Potential Factors Influencing Repeated SARS Outbreaks in China
Abstract
1. Introduction
2. Common Epidemiological Features for SARS-1 and SARS-2
2.1. Environmental Factors
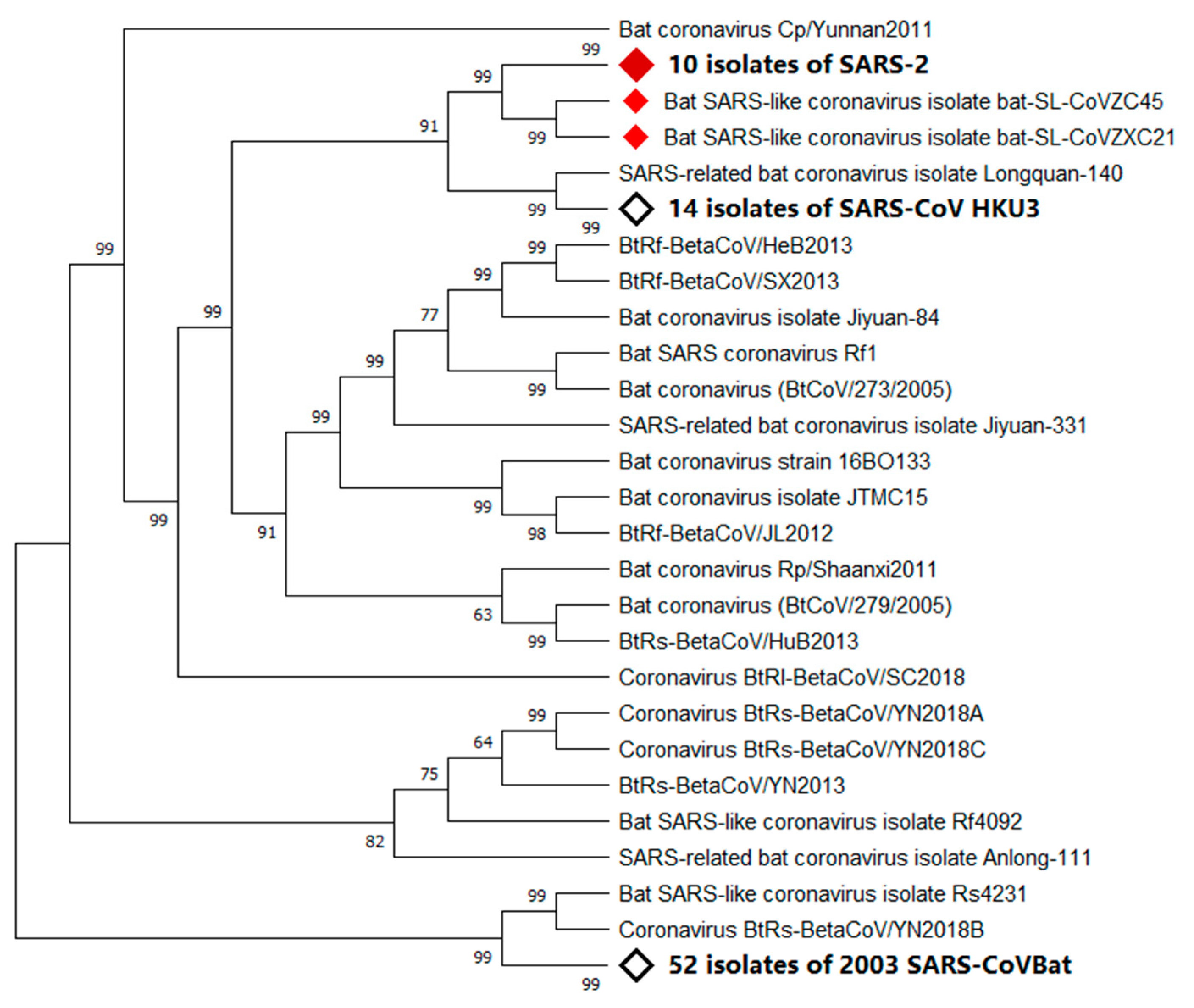
2.2. Natural Host
2.3. Intermediate Hosts
2.4. Ultimate Host
3. Potential Outbreak Scenarios for SARS-2
3.1. Single Outbreak Site and Single Source of Virus
3.2. Multiple Outbreak Sites and Multiple Sources of Viruses
3.3. Multiple Outbreak Sites and Single Unique Source of Virus
4. Recommendations for SARS Control and Prevention
4.1. Control Measures for the Ongoing SARS-2 Epidemic
4.2. Prevention Strategies for Potential SARS in Future
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.L.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. ARTIC Network, 17 February 2020. Available online: http://virological.org/t/the-proximal-origin-of-sars-cov-2/398 (accessed on 18 February 2020).
