Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products
Abstract
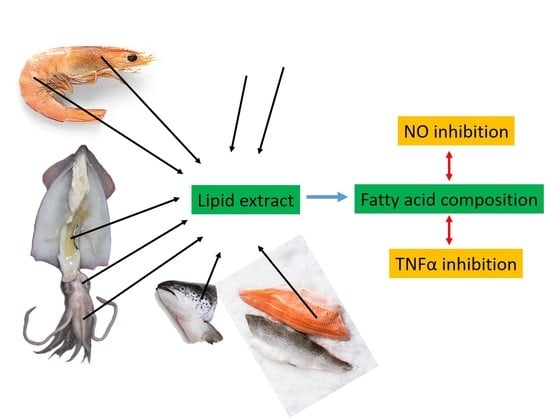
:1. Introduction
2. Results
2.1. Comparison of Fatty Acid Composition from Lipid Extracts of Different Seafood and Waste Products
2.2. Cytotoxicity
2.3. NO Inhibition
2.4. TNF-Alpha Inhibition
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Collection
4.3. Lipid Extraction
4.4. Fatty Acid Methyl Ester (FAME) Analysis
4.5. Cell Lines and Cell Culture
4.6. Lipid Extract Preparation
4.7. Cytotoxicity Assay
4.8. NO Inhibition Assay
4.9. TNF Alpha Inhibition Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharm. 2008, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B.; Amin, A.R.; Clancy, R.M.; Attur, M. The role of nitric oxide in tissue destruction. Best Pract. Res. Clin. Rheumatol. 2001, 15, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H. Nitric oxide: What role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995, 47, 107–116. [Google Scholar] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.M.; Sakata, R.K.; Issy, A.M.; Gerola, L.R.; Salomao, R. Cytokines and pain. Rev. Bras. Anestesiol. 2011, 61, 255–265. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, E.T.; Thomsen, M.; Hall, S.; Vitetta, L. Perna canaliculus and the intestinal microbiome. Mar. Drugs 2017, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Muller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Ozogul, F.; Ozogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Comp. Rev. Food Sci. Fod Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S14–S19. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, J.; Bowden, R.; Shelmadine, B. Fish Oil and c-Reactive Protein. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: San Diego, CA, USA, 2012; pp. 393–405. [Google Scholar]
- Zheng, J.S.; Hu, X.J.; Zhao, Y.M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. Br. Med. J. 2013, 346, f3706. [Google Scholar] [CrossRef] [PubMed]
- Yam, D.; Peled, A.; Shinitzky, M. Suppression of tumor growth and metastasis by dietary fish oil combined with vitamins E and C and cisplatin. Cancer Chemother. Pharmacol. 2001, 47, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Avula, C.P.; Fernandes, G.; Cameron, I.L. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against mda-mb 231 breast cancer xenografts. Clin. Cancer Res. 2001, 7, 2041–2049. [Google Scholar] [PubMed]
- Li, J.J.; Huang, C.J.; Xie, D. Anti-obesity effects of conjugated linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid. Mol. Nutr. Food Res. 2008, 52, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Kim, H.J.; Chiba, H.; Matsumoto, A. Anti-obesity effect of fish oil and fish oil-fenofibrate combination in female kk mice. J. Atheroscler. Thromb. 2009, 16, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Cho, L. What can we expect from omega-3 fatty acids? Cleve Clin. J. Med. 2009, 76, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V. Omega-3 fatty acids and the cardiometabolic syndrome. J. Cardiometab. Syndr. 