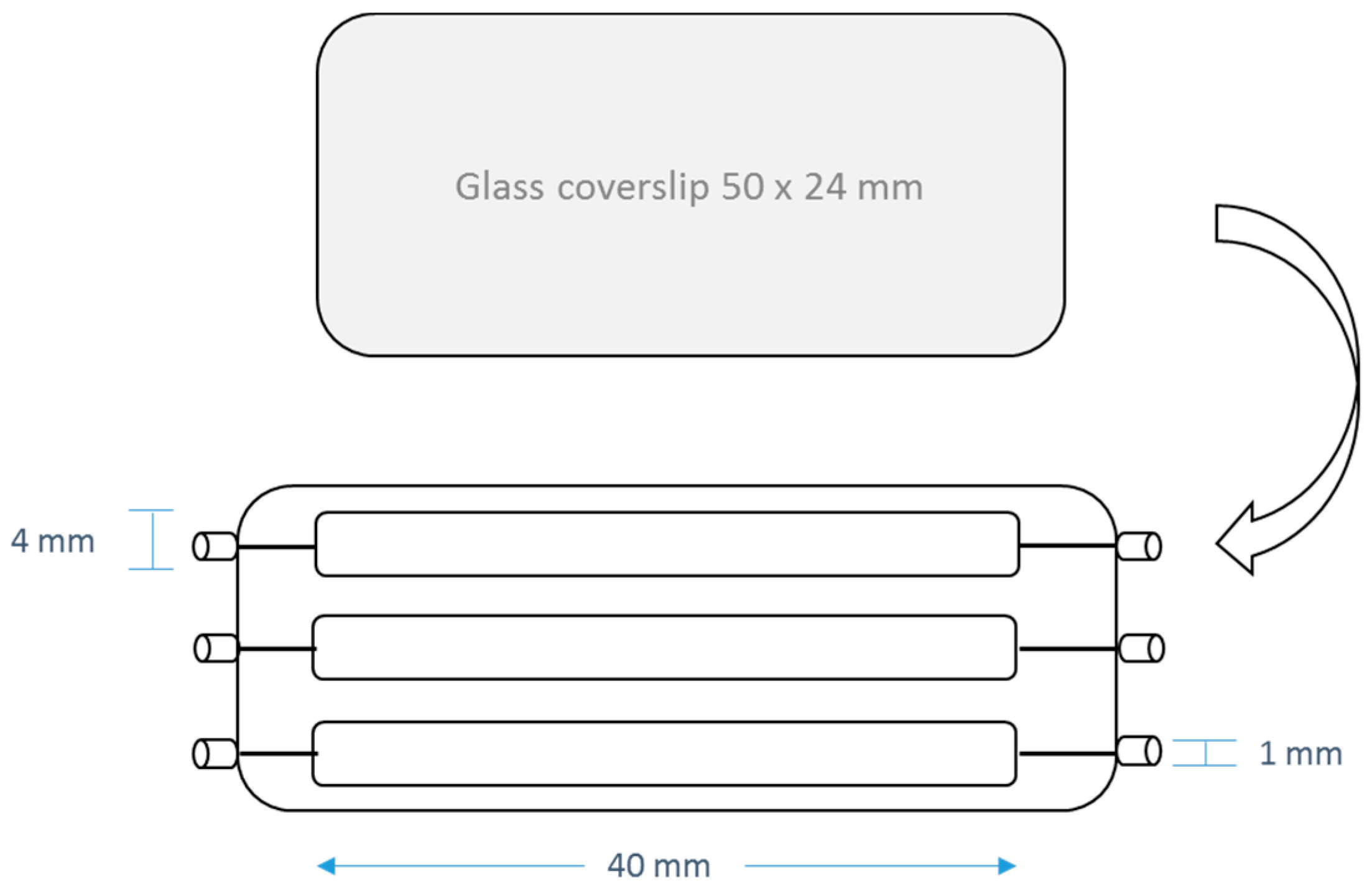
4.4. Anti-Adhesion Activities
4.5. Anti-Biofilm Acitivities
4.6. Anti-Quorum Sensing Activities
4.7. Statistical Analysis of Data
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Rubio, C. Compréhension des Mécanismes D’adhésion des Biofilms en Milieu Marin en vue de la Conception de Nouveaux Moyens de Prévention; Université Paris 6: Paris, France, 2002. (In French) [Google Scholar]
- Qian, P.-Y.; Lau, S.C.K.; Dahms, H.-U.; Dobretsov, S.; Harder, T. Marine biofilms as mediators of colonization by marine macroorganisms: Implications for antifouling and aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, S.; Aikawa, H.; Hayashi, H.; Yuasa, A.; Hirata, A. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol. Lett. 2003, 223, 287–292. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Lu, W.; Rabinowitz, J.D.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic Di-GMP levels and repression of vpsT. J. Bacteriol. 2008, 190, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.L.; Hanzelka, B.L.; Eberhard, A.; Greenberg, E.P. Quorum sensing in Vibrio fischeri: Probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol. 1996, 178, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.C.; Federle, M.J. Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol. Rev. 2014, 38, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of Cell-to-Cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 2002, 148, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Kociolek, M.G. Quorum-sensing inhibitors and biofilms. Anti-Infect. Agents Med. Chem. 2009, 8, 315–326. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Peter, S.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Zhang, L.-H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar] [PubMed]
- Yada, S.; Kamalesh, B.; Sonwane, S.; Guptha, I.; Swetha, R.K. Quorum sensing inhibition, relevance to periodontics. J. Int. Oral Health 2015, 7, 67–69. [Google Scholar] [PubMed]
- Fusetani, N. Antifouling marine natural products. Nat. Prod. Rep. 2011, 28, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Johnson, A.-L.; Hedner, E.; Dahlström, M.; Göransson, U.; Shirani, H.; Bergman, J.; Jonsson, P.R.; Bohlin, L. Antifouling activity of synthesized peptide analogs of the sponge metabolite barettin. Peptides 2006, 27, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Perdicaris, S.; Vlachogianni, T.; Valavanidis, A. Bioactive natural substances from marine sponges: New developments and prospects for future pharmaceuticals. Nat. Prod. Chem. Res. 2013, 1, 2329–6836. [Google Scholar] [CrossRef]
- Laport, M.S.; Santos, O.C.S.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H. Potential of sponges and microalgae for marine biotechnology. Trends Biotechnol. 2008, 26, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Dahlström, M.; Hedner, E.; Jonsson, P.R.; Vik, A.; Gundersen, L.-L.; Bohlin, L. Antifouling activity of the sponge metabolite agelasine D and synthesised analogs on Balanus improvisus. Biofouling 2008, 24, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Nir, S.; Reches, M. Bio-inspired antifouling approaches: The quest towards non-toxic and non-biocidal materials. Curr. Opin. Biotechnol. 