The behavior of the adsorbents under humid conditions was assessed by different experiments and compared to the previous results with dry flue gas mixtures.
4.2.1. Cyclic Operation
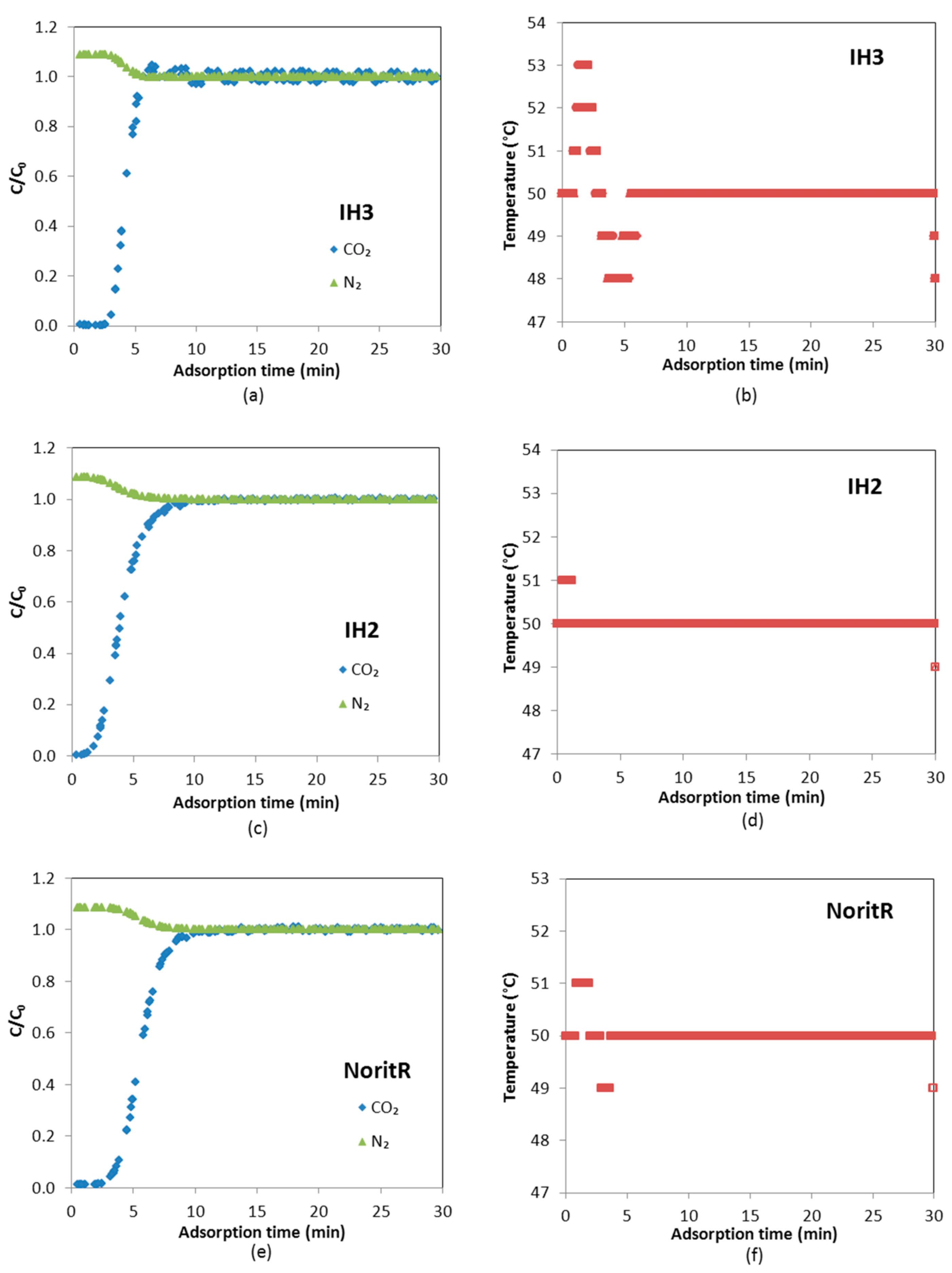
The CO
2, N
2 and H
2O breakthrough curves were measured for the activated carbons under study and
C/
C0 (ratio between the outlet CO
2, N
2 or H
2O concentration at a given time and that in the feed) was plotted versus time. In
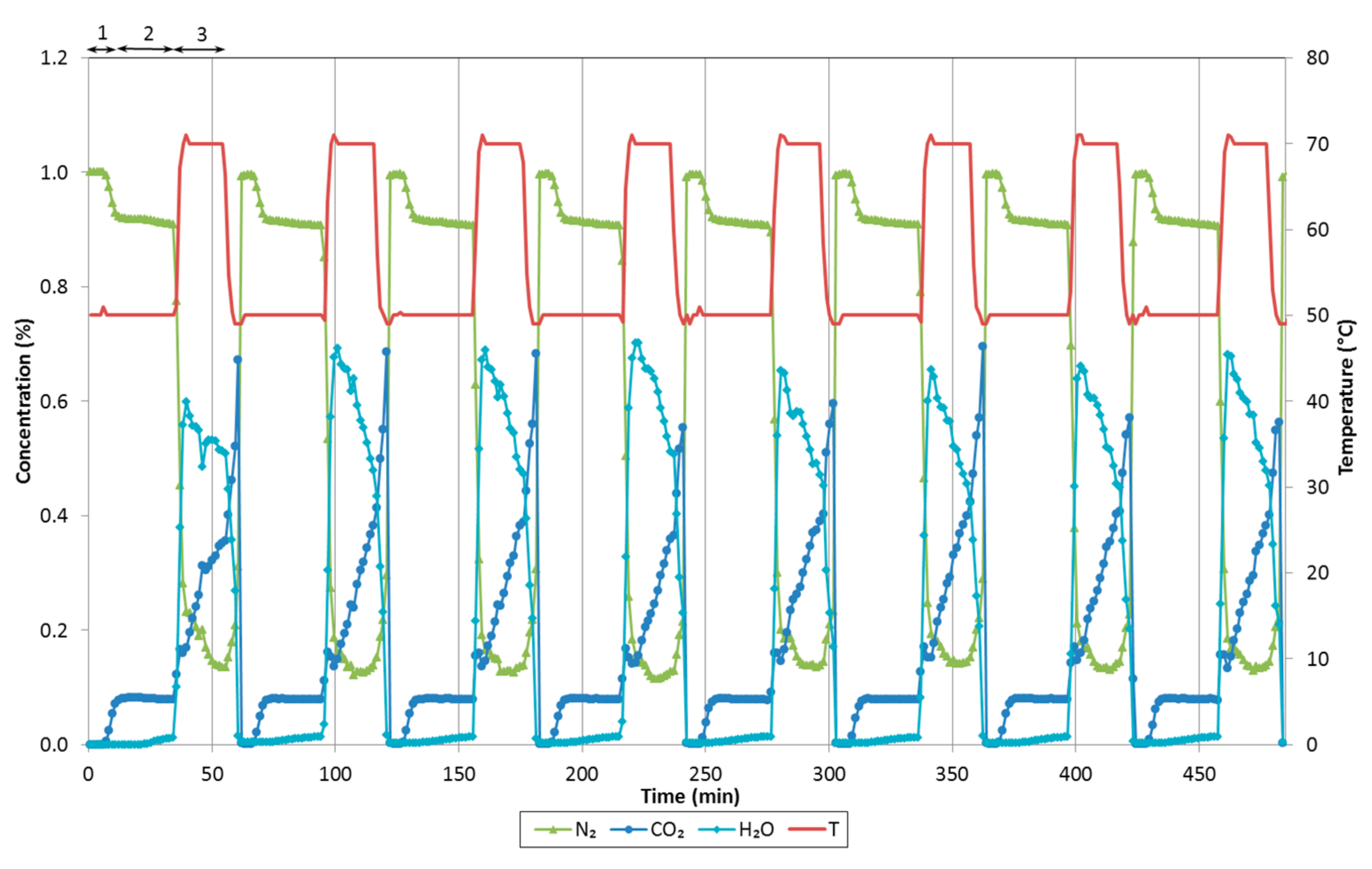
Figure 9 the last five consecutive cycles (Cycles 4–8), in which concentration of both CO
2 and H
2O can be considered stable, are represented. As far as CO
2 is concerned, the adsorbents reached saturation (maximum adsorption capacity of the adsorbed components) during the adsorption step and they were practically fully regenerated during the desorption step. However, water adsorption and desorption was slower. Therefore, it is observed in
Figure 9 that
C/
C0 for H
2O at
t = 0 is around 0.2 for IH3 and IH2 and 0.6 for Norit R.