2008, 3, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Taylor, P.C.; Nelson, G.J.; Schmidt, P.C.; Ferretti, A.; Erickson, K.L.; Yu, R.; Chandra, R.K.; Mackey, B.E. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids 1999, 34, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Siegel, D.; Fedor, D.M.; Adkins, Y.; Mackey, B.E. Dha supplementation decreases serum c-reactive protein and other markers of inflammation in hypertriglyceridemic men. J. Nutr. 2009, 139, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.A.; Hatcher, L. The effect of lean fish consumption on triglyceride levels. Phys. Sports Med. 2009, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Elliott, N.G.; Mooney, B.D.; Scientific, C. Nutritional Value of Australian Seafood II: Factors Affecting Oil Composition of Edible Species; Division of Marine Research, Commonwealth Scientific and Industrial Research Organization (CSIRO): Hobart, TAS, Australia, 1998. [Google Scholar]
- Ackman, R. Seafood lipids. In Seafoods: Chemistry, Processing Technology and Quality; Springer: New York City, NY, USA, 1994; pp. 34–48. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.; Bakar, J.; Ghazali, H. Fatty acid profile of the oil extracted from fish waste (head, intestine and liver)(Sardinella lemuru). World Appl. Sci. J. 2009, 7, 127–131. [Google Scholar]
- Khoddami, A.; Ariffin, A.; Bakar, J.; Ghazali, H. Quality and fatty acid profile of the oil extracted from fish waste (head, intestine and liver)(Euthynnus affinis). Afr. J. Biotechnol. 2012, 11, 1683–1689. [Google Scholar] [CrossRef]
- Nichols, P.D.; Mooney, B.D.; Elliott, N.G. Value-adding to australian marine oils. In Developments in Food Science; Sakaguchi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 42, pp. 115–130. [Google Scholar]
- Vigerust, N.F.; Bjorndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-alpha. Eur. J. Nutr. 2013, 52, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, L. Evaluation of the effect of neptune krill oil on chronic inflammation and arthritic symptoms. J. Am. Coll. Nutr. 2007, 26, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Liu, L.; Kotiw, M.; Benkendorff, K. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J. Ethnopharmacol. 2018, 210, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Ohshima, T. Bioactive marine peptides: Nutraceutical value and novel approaches. Adv. Food Nutr. Res. 2012, 65, 73–105. [Google Scholar] [PubMed]
- Cobb, C.S.; Ernst, E. Systematic review of a marine nutriceutical supplement in clinical trials for arthritis: The effectiveness of the new zealand green-lipped mussel Perna canaliculus. Clin. Rheumatol. 2006, 25, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Chakraborty, M.; Bose, M.; Mukherjee, D.; Roychoudhury, A.; Dhar, P.; Mishra, R. Indian freshwater edible snail bellamya bengalensis lipid extract prevents t cell mediated hypersensitivity and inhibits lps induced macrophage activation. J. Ethnopharmacol. 2014, 157, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, Y.; Zheng, J.; Li, D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar. Drugs 2014, 12, 568–588. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K. Green mussel extract (GME) goes commercial first nutraceutical produced by an ICAR institute. Cmfri Newsl 2012, 135, 5–6. [Google Scholar]