2016, 39, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, X.; Proksch, P.; Perry, C.C.; Osinga, R.; Gardères, J.; Schröder, H.C. Principles of biofouling protection in marine sponges: A model for the design of novel biomimetic and bio-inspired coatings in the marine environment? Mar. Biotechnol. 2013, 15, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.; Hellio, C.; Maréchal, J.-P.; Frank, W.; Lin, W.; Weber, H.; Proksch, P. Antifouling bastadin congeners target mussel phenoloxidase and complex copper(II) ions. Mar. Biotechnol. 2011, 13, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Hagenow, J.; Chung, M.-Y.; Hellio, C.; Weber, H.; Proksch, P. SAR of sponge-inspired hemibastadin congeners inhibiting blue mussel phenoloxidase. Mar. Drugs 2015, 13, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Ortlepp, S.; Sjögren, M.; Dahlström, M.; Weber, H.; Ebel, R.; Edrada, R.; Thoms, C.; Schupp, P.; Bohlin, L.; Proksch, P. Antifouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar. Biotechnol. 2007, 9, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Tolker-Nielsen, T.; Sternberg, C. Methods for studying biofilm formation: Flow cells and confocal laser scanning microscopy. In Pseudomonas Methods and Protocols; Filloux, A., Ramos, J.-L., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; pp. 615–629. ISBN 978-1-4939-0472-3. [Google Scholar]
- Grasland, B. Etude du Rôle des Interactions Energétiques et des Communications Intercellulaires Dans la Formation de Biofilms Bactériens en Milieu Marin. Ph.D. Thesis, Université de Bretagne-Sud, Lorient, France, 2002. [Google Scholar]
- Jacobson, A.H.; Willingham, G.L. Sea-nine antifoulant: An environmentally acceptable alternative to organotin antifoulants. Sci. Total Environ. 2000, 258, 103–110. [Google Scholar] [CrossRef]
- Yang, J.; Evans, B.A.; Rozen, D.E. Signal diffusion and the mitigation of social exploitation in pneumococcal competence signalling. Proc. Biol. Sci. 2010, 277, 2991–2999. [Google Scholar] [CrossRef] [PubMed]
- Monnet, V.; Juillard, V.; Gardan, R. Peptide conversations in Gram-positive bacteria. Crit. Rev. Microbiol. 2016, 42, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.; Grasland, B.; Vallée-Réhel, K.; Dufau, C.; Haras, D. On-line high-performance liquid chromatography-mass spectrometric detection and quantification of N-acylhomoserine lactones, quorum sensing signal molecules, in the presence of biological matrices. J. Chromatogr. A 2003, 1002, 79–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, N.; Cottrell, M.T.; Kirchman, D.L. Contribution of major bacterial groups to bacterial biomass production along a salinity gradient in the South China Sea. Aquat. Microb. Ecol. 2006, 43, 233–241. [Google Scholar] [CrossRef]
- Hommen, U.; Baveco, J.M.; Galic, N.; van den Brink, P.J. Potential application of ecological models in the European environmental risk assessment of chemicals I: Review of protection goals in EU directives and regulations. Integr. Environ. Assess. Manag. 2010, 6, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, K.; Paul, D.; Kweon, J.H. Inhibition of quorum sensing mechanism and aeromonas hydrophila biofilm formation by vanillin. Environ. Eng. Sci. 2009, 26, 1359–1363. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Toutain, C.M.; Caizza, N.C.; Zegans, M.E.; O’Toole, G.A. Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Res. Microbiol. 2007, 158, 471–477. [Google Scholar] [CrossRef] [PubMed]
