Intravenous Lipid Emulsions to Deliver Bioactive Omega-3 Fatty Acids for Improved Patient Outcomes
Abstract
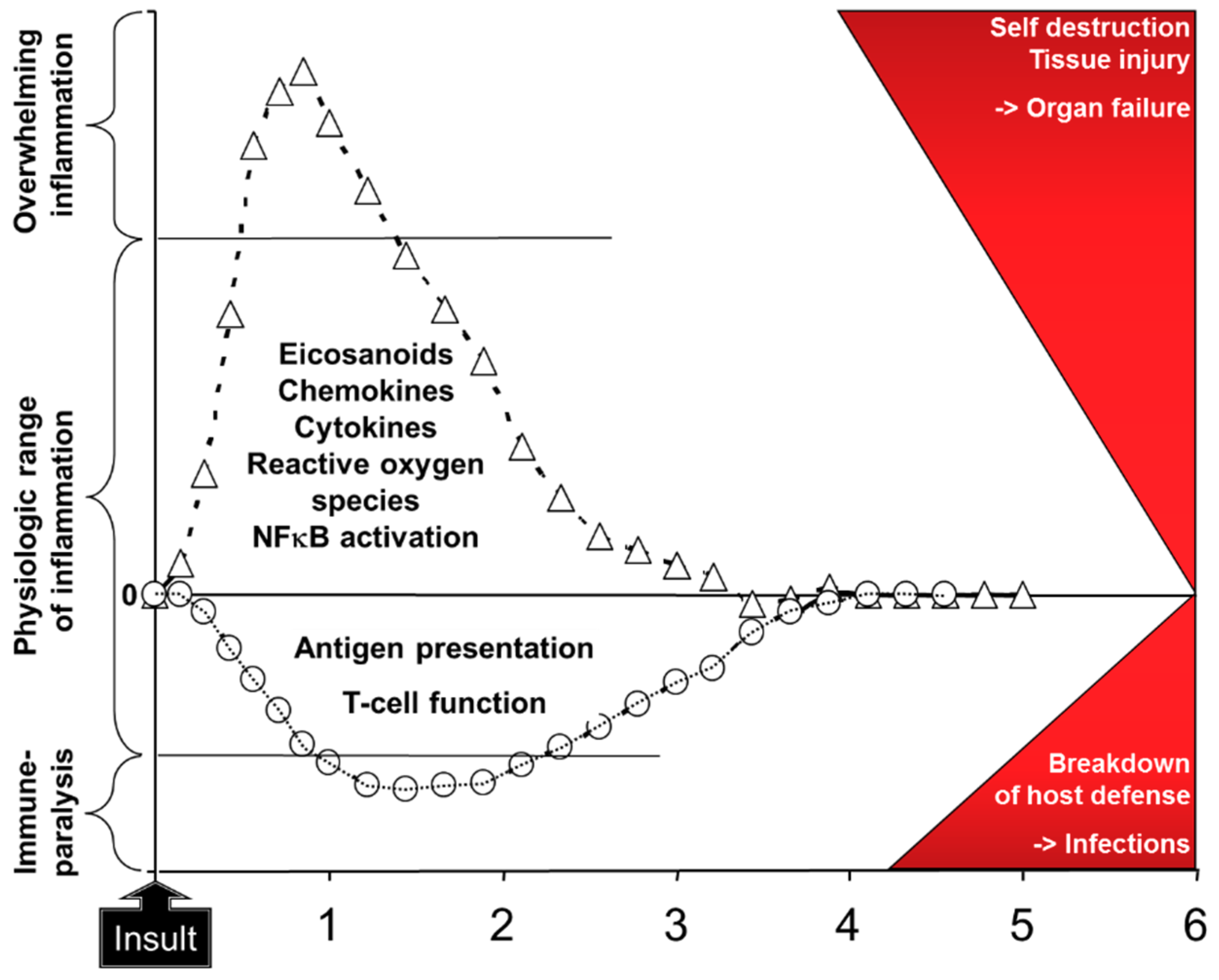
:1. Introduction
2. Fish Oil Containing LEs for Intravenous Use
2.1. The Role of LEs in Intravenous Nutrition Support
- a non-functional gastrointestinal tract due to:
- ○
- surgical removal because of disease
- ○
- intestinal blockage or leakage
- ○
- impaired absorptive capacity
- severe gastrointestinal disease
- severe malnutrition
- trauma or critical illness
2.2. Rationale for Fish Oil Containing LEs
2.3. Anti-Inflammatory and Immune Enhancing Effects of Fish Oil Containing LEs in Patients
3. Clinical Studies in Patients Undergoing Surgery
4. Clinical Studies in Patients Requiring Critical Care
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parent. Ent. Nutr. 2015, 39 (Suppl. 1), 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Omega-3: The good oil. Nutr. Bull. 2017, 42, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Katan, M.B.; Deslypere, J.P.; van Birgelen, A.P.J.M.; Penders, M.; Zegwaars, M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes and adipose tissue: An 18 month controlled study. J. Lipid Res. 1997, 38, 2012–2022. [Google Scholar]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.J.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef]
- Calder, P.C. The relationship between the fatty acid composition of immune cells and their function. Prostagl. Leukotr. Essent. Fatty Acids 2008, 79, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 through Toll-like receptor. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef]
- Wong, S.W.; Kwon, W.J.; Choi, A.M.; Kim, H.P.; Nakahira, K.; Hwang, D. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; DeLong, C.J.; Hong, Y.H.; Rieke, C.J.; Song, I.; Sidhu, R.S.; Yuan, C.; Warnock, M.; Schmaier, A.H.; Yokoyama, C.; et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J. Biol. Chem. 2007, 282, 22254–22266. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.; Serhan, C.N. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1260–1273. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Brit. J. Nutr. 2012, 107, S171–S184. [Google Scholar] [CrossRef] [Green Version]
- Abdulrazaq, M.; Innes, J.K.; Calder, P.C. Effect of ω-3 polyunsaturated fatty acids on arthritic pain: A systematic review. Nutrition 2017, 39-40, 57–66. [Google Scholar] [CrossRef]
- Senftleber, N.K.; Nielsen, S.M.; Andersen, J.R.; Bliddal, H.; Tarp, S.; Lauritzen, L.; Furst, D.E.; Suarez-Almazor, M.E.; Lyddiatt, A.; Christensen, R. Marine oil supplements for arthritis pain: A systematic review and meta-analysis of randomized trials. Nutrients 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Lipids for intravenous nutrition in hospitalised adult patients: A multiple choice of options. Proc. Nutr. Soc. 2013, 72, 263–276. [Google Scholar] [CrossRef]