- Pereira, R.B.; Taveira, M.; Valentao, P.; Sousa, C.; Andrade, P.B. Fatty acids from edible sea hares: Anti-inflammatory capacity in lps-stimulated raw 264.7 cells involves inos modulation. RSC Adv. 2015, 5, 8981–8987. [Google Scholar] [CrossRef]
- Chakraborty, K.; Vijayagopal, P.; Vijayan, K.K.; Syda Rao, G.; Joseph, J.; Chakkalakal, S.J. A Product Containing Anti-Inflammatory Principles from Green Mussel Perna viridis L. And a Process Thereof. Indian Patent IP 2066/CHE/2010, 22 March 2013. [Google Scholar]
- Simopoulos, A.P. The importance if the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; Bechtel, P.J. Lipid composition of alaska pink salmon (Oncorhynchus gorbuscha) and Alaska Walleye pollock (Theragra chalcogramma) byproducts. J. Aquat. Food Prod. Technol. 2005, 14, 73–91. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Sankar, T.V.; Ananda, R.; Chakraborty, K.; Sridhar, N. DHA and EPA content and fatty acid profile of 39 food fishes from India. Biomed Res. Int. 2016, 2016, 4027437. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Lirk, P.; Rieder, J. In Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Cahill, C. Tnf-α and neuropathic pain—A review. J. Neuroinflammation 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Halpern, G. Novel anti-inflammatory mechanism of action of lyprinol in the aia rat model. Prog. Nutr. 2008, 10, 146–152. [Google Scholar]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Johnsen, L. Krill products: An overview of animal studies. Nutrients 2015, 7, 3300–3321. [Google Scholar] [CrossRef] [PubMed]
- Ohata, T.; Fukuda, K.; Takahashi, M.; Sugimura, T.; Wakabayashi, K. Suppression of nitric oxide production in lipopolysaccharide-stimulated macrophage cells by omega 3 polyunsaturated fatty acids. Jpn. J. Cancer Res. 1997, 88, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, W.; Ishihara, K.; Murata, M.; Saito, H.; Shinohara, K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-γ plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic. Biol. Med. 2002, 34, 1006–1016. [Google Scholar] [CrossRef]
- Honda, K.L.; Lamon-Fava, S.; Matthan, N.R.; Wu, D.; Lichtenstein, A.H. EPA and DHA Exposure Alters the Inflammatory Response but not the Surface Expression of Toll-like Receptor 4 in Macrophages. Lipids 2015, 50, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Soliman Mimura, T.; Itoh, S.; Tsujikawa, K.; Nakajima, H.; Satake, M.; Kohama, Y.; Okabe, M. Studies on biological activities of melanin from marine animals. V. Antiinflammatory activity of low-molecular-weight melanoprotein from squid (Fr. Sm ii). Chem. Pharm. Bull. 1987, 35, 1144–1150. [Google Scholar] [CrossRef]
