Pyrolysis of Grape Marc from Tunisian Wine Industry: Feedstock Characterization, Thermal Degradation and Kinetic Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Kinetic Approach
4. Results and Discussions
4.1. Grape Marc Characterization
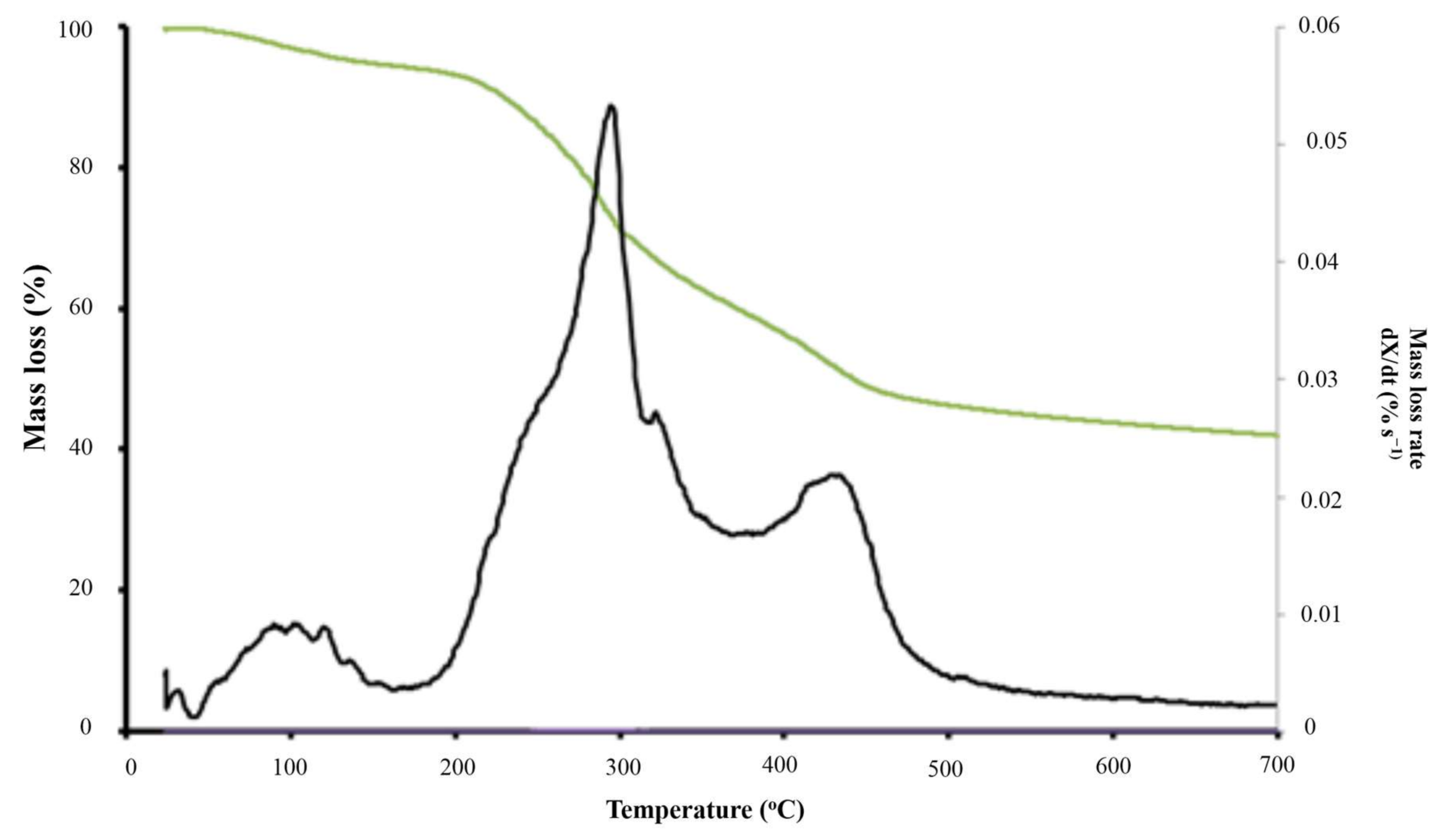
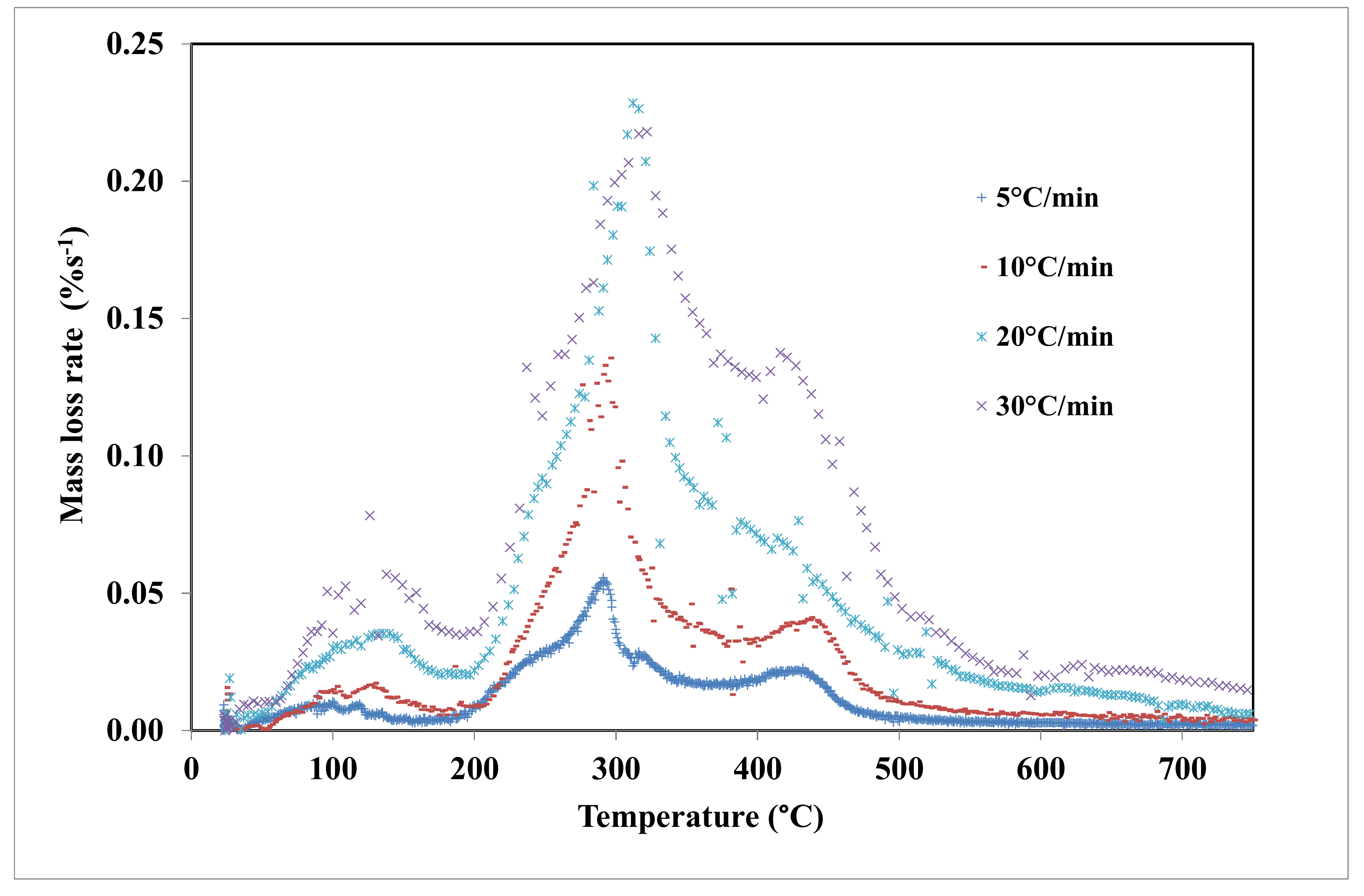
4.2. Thermal Degradation of Grape Marc under Inert Atmosphere
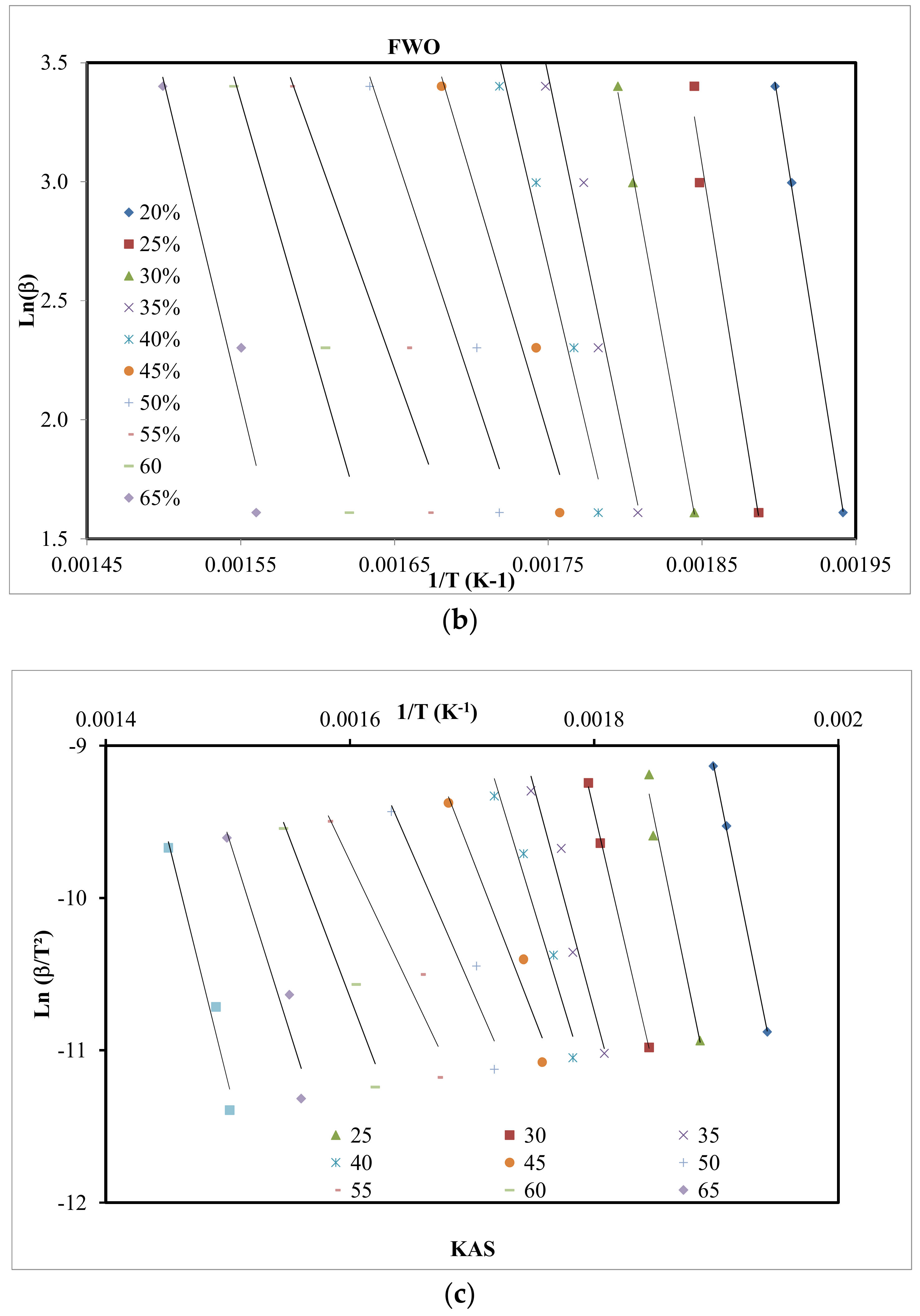
4.3. Kinetic Study
5. Conclusions
Author Contributions
Conflicts of Interest
Nomenclature
| A | pre-exponential factor (s−1) |
| Ea | activation energy (kJ/mol) |
| k | rate constant (s−1) |
| R | universal gas constant (J/K mol) |
| RM | mean reactivity (%·s−1·°C−1) |
| t | time (s) |
| T | absolute temperature (K) |
| Tpeak | peak temperature (K) |
| W0 | initial weight of the sample (mg) |