- Calder, P.C.; Adolph, M.; Deutz, N.E.; Grau, T.; Innes, J.K.; Klek, S.; Lev, S.; Mayer, K.; Michael-Titus, A.T.; Pradelli, L.; et al. Lipids in the intensive care unit: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2018, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Paulsrud, J.R.; Pensler, L.; Whitten, C.F.; Stewart, S.; Holman, R.T. Essential fatty acid deficiency in infants induced by fat-free intravenous feeding. Am. J. Clin. Nutr. 1972, 25, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Yamamori, H.; Takagi, K.; Hayashi, N.; Suzuki, R.; Nakajima, N.; Tashiro, T. Influences of soybean oil emulsion on stress response and cell-mediated immune function in moderately or severely stressed patients. Nutrition 2002, 18, 235–240. [Google Scholar] [CrossRef]
- Arnalich, F.; Garcia-Palomero, E.; López, J.; Jiménez, M.; Madero, R.; Renart, J.; Vázquez, J.J.; Montiel, C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect. Immun. 2000, 68, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef]
- Andaluz-Ojeda, D.; Bobillo, F.; Iglesias, V.; Almansa, R.; Rico, L.; Gandía, F.; Resino, S.; Tamayo, E.; de Lejarazu, R.O.; Bermejo-Martin, J.F. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine 2012, 57, 332–336. [Google Scholar] [CrossRef]
- Heller, A.R. Intravenous fish oil in adult intensive care unit patients. World Rev. Nutr. Dietet. 2015, 112, 127–140. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Edidin, M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. Am. J. Clin. Nutr. 2006, 84, 1277–1289. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P.; Thies, F.; Wallace, F.A.; Miles, E.A. Fatty acids and lymphocyte functions. Brit. J. Nutr. 2002, 87, S31–S48. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. N-3 fatty acids, inflammation and immunity—relevance to postsurgical and critically ill patients. Lipids 2004, 39, 1147–1161. [Google Scholar] [CrossRef]
- Mascioli, E.; Leader, L.; Flores, E.; Trimbo, S.; Bistrian, B.; Blackburn, G. Enhanced survival to endotoxin in guinea pigs fed IV fish oil emulsion. Lipids 1988, 23, 623–625. [Google Scholar] [CrossRef]
- Sadeghi, S.; Wallace, F.A.; Calder, P.C. Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology 1999, 96, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Fan, X.H.; Wu, Y.P.; Zhu, J.L.; Wang, F.; Bo, L.L.; Li, J.B.; Bao, R.; Deng, X.M. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 457–464. [Google Scholar] [CrossRef]