- Soliman, A.; Fahmy, S. in vitro antioxidant, analgesic and cytotoxic activities of Sepia officinalis ink and Coelatura aegyptiaca extracts. Afr. J. Pharm. Pharmacol. 2013, 7, 1512–1522. [Google Scholar]
- Joseph, S.; George, M.; Nair, J.; Senan, V.; Pillai, D.; Sherief, P. Effect of feeding cuttlefish liver oil on immune function, inflammatory response and platelet aggregation in rats. Curr. Sci. 2005, 88, 507–510. [Google Scholar]
- Emery, T.; Curtotti, R. Chapter 13: Southern squid jig Fishery. In Fishery Status Reports; Australian Bureau Agricultural and Resource Economics and Sciences: Canberra, Australia, 2018. Available online: http://www.agriculture.gov.au/abares/research-topics/fisheries/fishery-status/southern-squid-jig-fishery (accessed on 8 February 2019).
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Chan, P.; Steinhart, A.; SZlotkin, S.; Griffiths, A. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): A systematic review and meta-analyses. Inflamm. Bowel Dis. 2010, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.; Thien, F.; Abramson, M. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochranes Database Syst. Rev. 2002, 2002, CD001283. [Google Scholar]
- Choi, J.; Jang, J.; Son, D.; Im, H.-K.; Kim, J.; Patk, J.; Choi, W.; Han, S.; Hong, J. Antarctic krill oil diet protects against lipopolysaccharide-inducde oxidative stress, neuroinflammation and cognitive impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef] [PubMed]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Cheng, B.; Cho, S.; Lee, H. Green lipped mussel oil complex suppresses lipopolysaccharide stimulated inflammation via regulating nuclear factor kb and mitogen activated kinases signalling in RAW 264.7 murine macrophages. Food Sci. Biotech. 2017, 26, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Lawson, B.R.; Belkowski, S.M.; Whitesides, J.F.; Davis, P.; Lawson, J.W. Immunomodulation of murine collagen-induced arthritis by N,N-dimethylglycine and a preparation of Perna canaliculus. BMC Comp. Alt. Med. 2007, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Valles-Regino, R.; Tate, R.; Kelaher, B.; Savins, D.; Dowell, A.; Benkendorff, K. Ocean warming and CO2-induced acidification impact the lipid content of a marine predatory gastropod. Mar. Drugs 2015, 13, 6019–6037. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Rudd, D.; Smith, J.; Kotiw, M.; Mouatt, P.; Seymour, L.M.; Liu, L.; Benkendorff, K. Anti-inflammatory activity and structure-activity relationships of brominated indoles from a marine mollusc. Mar. Drugs 2017, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016, pdb prot087379. [Google Scholar] [CrossRef]


| (A) Fatty Acid | Trivial Name | Octopus tetricus | Sepioteuthis australis | Sardinops sagax | Salmo salar | Penaeus plebejus | |||||