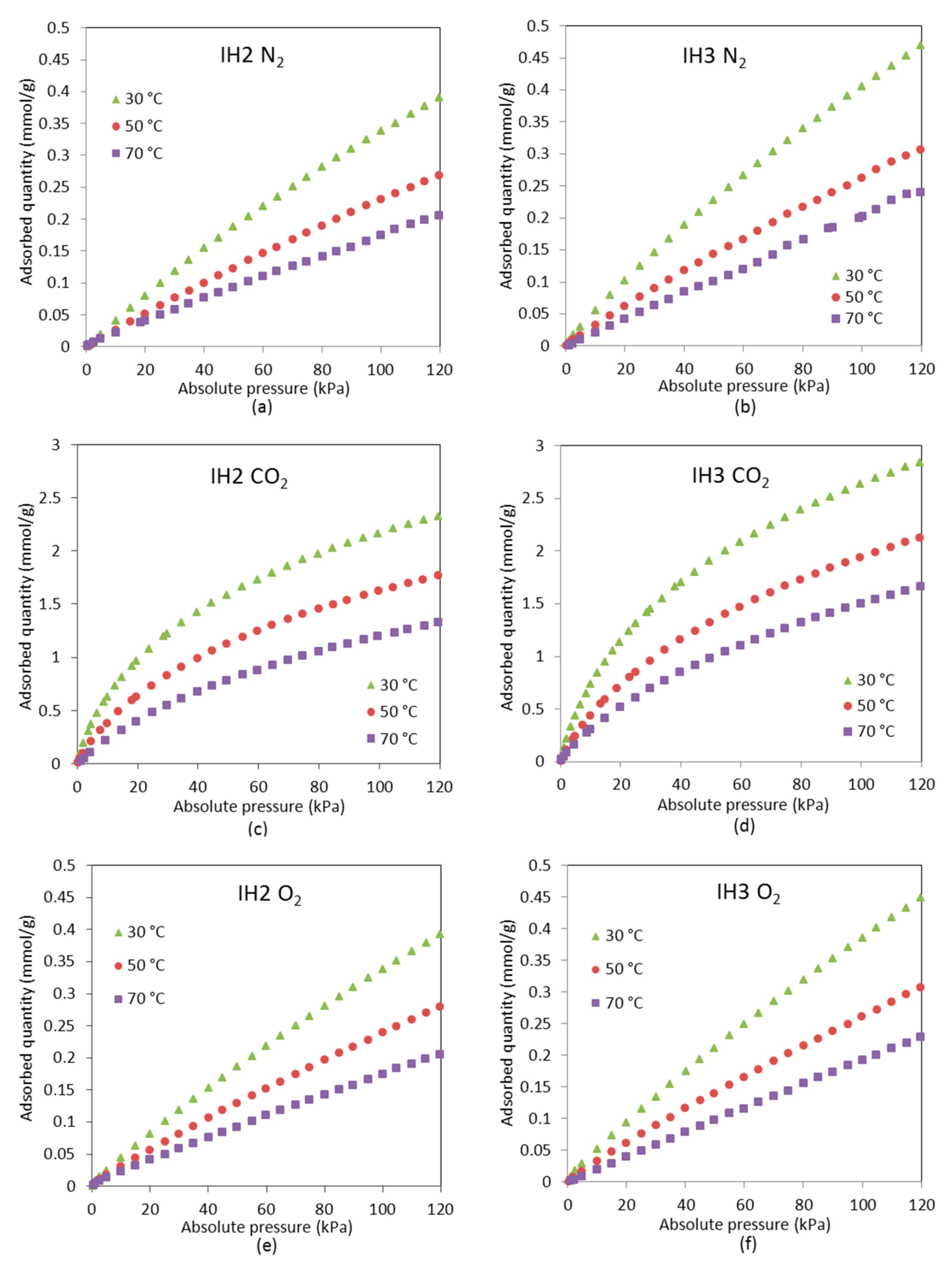
Both CO2 and water vapor breakthrough curves are steeper for IH3 than for IH2, showing that the latter has greater mass transfer resistance due to diffusion based constraints associated to the less developed porosity. Norit R presents similar diffusional resistance to IH2 for CO2; however, the breakthrough times are bigger.
It is noteworthy that kinetics of H2O adsorption/desorption on Norit R are particularly slow. At 50 °C and a working partial pressure of H2O around 2.4 kPa, the adsorption capacity of Norit R, as expected from the equilibrium adsorption isotherm, is about 1.20 mmol/g. Thus, it is larger than for the other two samples: 0.79 mmol/g for IH3 and 0.56 mmol/g for IH2. Nevertheless, considerable differences can be observed between partial pressures of H2O at the beginning and the end of the adsorption stage under cyclic operation. The initial and final partial H2O pressures are 0.44 and 2.21 kPa for IH3 and 0.35 and 1.75 kPa for IH2, whereas the values obtained for Norit R are 0.56 and 0.79 kPa, respectively. This is most probably the reason why breakthrough curves of CO2 for Norit R show significant differences between the dry and wet cyclic operation and, therefore, there is a greater reduction in breakthrough times.