- Barbosa, V.M.; Miles, E.A.; Calhau, C.; Lafuente, E.; Calder, P.C. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: A randomized, controlled clinical trial. Crit. Care 2010, 14, R5. [Google Scholar] [CrossRef]
- Al-Taan, O.; Stephenson, J.A.; Spencer, L.; Pollard, C.; West, A.L.; Calder, P.C.; Metcalfe, M.; Dennison, A.R. Changes in plasma and erythrocyte omega-6 and omega-3 fatty acids in response to intravenous supply of omega-3 fatty acids in patients with hepatic colorectal metastases. Lipids Health Dis. 2013, 12, 64. [Google Scholar] [CrossRef]
- Barros, K.V.; Cassulino, A.P.; Schalch, L.; Della Valle Munhoz, E.; Manetta, J.A.; Noakes, P.S.; Miles, E.A.; Calder, P.C.; Flor Silveira, V.L. Supplemental intravenous n-3 fatty acids and n-3 fatty acid status and outcome in critically ill elderly patients in the ICU receiving enteral nutrition. Clin. Nutr. 2013, 32, 599–605. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Brit. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Wachtler, P.; König, W.; Senkal, M.; Kemen, M.; Köller, M. Influence of a total parenteral nutrition enriched with omega-3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J. Trauma. 1997, 42, 191–198. [Google Scholar] [CrossRef]
- Grimm, H.; Mertes, N.; Goeters, C.; Schlotzer, E.; Mayer, K.; Grimminger, F.; Fürst, P. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur. J. Nutr. 2006, 45, 55–60. [Google Scholar] [CrossRef]
- Weiss, G.; Meyer, F.; Matthies, B.; Pross, M.; Koenig, W.; Lippert, H. Immunomodulation by perioperative administration of n-3 fatty acids. Brit. J. Nutr. 2002, 87 (Suppl. 1), S89–S94. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: A meta-analysis of randomized control trials in China. Medicine (Baltimore) 2018, 97, e0472. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, Y.; Yang, P.; Wan, H.W.; Wu, X.T. Safety and efficacy of fish oil-enriched parenteral nutrition regimen on postoperative patients undergoing major abdominal surgery: A meta-analysis of randomized controlled trials. J. Parent Enteral. Nutr. 2010, 34, 387–394. [Google Scholar] [CrossRef]
- Wie, C.; Hua, J.; Bin, C.; Klassen, K. Impact of lipid emulsion containing fish oil on outcomes of surgical patients: Systematic review of randomized controlled trials from Europe and Asia. Nutrition 2010, 26, 474–481. [Google Scholar]
- Pradelli, L.; Mayer, K.; Muscaritoli, M.; Heller, A.R. N-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: A meta-analysis. Crit. Care 2012, 16, R184. [Google Scholar] [CrossRef]
- Li, N.N.; Zhou, Y.; Qin, X.P.; Chen, Y.; He, D.; Feng, J.Y.; Wu, X.T. Does intravenous fish oil benefit patients post-surgery? A meta-analysis of randomised controlled trials. Clin. Nutr. 2014, 33, 226–239. [Google Scholar] [CrossRef]