| Flesh | Viscera | Flesh | Head | Flesh | Viscera & Head | Flesh | Head | Flesh | Head & Viscera | ||
| Saturated Fatty Acids (SFAs) | |||||||||||
| C12:0 | Lauric | 0.7 | 0 | 0 | 0 | 0.8 | 0.8 | 0.8 | 0.8 | 0.7 | 0 |
| C13:0 | tridecanoic | 0 | 0 | 0 | 0 | 0.7 | 0.8 | 0 | 0 | 0 | 1.6 |
| C14:0 | myristic | 43.0 | 20.0 | 11.3 | 14.8 | 57.8 | 59.7 | 16.5 | 20.1 | 17.8 | 16.1 |
| C15:0 | pentadecanoic | 4.1 | 4.6 | 4.2 | 5.9 | 5.0 | 6.0 | 1.6 | 2.3 | 5.7 | 41.0 |
| C16:0 | palmitic | 146.9 | 132.0 | 160.9 | 176.4 | 163.1 | 173.0 | 142.9 | 132.2 | 121.8 | 112.3 |
| C17:0 | heptadecanoic | 8.0 | 14.0 | 11.1 | 11.6 | 65.6 | 6.9 | 3.1 | 4.0 | 7.4 | 26.0 |
| C18:0 | Stearic | 43.7 | 69.1 | 69.4 | 67.2 | 34.6 | 37.0 | 40.1 | 39.4 | 39.2 | 44.5 |
| C20:0 | arachidic | 3.6 | 4.1 | 1.5 | 1.5 | 4.5 | 4.8 | 0.8 | 0.8 | 1.5 | 3.2 |
| C21:0 | henicosanoic | 0.7 | 0 | 0.8 | 0 | 0.8 | 0.8 | 0.8 | 0.8 | 3.1 | 21.4 |
| C22:0 | Behenic | 2.2 | 2.5 | 1.6 | 2.4 | 2.4 | 1.7 | 0.9 | 0.8 | 1.6 | 3.4 |
| C23:0 | tricosanoic | 0 | 0 | 0 | 0 | 0 | 0 | 1.0 | 0.9 | 0.9 | 1.9 |
| C24:0 | lignoceric | 6.2 | 2.6 | 1.6 | 4.2 | 0.8 | 0.9 | 0.9 | 0.9 | 0.8 | 1.7 |
| Monounsaturated Fatty Acids (MUFAs) | |||||||||||
| C14:1 | myristoleic | 6.2 | 2.3 | 0 | 0 | 1.5 | 1.5 | 1.6 | 1.5 | 0.7 | 2.3 |
| C15:1 | pentadecanoic | 0.7 | 1.5 | 0 | 0 | 0.7 | 0.8 | 0.8 | 0.7 | 0.7 | 1.5 |
| C16:1 | palmitoleic | 44.1 | 22.5 | 5.6 | 10.9 | 59.4 | 60.7 | 48.5 | 42.3 | 39.8 | 36.8 |
| C17:1 | heptadecanoic | 0.7 | 1.5 | 0.7 | 2.2 | 0.7 | 1.5 | 2.3 | 2.2 | 7.0 | 50.6 |
| C18:1n9t | elaidic | 2.1 | 3.1 | 1.4 | 3.0 | 0.7 | 0.8 | 0.8 | 0.7 | 1.5 | 3.9 |
| C18:1n9c | oleic | 42.2 | 43.7 | 16.7 | 55.9 | 50.3 | 54.2 | 301.9 | 261.7 | 230.3 | 93.4 |
| C20:1n9 | eicosenoic | 19.2 | 14.4 | 12.6 | 0 | 3.8 | 3.9 | 15.6 | 16.8 | 16.3 | 8.0 |
| C22:1n9 | erucic | 8.2 | 7.6 | 1.6 | 4.0 | 9.5 | 9.1 | 4.3 | 4.8 | 5.5 | 5.0 |
| C24:1n9 | nervonic | 5.3 | 6.0 | 1.6 | 2.5 | 4.8 | 5.0 | 1.7 | 2.4 | 3.2 | 4.3 |
| Polyunsaturated Fatty Acids (PUFAs) | |||||||||||
| C18:2n6c | Linoleic (LA) | 12.3 | 8.5 | 2.8 | 0 | 15.2 | 0.8 | 82.8 | 76.3 | 67.2 | 20.8 |
| C18:3n6 | Ƴ-linolenic (GLA) | 1.4 | 5.4 | 0.7 | 11.2 | 2.2 | 2.3 | 1.6 | 2.2 | 2.2 | 1.5 |
| C18:3n3 | α-linolenic (ALA) | 10.6 | 4.7 | 0.7 | 2.2 | 13.9 | 14.5 | 10.3 | 8.9 | 8.6 | 3.1 |
| C20:2 | eicosadienoic | 1.4 | 5.5 | 2.2 | 12.2 | 1.5 | 1.6 | 4.8 | 7.5 | 6.6 | 6.3 |
| C20:3n3 | eicosatrienoic | 2.2 | 3.3 | 2.3 | 4.8 | 3.1 | 3.3 | 4.2 | 4.7 | 5.4 | 5.0 |
| C20:4n6 | arachidonic (ARA) | 21.6 | 56.7 | 77.1 | 63.2 | 11.4 | 12.0 | 4.8 | 7.0 | 10.5 | 34.0 |
| C20:5n3 | eicosapentanoic (EPA) | 120.3 | 107.0 | 62.5 | 53.6 | 123.2 | 125.6 | 14.0 | 19.6 | 26.2 | 75.3 |
| C22:2 | docosadienoic | 1.5 | 0.9 | 1.6 | 0 | 0.8 | 0.8 | 0 | 0.8 | 0.8 | 1.7 |
| C22:5n3 | docosapentanoic (DPA) | 24.4 | 22.9 | 10.1 | 7.9 | 18.1 | 18.0 | 7.5 | 12.2 | 14.4 | 33.8 |
| C22:6n3 | docosahexanoic (DHA) | 141.9 | 192.9 | 229.8 | 205.7 | 105.1 | 111.0 | 48.0 | 50.7 | 51.9 | 66.7 |