In addition, it is found that the shape of the water breakthrough curve of Norit R under cyclic operation is very different in comparison to biomass adsorbents IH2 and IH3. The experiment with Norit R reached low water vapor partial pressure meaning that, at the end of the adsorption step, working pressure conditions correspond to the first region of the water vapor adsorption isotherm before the steep H
2O uptake.
Table 6 summarizes the average CO
2 uptakes and breakthrough times attained during the wet cyclic breakthrough experiments. Both IH3 and IH2 show enhanced performance in terms of CO
2 adsorption under humid conditions when compared to Norit R.
A small roll-up phenomenon can be observed in the detail of the CO
2 curve in
Figure 9: as the front of H
2O travels through the bed, part of the CO
2 initially adsorbed is displaced by the adsorption of H
2O. However, this is not observed for Norit R because the quantity adsorbed of H
2O in each cycle is small given that the adsorption time is limited to 30 min.
4.2.2. Non-Cyclic Operation
Ternary breakthrough curves were carried out using mixtures of N2, CO2 and H2O, and these were compared to binary breakthrough curves carried out with N2 and H2O mixtures to assess the influence of CO2 on H2O adsorption departing from a fully regenerated adsorbent. Moreover, the influence of H2O on CO2 adsorption was studied comparing the behavior of a ternary N2/H2O/CO2 mixture fed to a regenerated adsorbent and to an adsorbent initially saturated with H2O. To evaluate the dynamic adsorption at different relative humidity (RH), experiments were performed at 30 °C (RH ≈ 60%) and 50 °C (RH ≈ 22%).
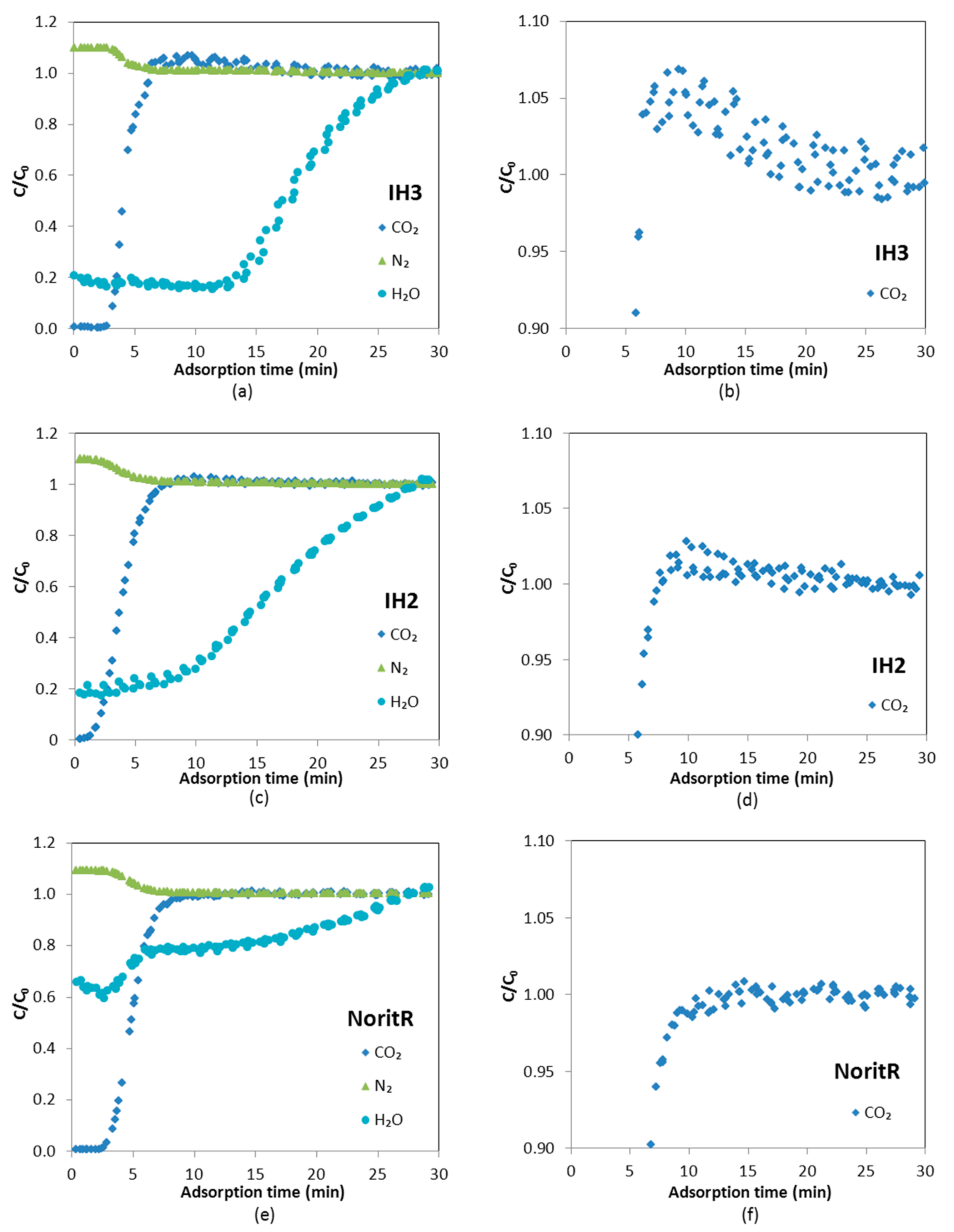
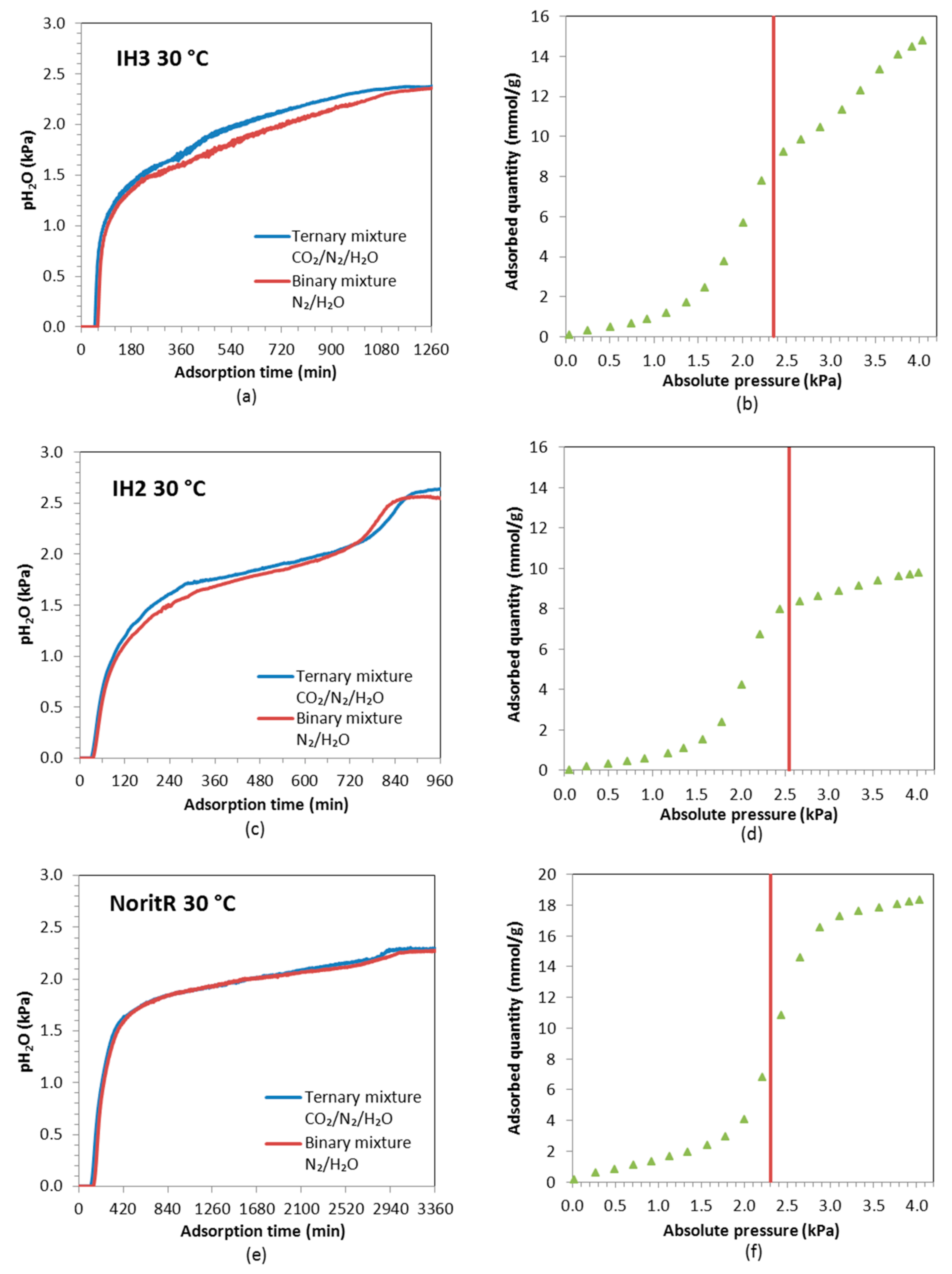
Dynamic adsorption experiments with gas mixtures at high relative humidity were carried out. A breakthrough curve of water vapor for a binary mixture of 98% N
2 and 2% H
2O obtained at 30 °C is compared with the breakthrough curve for a ternary mixture of 8% CO
2, 90 % N
2 and 2% H
2O. As can be seen in
Figure 10, the shape of the breakthrough curve of H
2O for a binary H
2O/N
2 mixture is similar to that of the ternary N
2/CO
2/H
2O mixture fed to a fresh bed. These results indicate that H
2O adsorption is little affected by CO
2 adsorption.