- DiRienzo, J.M.; Nakamura, K.; Inouye, M. The outer membrane proteins of gram-negative bacteria: Biosynthesis, assembly, and functions. Annu. Rev. Biochem. 1978, 47, 481–532. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1996, 178, 5853–5859. [Google Scholar] [CrossRef] [PubMed]
- Braekman, J.C.; Daloze, D. Chemical defence in sponges. Pure Appl. Chem. 2009, 58, 357–364. [Google Scholar] [CrossRef]
- Engel, S.; Pawlik, J.R. Allelopathic activities of sponge extracts. Mar. Ecol. Prog. Ser. 2000, 207, 273–281. [Google Scholar] [CrossRef]
- Assmann, M.; Lichte, E.; Pawlik, J.R.; Kck, M. Chemical defenses of the Caribbean sponges Agelas wiedenmayeri and Agelas conifera. Mar. Ecol. Prog. Ser. 2000, 207, 255–262. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E.G.; Proksch, P. From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Huigens, R.W.; Richards, J.J.; Parise, G.; Ballard, T.E.; Zeng, W.; Deora, R.; Melander, C. Inhibition of Pseudomonas aeruginosa biofilm formation with bromoageliferin analogues. J. Am. Chem. Soc. 2007, 129, 6966–6967. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Lin, W.; Proksch, P. Bioactive sesterterpenes and triterpenes from marine sponges: Occurrence and pharmacological significance. Mar. Drugs 2010, 8, 313–346. [Google Scholar] [CrossRef] [PubMed]
- Melander, C.; Moeller, P.D.R.; Ballard, T.E.; Richards, J.J.; Huigens, R.W., III; Cavanagh, J. Evaluation of dihydrooroidin as an antifouling additive in marine paint. Int. Biodeterior. Biodegrad. 2009, 63, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Hellio, C.; Pavia, H.; Stensen, W.; Stensvåg, K.; Svendsen, J.-S.; Haug, T.; Svenson, J. Antifouling compounds from the sub-arctic ascidian synoicum pulmonaria: Synoxazolidinones A and C, pulmonarins A and B, and synthetic analogues. J. Nat. Prod. 2014, 77, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.E.; Richards, J.J.; Wolfe, A.L.; Melander, C. Synthesis and antibiofilm activity of a second-generation reverse-Amide oroidin library: A structure-activity relationship study. Chem. Eur. J. 2008, 14, 10745–10761. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, C.M.; Grumezescu, M.A.; Lazar, V. Quorum sensing inhibitors from the sea: Lessons from marine symbiotic relationships. Curr. Organ. Chem. 2014, 18, 823–839. [Google Scholar] [CrossRef]
- Nithya, C.; Begum, M.F.; Pandian, S.K. Marine bacterial isolates inhibit biofilm formation and disrupt mature biofilms of Pseudomonas aeruginosa PAO1. Appl. Microbiol. Biotechnol. 2010, 88, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Iii, R.W.H.; Ma, L.; Gambino, C.; Moeller, P.D.R.; Basso, A.; Cavanagh, J.; Wozniak, D.J.; Melander, C. Control of bacterial biofilms with marine alkaloid derivatives. Mol. BioSyst. 2008, 4, 614–621. [Google Scholar] [CrossRef]