- Human Coronavirus Types. In Centers for Disease Control and Prevention; 15 February 2020. Available online: https://www.cdc.gov/coronavirus/types.html (accessed on 18 February 2020).
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef]
- Li, W.H.; Moore, M.J.; Vasilieva, N.; Sui, J.H.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Van Doremlen, N.; Miazgowicz, K.; Milne-Price, S.; Bushmaker, T.; Robertson, S.; Scott, D.; Kinne, J.; McLellan, J.S.; Zhu, J.; Munster, V. Host Species Restriction of Middle East Respiratory Syndrome Coronavirus through Its Receptor, Dipeptidyl Peptidase 4. J. Virol 2014, 88, 9220–9232. [Google Scholar] [CrossRef]
- Xu, R.H.; He, J.F.; Evans, M.R.; Peng, G.W.; Field, H.E.; Yu, D.W.; Lee, C.K.; Luo, H.M.; Lin, W.S.; Lin, P.; et al. Epidemiologic clues to SARS origin in China. Emerg. Infect. Dis. 2004, 10, 1030–1037. [Google Scholar] [CrossRef]
- Lu, H.; Stratton, C.W.; Tang, Y.W. Outbreak of Pneumonia of Unknown Etiology in Wuhan China: The Mystery and the Miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef]
- NBSC. National Bureau of Statistics PRC: China Statistical Yearbook 2018 (Chinese-English Edition); China Statistics Press: Beijing, China, 2018. [Google Scholar]
- NBSC. National Bureau of Statistics PRC: China Statistical Yearbook 2019 (Chinese-English Edition); China Statistics Press: Beijing, China, 2019. [Google Scholar]
- Shi, N.N.; Liu, J.Y.; Kuang, Y.L.; Zhou, Y.Y.; Liao, Q.; Liu, Z.F. Characteristics and Influences of Precipitation Tendency in Foshan under Environmental Varia. J. Water Resour. 2014, 03, 41–49. [Google Scholar] [CrossRef]
- Drought stress in 2002. China Weather Network, 6 May 2010. Available online: http://www.weather.com.cn/drought/ghsj/2002/05/443027.shtml (accessed on 18 February 2020).
- Ding, Y.T. Heavy drought in the middle and lower reaches of Yangtze River. People Net, 7 November 2019. Available online: http://paper.people.com.cn/rmrb/html/2019-11/07/nw.D110000renmrb_20191107_2-17.htm (accessed on 18 February 2020).
- The average rainfall in Wuhan in December was 26 millimeters, and there were only scattered light rain on the 3rd. Wuhan Weather News, 19 January 2020. Available online: https://weather.mipang.com/wuhan/news-1549253.html# (accessed on 18 February 2020).
- Schneider, U.; Becker, A.; Finger, P.; Meyer-Christoffer, A.; Ziese, M.; Rudolf, B. GPCC’s new land surface precipitation climatology based on quality-controlled in situ data and its role in quantifying the global water cycle. Theor. Appl. Climatol. 2014, 115, 15–40. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010, 76, 2712–2717. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Peiris, J.S.; Lam, S.Y.; Poon, L.L.; Yuen, K.Y.; Seto, W.H. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, K.; Saukkoriipi, A.; Jokelainen, J.; Juvonen, R.; Kauppila, J.; Vainio, O.; Ziegler, T.; Ronkko, E.; Jaakkola, J.J.; Ikaheimo, T.M.; et al. Decline in temperature and humidity increases the occurrence of influenza in cold climate. Environ. Health 2014, 13, 22. [Google Scholar] [CrossRef]
- Kudo, E.; Song, E.; Yockey, L.J.; Rakib, T.; Wong, P.W.; Homer, R.J.; Iwasaki, A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. USA 2019, 116, 10905–10910. [Google Scholar] [CrossRef]
- Relative Humidity. Wikipedia. Available online: https://en.wikipedia.org/wiki/Relative_humidity (accessed on 18 February 2020).