Comparing the H
2O breakthrough curves for IH3, IH2 and Norit R, it can be observed that they have slightly different shapes. This can be directly ascribed to the shape of the equilibrium adsorption isotherms. The H
2O breakthrough curve of IH2 presents two uptake zones with an intermediate plateau, while, in the curves of IH3 and Norit R, the second uptake is not observed because, at the working partial pressure of water vapor (≈2.4 and 2.3 kPa, respectively), a steep rise in the H
2O uptake is to take place according to the corresponding adsorption isotherms in
Figure 10.
The experimental water vapor partial pressure at saturation is slightly lower for IH3 experiments than for IH2 (2.35–2.4 kPa vs. 2.55–2.6 kPa), thus, according to the pure component adsorption isotherms of H2O, both samples are expected to adsorb approximately the same amount of water (≈8 mmol/g). However, water vapor adsorption on carbon IH3 is somewhat smaller than on IH2. To the authors’ knowledge this could only be due to the cumulative error associated with the integration of the experimental data over longer times in the experiments with IH3 (1260 min for IH3 vs. 920 min for IH2). Norit R presents greater water vapor adsorption capacity and slower kinetics than the pine sawdust carbon pellets, hence water vapor breakthrough is postponed and the saturation point is reached after more than 3200 min from the beginning of the water vapor feeding.
Table 7 summarizes the water adsorption uptake and breakthrough times for experiments at 30 °C (
RH ≈ 60%) and 50 °C (
RH ≈ 22%). It is worth noting that the total feed flow rate and its content in H
2O was the same for both experiments. Norit R presents the highest H
2O adsorption capacity and much longer breakthrough times both at low and at high relative humidity conditions. On the contrary, IH2 shows shorter breakthrough times and lower water vapor uptake at a relative humidity of 22%, whereas IH3 adsorbs less water vapor under high humidity conditions. The H
2O uptakes calculated for the ternary and binary mixtures at each temperature confirm the little influence of CO
2 on the H
2O adsorption capacity.
Under high relative humidity condition the evaluated carbons tend to lose H2O adsorption capacity when compared to the uptake estimated from the H2O equilibrium adsorption isotherms. On the contrary, low RH conditions seem to enhance the H2O uptake during the breakthrough testing, although this could be due to more uncertainty on the equilibrium data for H2O adsorption at these low partial pressures.
In addition, to further assess the influence that water holdup can represent to CO
2 adsorption, a ternary mixture with 90% N
2, 8% CO
2 and 2% H
2O was fed to the adsorbent bed previously saturated with H
2O. The obtained CO
2 breakthrough curve is compared to that from the ternary mixture departing from a fully regenerated adsorbent bed in
Figure 11.
The CO
2 concentration profiles of the adsorbents under the two evaluated conditions substantially differ and point out the influence of humidity conditions of the adsorbent bed on its CO
2 capture performance. However, the slope of the CO
2 breakthrough curves might suggest that mass transfer would be favored when the bed is initially saturated with H
2O. A roll up effect (
C/
C0 > 1) that indicates the displacement of CO
2 by the adsorption of H
2O can be observed in
Figure 11 in the case of IH2 and IH3; however, Norit R requires a longer time to discern it (around 90 min) due to its higher adsorption capacity for H
2O.
Table 8 reports the CO
2 uptakes and breakthrough times estimated from the ternary breakthrough experiments with initially fully wet and fresh beds. Experimental breakthrough results (
qCO
2) are also compared to the data from the pure component adsorption isotherms (
).
The dynamic experiments carried out using ternary mixtures of CO2, N2 and H2O over a fully regenerated adsorbent bed (free of CO2 and H2O at t = 0) show no reduction in the breakthrough time for CO2, compared to the dry cases.
The CO2 adsorption capacities and breakthrough times are similar for both ternary experiments (bed initially saturated with water vapor and fully regenerated) at 50 °C and to the values previously estimated for the binary mixture experiments conducted at 50 °C. A maximum reduction of ≈20% when compared to the CO2 uptake estimated from the equilibrium CO2 adsorption isotherms is observed. However, at 30 °C CO2 adsorption capacity decreases to a great extent (a minimum reduction of ≈50% from the equilibrium CO2 adsorption isotherms) by the co-adsorption of H2O when feeding a humid gas stream to a bed saturated with water vapor. Therefore, the breakthrough time of CO2 is also reduced. This performance accounts for the equilibrium adsorption capacity for pure water vapor at the relative humidity of the feed (e.g., RH ≈ 60%) that is more than 12 times greater than the adsorption capacity for pure CO2 at the partial pressure of CO2 in the feed.