| (B) Fatty Acid | Trivial Name | Octopus tetricus | Sepioteuthis australis | Sardinops sagax | Salmo salar | Penaeus plebejus | |||||
| Flesh | Viscera | Flesh | Head | Flesh | Viscera & Head | Flesh | Head | Flesh | Head & Viscera | ||
| Dimethyl Acetal Aldehydes | |||||||||||
| dimethyl acetal octadecan-1-al | 0 | 2.5 | 3.0 | 2.9 | 0 | 0 | 0 | 0 | 0 | 0 | |
| dimethyl acetal nonadecan-1-al | 3.3 | 4 | 1.4 | 1.8 | 3.6 | 3.7 | 3.4 | 3.6 | 3.9 | 3.0 | |
| Categories | |||||||||||
| SFAs | 25.5 | 24.9 | 26.2 | 28.4 | 27.7 | 29.2 | 20.9 | 20.3 | 20.0 | 27.3 | |
| MUFAs | 12.8 | 10.3 | 4.0 | 7.8 | 13.1 | 13.8 | 37.8 | 33.3 | 30.5 | 20.6 | |
| PUFAs | 33.7 | 40.8 | 39.0 | 36.1 | 29.4 | 29.0 | 17.9 | 19.0 | 19.3 | 24.8 | |
| Total ω-3 | 29.9 | 33.1 | 30.5 | 27.4 | 26.3 | 27.2 | 8.4 | 9.6 | 10.7 | 18.4 | |
| Total ω-6 | 3.5 | 17.1 | 8.6 | 7.4 | 2.9 | 1.5 | 9.1 | 8.5 | 8.0 | 5.6 | |
| Total ω-9 | 15.0 | 7.5 | 3.4 | 6.5 | 6.9 | 7.3 | 32.4 | 28.6 | 25.7 | 11.5 | |
| Total unidentified | 24.2 | 17.6 | 26.4 | 23.0 | 26.1 | 24.3 | 20.0 | 23.8 | 26.2 | 24.3 | |
| Saturated/unsaturated ratio | 0.6 | 0.5 | 0.6 | 0.6 | 0.7 | 0.7 | 0.4 | 0.4 | 0.4 | 0.6 | |
| ω-6/ω-3 ratio | 0.1 | 0.2 | 0.3 | 0.3 | 0.1 | 0.1 | 1.1 | 1.0 | 0.7 | 0.3 | |
| EPA per 100 g tissue | 64.2 | 395.7 | 88.7 | 99.3 | 1845.5 | 2972.5 | 104.2 | 238.5 | 29.9 | 138.2 | |
| DPA per 100 g tissue | 13.1 | 84.7 | 14.3 | 14.6 | 268.8 | 426.0 | 55.8 | 148.5 | 16.4 | 62.0 | |
| DHA per 100 g tissue | 75.7 | 713.4 | 326.0 | 381.2 | 1560.7 | 2627.0 | 357.2 | 617.0 | 59.1 | 122.4 | |
| Organism | Extract | 3T3 ccl-92 Fibroblasts Viability at 50 µg/mL | RAW 264.7 Macrophages Viability at 50 µg/mL | NO Inhibition IC50 (µg/mL) | TNFα Inhibition IC50 (µg/mL) |
|---|---|---|---|---|---|
| Octopus tetricus (Octopus) | Viscera | 100% | 100% | 64.6 | 51.0 |
| Flesh | 100% | 100% | 71.2 | 71.0 | |
| Sepioteuthis australis (Squid) | Head | 100% | 100% | 91.1 | 67.7 |
| Flesh | 100% | 100% | 114.2 | 78.8 | |
| Sardinops sagax (Australian Sardine) | Viscera/head | 100% | 100% | 84.6 | 71.1 |
| Fillet | 100% | 100% | 66.5 | 147.7 | |
| Salmo salar (Salmon) | Head | 100% | 100% | 97.3 | 85.8 |
| Fillet | 100% | 100% | 157.9 | 157.1 | |
| Penaeus plebejus (School Prawn) | Head/viscera | 100% | 100% | 88.0 | 71.2 |
| Body | 100% | 100% | 306.4 | 201.7 | |
| Euphausia superba | Krill Oil | 100% | 100% | 337.8 | 99.8 |
| Perna canaliculus (NZ Green-Lipped Mussel) | Oil (Lyprinol) | 100% | 100% | No detectable activity | >> max test dose 587.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. https://doi.org/10.3390/md17030155
Ahmad TB, Rudd D, Kotiw M, Liu L, Benkendorff K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Marine Drugs. 2019; 17(3):155. https://doi.org/10.3390/md17030155
Chicago/Turabian StyleAhmad, Tarek B., David Rudd, Michael Kotiw, Lei Liu, and Kirsten Benkendorff. 2019. "Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products" Marine Drugs 17, no. 3: 155. https://doi.org/10.3390/md17030155