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Monthly Weather Forecast and Climate Jeddah, Saudi Arabia. Weather Atlas. Available online: https://www.weather-atlas.com/en/saudi-arabia/jeddah-climate (accessed on 15 February 2020).
- Hu, B.; Zeng, L.P.; Yang, X.L.; Ge, X.Y.; Zhang, W.; Li, B.; Xie, J.Z.; Shen, X.R.; Zhang, Y.Z.; Wang, N.; et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017, 13, e1006698. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Y.; Li, J.L.; Yang, X.L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Hu, B.; Wang, B.; Wang, M.N.; Zhang, Q.; Zhang, W.; Wu, L.J.; Ge, X.Y.; Zhang, Y.Z.; Daszak, P.; et al. Isolation and characterization of a novel bat coronavirus closely related to the direct progenitor of severe acute respiratory syndrome coronavirus. J. Virol. 2015, 90, 3253–3256. [Google Scholar] [CrossRef]
- Hu, D.; Zhu, C.Q.; Ai, L.L.; He, T.; Wang, Y.; Ye, F.Q.; Yang, L.; Ding, C.X.; Zhu, X.H.; Lv, R.C.; et al. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Biorxiv 2020. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Kulkarni, D.D.; Tosh, C.; Venkatesh, G.; Senthil Kumar, D. Nipah virus infection: Current scenario. Indian. J. Virol. 2013, 24, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Widagdo, W.; Sooksawasdi Na Ayudhya, S.; Hundie, G.B.; Haagmans, B.L. Host Determinants of MERS-CoV Transmission and Pathogenesis. Viruses 2019, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.; Chan, M.C. Tracing the SARS-coronavirus. J. Thorac. Dis. 2013, 5 (Suppl. 2), S118–S121. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wang, W.; Zhao, X.; Zai, J.; Li, X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J. Med. Virol. 2020. [CrossRef]
- Callaway, E.; Cyranoski, D. Why snakes probably aren’t spreading the new China virus. Nature 2020. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W.; Chen, J.-P. Viral Metagenomics Revealed Sendai Virus and Coronavirus Infection of Malayan Pangolins (Manis javanica). Viruses 2019, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.-Y.; Shum, M.H.-H.; Zhu, H.C.; Tong, Y.G.; Ni, X.B.; Liao, Y.S.; Wei, W.; Cheung, W.Y.-M.; Li, W.J.; Li, L.F.; et al. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. Biorxiv 2020. [Google Scholar] [CrossRef]
- Sequence similarity between pangolin and infected human strain is 99%. sohu. Available online: https://www.sohu.com/a/371763329_313745 (accessed on 18 February 2020).
- Zhong Nanshan: The new coronavirus is likely to come from game products such as bamboo rats and tadpoles. Netease News, 20 January 2020. Available online: https://news.163.com/20/0120/22/F3C9KSI50001899O.html (accessed on 18 February 2020).
- Chinese Cobra. Wikipedia. Available online: https://en.wikipedia.org/wiki/Chinese_cobra (accessed on 18 February 2020).