Under high relative humidity conditions, which represent the worst case scenario for the adsorption of CO2, the capacity to adsorb CO2 on Norit R seems less affected than that of IH2 and IH3 when the CO2 mixture is fed to a saturated bed. However, it can be noted that under all the evaluated conditions of relative humidity and initial conditions of the bed, IH3 generally shows higher CO2 uptake on a mass basis than the other two adsorbents.
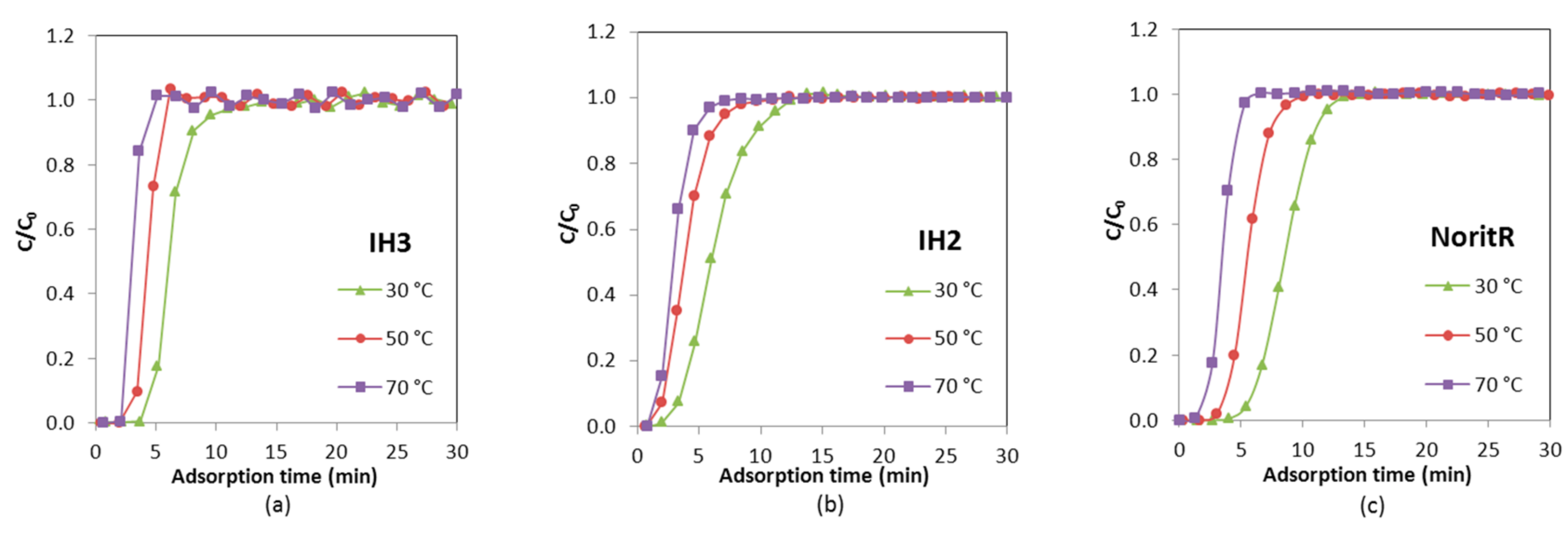
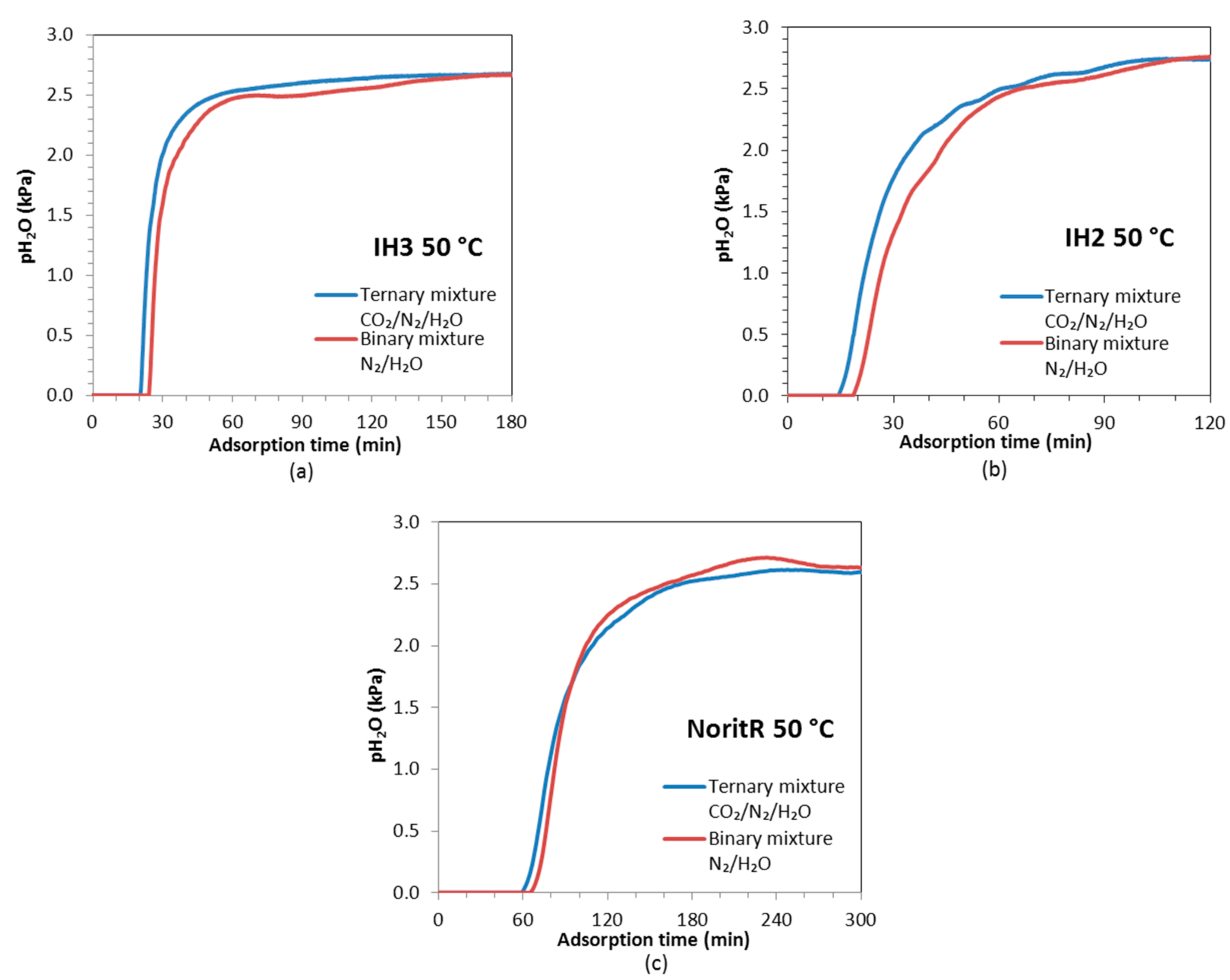
The breakthrough curves of H
2O were also tested at low relative humidity (
RH ≈ 22%) conditions showing a single uptake zone with a steep breakthrough (see
Figure 12). When the relative humidity of the feed is low, the working pressure of water vapor is located within the first region of the adsorption isotherm before the steep uptake takes place. The shape of the H
2O curves is similar for both the binary N
2/H
2O and ternary N
2/CO
2/H
2O experiments; however, the H
2O breakthrough is slightly anticipated for the ternary mixture due to the competitive co-adsorption of CO
2.