- Bae, H.J.; Lee, G.Y.; Seong, J.M.; Gwak, H.S. Outcomes with perioperative fat emulsions containing omega-3 fatty acid: A meta-analysis of randomized controlled trials. Am. J. Health Syst. Pharm. 2017, 74, 904–918. [Google Scholar] [CrossRef]
- Mayer, K.; Schaefer, M.B.; Seeger, W. Fish oil in the critically ill: From experimental to clinical data. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 140–148. [Google Scholar] [CrossRef]
- Mayer, K.; Schaefer, M.B.; Hecker, M. Intravenous n-3 fatty acids in the critically ill. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 124–128. [Google Scholar] [CrossRef]
- Palmer, A.J.; Ho, C.K.; Ajibola, O.; Avenell, A. The role of ω-3 fatty acid supplemented parenteral nutrition in critical illness in adults: A systematic review and meta-analysis. Crit. Care Med. 2013, 41, 307–316. [Google Scholar] [CrossRef]
- Manzanares, W.; Dhaliwal, R.; Jurewitsch, B.; Stapleton, R.D.; Jeejeebhoy, K.N.; Heyland, D.K. Parenteral fish oil lipid emulsions in the critically ill: A systematic review and meta-analysis. J. Parenter Enteral. Nutr. 2014, 38, 20–28. [Google Scholar] [CrossRef]
- Manzanares, W.; Langlois, P.L.; Dhaliwal, R.; Lemieux, M.; Heyland, D.K. Intravenous fish oil lipid emulsions in critically ill patients: An updated systematic review and meta-analysis. Crit. Care 2015, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, C.E.; Brody, R.A.; Parrott, J.S.; Stankorb, S.M.; Heyland, D.K. The effects of different IV fat emulsions on clinical outcomes in critically ill patients. Crit. Care Med. 2014, 42, 1168–1177. [Google Scholar] [CrossRef] [PubMed]






| Pure Soybean Oil | Soybean Oil MCT Oil Blend | Restructured Soybean Oil MCT Oil Blend | Pure Fish Oil | Olive Oil Based | Fish Oil Blend 1 | Fish Oil Blend 2 | |
|---|---|---|---|---|---|---|---|
| Oil source (%): | |||||||
| Soybean | 100 | 50 | 64 | - | 20 | 40 | 30 |
| MCT | - | 50 | 36 | - | - | 50 | 30 |
| Olive | - | - | - | - | 80 | - | 25 |
| Fish | - | - | - | 100 | - | 10 | 15 |
| Fatty acids (%) | |||||||
| Saturated | 15 | 58 | 46 | 21 | 14 | 49 | 37 |
| Monounsaturated * | 24 | 11 | 14 | 23 | 64 | 14 | 33 |
| Polyunsaturated | 61 | 31 | 40 | 56 | 22 | 37 | 30 |
| Omega-3 | 8 | 4 | 5 | 48 | 3 | 10 | 7 |
| ALA | 8 | 4 | 5 | 1 | 3 | 4 | 2 |
| EPA | - | - | - | 20 | - | 3.5 | 3 |
| DHA | - | - | - | 19 | - | 2.5 | 2 |
| Omega-6 ** | 53 | 27 | 35 | 5 | 19 | 27 | 23 |
| Effect of Fish Oil LE On | |||
|---|---|---|---|
| Meta-Analysis and Year | Infections | Length of ICU Stay | Length of Hospital Stay |
| Chen et al. (2010) [43] | Odds ratio 0.56 (0.32, 0.98) P = 0.04 n = 7 studies | −1.80 days (−3.04, −0.56) P = 0.004 n = 5 studies | −2.98 days (−4.65, −1.31) P = 0.0005 n = 7 studies |