- Zhang, X.R.; Pan, J.; Yue, C.L.; Li, H.P.; Wang, J. Analysis of the Mammal Diversity and Fauna in Zhejiang Province. Chin. J. Wildl. 2019, 40, 37–47. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, W.B.; Wang, J. Mammalian fauna and distribution of Putuoshan Island in Zhoushan. J. Zhejiang A&F Univ. 2010, 27, 110–115. [Google Scholar] [CrossRef]
- Letko, M.; Miazgowicz, K.; McMinn, R.; Seifert, S.N.; Sola, I.; Enjuanes, L.; Carmody, A.; van Doremalen, N.; Munster, V. Adaptive Evolution of MERS-CoV to Species Variation in DPP4. Cell Rep. 2018, 24, 1730–1737. [Google Scholar] [CrossRef]
- Hilary, F.F. Vanishing Borders: Protecting the Planet in the Age of Globalization; Chapters 1–6; W. W. Norton & Company: New York, NY, USA, 2000. [Google Scholar]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Tan, W.J.; Zhao, X.; Ma, X.J.; Wang, W.L.; Niu, P.H.; Xu, W.B.; Gao, G.F.; Wu, G.Z. A Novel Coronavirus Genome Identified in a Cluster of Pneumonia Cases—Wuhan, China 2019−2020. China CDC Weekly, 21 January 2020. Available online: http://weekly.chinacdc.cn/en/article/id/a3907201-f64f-4154-a19e-4253b453d10c (accessed on 18 February 2020).
- The largest bamboo rat Chinese silver star bamboo rat breeding base in Zhoushan. Shanghang.net, 3 July 2018. Available online: http://www.shanghang.net/q-2382.html (accessed on 18 February 2020).
- Dechmann, D.K.N.; Wikelski, M.; Ellis-Soto, D.; Safi, K.; O’Mara, M.T. Determinants of spring migration departure decision in a bat. Biol. Lett. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.C.; Roeleke, M.; Marggraf, L.; Petersons, G.; Voigt-Heucke, S.L. Migratory bats respond to artificial green light with positive phototaxis. PLoS ONE 2017, 12, e0177748. [Google Scholar] [CrossRef]
- Deka, M.A.; Morshed, N. Mapping Disease Transmission Risk of Nipah Virus in South and Southeast Asia. Trop. Med. Infect. Dis. 2018, 3, 57. [Google Scholar] [CrossRef]
- Xi, G.H. Adventures of Huanong Brothers: Shooting bamboo rat videos became popular, fans exceeded 5 million and annual income exceeded 300,000. Tencent, 5 February 2020. Available online: https://new.qq.com/omn/20190205/20190205A0G1Q7.html (accessed on 18 February 2020).
- 1st death in US from coronavirus: Live updates on COVID-19. Live Science, 1 March 2020. Available online: https://www.livescience.com/new-china-coronavirus-faq.html (accessed on 1 March 2020).
- Lau, S.K.P.; Li, K.S.M.; Huang, Y.; Shek, C.-T.; Tse, H.; Wang, M.; Choi, G.K.Y.; Xu, H.F.; Lam, C.S.F.; Guo, R.T.; et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J. Virol. 2010, 84, 2808–2819. [Google Scholar] [CrossRef]
- Voigt, C.C.; Rehnig, K.; Lindecke, O.; Pētersons, G. Migratory bats are attracted by red light but not by warm-white light: Implications for the protection of nocturnal migrants. Ecol. Evol. 2018, 8, 9353–9361. [Google Scholar] [CrossRef]
- Xiong, C.L.; Jiang, L.F.; Chen, Y.; Jiang, Q.W. Evolution and variation of 2019-novel coronavirus. Biorxiv 2020. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Thilakavathy, K.; Kumar, S.S.; He, G.; Liu, S.V. Potential Factors Influencing Repeated SARS Outbreaks in China. Int. J. Environ. Res. Public Health 2020, 17, 1633. https://doi.org/10.3390/ijerph17051633
Sun Z, Thilakavathy K, Kumar SS, He G, Liu SV. Potential Factors Influencing Repeated SARS Outbreaks in China. International Journal of Environmental Research and Public Health. 2020; 17(5):1633. https://doi.org/10.3390/ijerph17051633
Chicago/Turabian StyleSun, Zhong, Karuppiah Thilakavathy, S. Suresh Kumar, Guozhong He, and Shi V. Liu. 2020. "Potential Factors Influencing Repeated SARS Outbreaks in China" International Journal of Environmental Research and Public Health 17, no. 5: 1633. https://doi.org/10.3390/ijerph17051633
APA StyleSun, Z., Thilakavathy, K., Kumar, S. S., He, G., & Liu, S. V. (2020). Potential Factors Influencing Repeated SARS Outbreaks in China. International Journal of Environmental Research and Public Health, 17(5), 1633. https://doi.org/10.3390/ijerph17051633