The steeper slopes of the H2O breakthrough curves in the IH3 bed indicate less diffusional resistance for adsorption than in the other two adsorbent beds. On activated carbon IH2 the saturation point is reached after approximately 110 min, IH3 needs around 150 min and Norit R up to 240 min. In addition, the three samples adsorbed a little bit more of water vapor in the binary mixture experiment than in the ternary one, due to the absence of CO2 adsorption competition.
At 50 °C, the relative humidity of the feed is lower (
RH ≈ 22%) so the equilibrium adsorption capacity for pure H
2O is only 1.5 times greater than that for pure CO
2 at the partial pressure of CO
2 in the feed. Therefore, CO
2 adsorption is barely affected by H
2O adsorption in low humidity conditions. In fact, as could be observed in
Figure 11, the breakthrough curves of CO
2 over a fresh bed and over a bed saturated with H
2O almost overlap for all the samples in the experiments at 50 °C. CO
2 breaks first through the beds of IH2 and IH3 than through Norit R; however, IH3 reaches equilibrium first meaning that adsorption kinetics is faster on this sample.
The results presented herein are in good agreement with those from a previous work [
21] carried out with a biomass based carbon adsorbent evaluated under post-combustion capture conditions (14% CO
2 at ambient temperature). A reduction in the CO
2 adsorption capacity up to 64% was found for a relative humidity in the gas feed of 95%.