- Andjouh, S.; Blache, Y. Screening of bromotyramine analogues as antifouling compounds against marine bacteria. Biofouling 2016, 32, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.J.; Ballard, T.E.; Huigens, R.W.; Melander, C. Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. ChemBioChem 2008, 9, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Forte, B.; Malgesini, B.; Piutti, C.; Quartieri, F.; Scolaro, A.; Papeo, G. A submarine journey: The pyrrole-imidazole alkaloids. Mar. Drugs 2009, 7, 705–753. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-G.; O’Toole, G.A. c-di-GMP and its effects on biofilm formation and dispersion: A Pseudomonas aeruginosa review. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Anetzberger, C.; Pirch, T.; Jung, K. Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi. Mol. Microbiol. 2009, 73, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Kumar, P.; Pandian, S.T.K.; Sharma, P. Biofouling control by quorum quenching. In Springer Handbook of Marine Biotechnology; Prof, S.-K.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 431–440. ISBN 978-3-642-53970-1. [Google Scholar]
- Skindersoe, M.E.; Ettinger-Epstein, P.; Rasmussen, T.B.; Bjarnsholt, T.; de Nys, R.; Givskov, M. Quorum sensing antagonism from marine organisms. Mar. Biotechnol. 2007, 10, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Teplitski, M.; Paul, V. Mini-review: Quorum sensing in the marine environment and its relationship to biofouling. Biofouling 2009, 25, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Kievit, T.R.D.; Gillis, R.; Marx, S.; Brown, C.; Iglewski, B.H. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: Their role and expression patterns. Appl. Environ. Microbiol. 2001, 67, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Grasland, B.; Mitalane, J.; Briandet, R.; Quemener, E.; Meylheuc, T.; Linossier, I.; Vallee-Rehel, K.; Haras, D. Bacterial biofilm in seawater: Cell surface properties of early-attached marine bacteria. Biofouling 2003, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.A.; Bassler, B.L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 1999, 31, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.; Frommberger, M.; Rothballer, M.; Li, X.; Englmann, M.; Fekete, J.; Hartmann, A.; Eberl, L.; Schmitt-Kopplin, P. Identification of bacterial N-acylhomoserine lactones (AHLs) with a combination of ultra-performance liquid chromatography (UPLC), ultra-high-resolution mass spectrometry, and in-situ biosensors. Anal. Bioanal. Chem. 2007, 387, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program comstat. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.P.; Hastings, J.W.; Ulitzur, S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch. Microbiol. 1979, 120, 87–91. [Google Scholar] [CrossRef]
- Yang, C.; Fang, S.; Chen, D.; Wang, J.; Liu, F.; Xia, C. The possible role of bacterial signal molecules N-acyl homoserine lactones in the formation of diatom-biofilm (Cylindrotheca sp.). Mar. Pollut. Bull. 2016, 107, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Tait, K.; Havenhand, J. Investigating a possible role for the bacterial signal molecules N-acylhomoserine lactones in Balanus improvisus cyprid settlement. Mol. Ecol. 2013, 22, 2588–2602. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Strains | Gram | AHL Produced |