| Wei et al. (2010) [44] | Risk ratio 0.49 (0.26, 0.93) P = 0.03 n = 4 studies | −2.07 days (−3.47, −0.47) P = 0.004 n = 3 studies | |
| Pradelli et al. (2012) [45] (non-ICU patients) | Risk ratio 0.53 (0.34, 0.82) P = 0.004 n = 6 studies | −1.86 days (−3.13, −0.59) P = 0.0004 n = 6 studies | |
| Li et al. (2014) [46] | Odds ratio 0.53 (0.35, 0.81) P = 0.003 n = 9 studies | −2.14 days (−3.02, −1.27) P < 0.00001 n = 11 studies | |
| Bae et al. (2017) [47] | Odds ratio 0.44 (0.30, 0.65) P < 0.0001 n = 15 studies | −2.70 days (−3.60, −1.79) P < 0.00001 n = 10 studies | |
| Zhao and Wang (2018) [42] | Odds ratio 0.36 (0.20, 0.66) P = 0.0008 n = 8 studies | ||
| Effect of Fish Oil LE On | |||||
|---|---|---|---|---|---|
| Meta-Analysis and Year | Infections | Length of ICU Stay | Length of Hospital Stay | Ventilation Requirement | Mortality |
| Pradelli et al. (2012) [45] (ICU patients) | Odds ratio 0.71 (0.45, 1.12) P = 0.14 n = 5 studies | −1.92 days (−3.27, −0.58) P = 0.005 n = 8 studies | −5.17 days (−8.35, −1.99) P = 0.001 n = 8 studies | ||
| Palmer et al. (2013) [50] | Risk ratio 0.78 (0.43, 1.41) P = 0.41 n = 5 studies | −0.57 days (−5.05, 3.90) P = 0.80 n = 6 studies | −9.49 days (−16.51, −2.47) P = 0.008 n = 3 studies | Risk ratio 0.83 (0.57, 1.20) P = 0.32 n = 8 studies | |
| Manzanares et al. (2014) [51] | Risk ratio 0.76 (0.42, 1.36) P = 0.35 n = 3 studies | −1.13 days (−8.96, 6.69) P = 0.78 n = 3 studies | −1.81 days (−3.98, 0.36) P = 0.10 n = 3 studies | Risk ratio 0.71 (0.49, 1.04) P = 0.08 n = 5 studies | |
| Manzanares et al. (2015) [52] | Risk ratio 0.64 (0.44, 0.92) P = 0.02 n = 5 studies | −1.42 days (−4.53, 1.69) P = 0.37 n = 7 studies | −3.71 days (−9.31, 1.88) P = 0.19 n = 7 studies | −1.14 days (−2.67, 0.38) P = 0.14 n = 6 studies | Risk ratio 0.90 (0.67, 1.20) P = 0.46 n = 9 studies |
| Outcome | Soybean Oil | Soybean Oil MCT Oil Blend | Fish Oil Blend |
|---|---|---|---|
| Patient died within 60 days (%) | 28.3 | 30.8 | 10.5 |
| Duration of mechanical ventilation (median days) | 4.9 | 5.3 | 5.0 |
| Length of ICU stay (median days) | 10.9 | 9.6 | 7.05 |
| Length of hospital stay (median days) | 28.1 | 31.9 | 14.1 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calder, P.C. Intravenous Lipid Emulsions to Deliver Bioactive Omega-3 Fatty Acids for Improved Patient Outcomes. Mar. Drugs 2019, 17, 274. https://doi.org/10.3390/md17050274
Calder PC. Intravenous Lipid Emulsions to Deliver Bioactive Omega-3 Fatty Acids for Improved Patient Outcomes. Marine Drugs. 2019; 17(5):274. https://doi.org/10.3390/md17050274
Chicago/Turabian StyleCalder, Philip C. 2019. "Intravenous Lipid Emulsions to Deliver Bioactive Omega-3 Fatty Acids for Improved Patient Outcomes" Marine Drugs 17, no. 5: 274. https://doi.org/10.3390/md17050274