|---|---|---|
| Pseudomonas aeruginosa PAO1 | Negative | C4-HSL, C6-HSL and 3-oxo-C12-HSL |
| Paracoccus sp. 4M6 | Negative | C4-HSL, C6-HSL, C8-HSL and 3-oxo-C10-HSL |
| Bacillus sp. 4J6 | Positive | Autoinducer-2 |
| Pseudoalteromonas sp. 5M6 | Negative | No. AHL |
| Bacterial strains | Control | 16 µM DBHB | 32 µM DBHB |
|---|---|---|---|
| 4J6 biovolumes (µm3/µm2) | 18.9 ± 1.8 | 21.5 ± 1.9 | 22.5 ± 1.7 |
| 5M6 biovolumes (µm3/µm2) | 22.0 ± 1.5 | 21.9 ± 1.3 | 22.1 ± 1.8 |
| ANOVA | - | - | |
| TUKEY | - | - |
| Biovolumes (µm3/µm2) | Paracoccus sp. 4M6 | Pseudomonas aeruginosa PAO1 | ||
|---|---|---|---|---|
| Control | 32 µM DBHB | Control | 32 µM DBHB | |
| Living cells (Syto9®) | 12.7 ± 1.3 | 4.9 ± 0.1 | 14.7 ± 1.0 | 4.3 ± 1.0 |
| Dead cells (SytoxRed®) | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.02 | 0.2 ± 0.03 |
| Mortality percentage | 0.98 | 0.94 | 0.98 | 0.95 |
| ANOVA | - | - | ||
| TUKEY | - | - | ||
| Classes | Compounds | Activity | References |
|---|---|---|---|
| Terpenoids | Ageloxime-D | antibiofilm, antimacrofouling | [17,46] |
| Manoalide | antibiotic, anti-inflammatory, anti-quorum sensing | [48] | |
| Pyrrole-imidazole alkaloids | Oroidin | antibiofilm, antifouling | [49,50,51] |
| Sceptrin | antifouling, anti-quorum sensing | [52] | |
| Bromoageliferin | anti-adhesion, antibiofilm | [47,53] |
| Activities | Concentrations | Bacteria | Conditions | |
|---|---|---|---|---|
| Anti-adhesion | 16 µM | P. aeruginosa PAO1 Paracoccus sp. 4M6 | 1 | Addition in the bacterial suspension |
| 2 | Conditioning of the adhesion surface | |||
| Anti-biofilm | 2, 8, 16 and 32 µM | P. aeruginosa PAO1 Paracoccus sp. 4M6 | Biofilm formation: addition in the growth medium flow (dynamic: 150 µL/min) | |
| 16 and 32 µM | Pseudoalteromonas sp. 5M6 Bacillus sp. 4J6 | |||
| 16 µM | P. aeruginosa PAO1 Paracoccus sp. 4M6 | Biofilm degradation: injection of media containing the compound on a biofilm formed (static: 2 h) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Norcy, T.; Niemann, H.; Proksch, P.; Tait, K.; Linossier, I.; Réhel, K.; Hellio, C.; Faÿ, F. Sponge-Inspired Dibromohemibastadin Prevents and Disrupts Bacterial Biofilms without Toxicity. Mar. Drugs 2017, 15, 222. https://doi.org/10.3390/md15070222
Le Norcy T, Niemann H, Proksch P, Tait K, Linossier I, Réhel K, Hellio C, Faÿ F. Sponge-Inspired Dibromohemibastadin Prevents and Disrupts Bacterial Biofilms without Toxicity. Marine Drugs. 2017; 15(7):222. https://doi.org/10.3390/md15070222
Chicago/Turabian StyleLe Norcy, Tiffany, Hendrik Niemann, Peter Proksch, Karen Tait, Isabelle Linossier, Karine Réhel, Claire Hellio, and Fabienne Faÿ. 2017. "Sponge-Inspired Dibromohemibastadin Prevents and Disrupts Bacterial Biofilms without Toxicity" Marine Drugs 15, no. 7: 222. https://doi.org/10.3390/md15070222
APA StyleLe Norcy, T., Niemann, H., Proksch, P., Tait, K., Linossier, I., Réhel, K., Hellio, C., & Faÿ, F. (2017). Sponge-Inspired Dibromohemibastadin Prevents and Disrupts Bacterial Biofilms without Toxicity. Marine Drugs, 15(7), 222. https://doi.org/10.3390/md15070222