| Wf | final weight of the sample (mg) |
| Wt | time t weight of the sample (mg) |
| X | mass loss (%) |
| β | heating rate (K/s) |
References
- Moncayo, J.R.; Aurand, J.M. Table and Dried Grapes; Food and Agriculture Organisation (FAO): Rome, Italy, 2016; ISBN 978-92-5-109708-3. [Google Scholar]
- Celma, A.R.; Rojas, S.; Lopez-Rodriguez, F. Waste-to-energy possibilities for industrial olive and grape by-products in Extremadura. Biomass Bioenergy 2007, 31, 522–534. [Google Scholar] [CrossRef]
- Toscano, G.; Riva, G.; Duca, D.; Pedretti, E.F.; Corinaldesi, F.; Rossini, G. Analysis of the characteristics of the residues of the wine production chain finalized to their industrial and energy recovery. Biomass Bioenergy 2013, 55, 260–267. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Souza, E.C.; Uchoa-Thomaz, A.M.A.; Carioca, J.O.B.; Lima, A.; Martin, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; Silva, J.N.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera, L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol, Campinas 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Perez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, C.; Tripodo, M.M.; Lo Curto, R.B.; Cimino, G. The effects of NaOH treatments of grape-marc, vinasse, and wheat-straw mixtures on their degradability in vitro. Bioresour. Technol. 1993, 44, 197–202. [Google Scholar] [CrossRef]
- Lo Curto, R.B.; Tripodo, M.M. Yeast production from virgin grape marc. Bioresour. Technol. 2001, 78, 5–9. [Google Scholar] [CrossRef]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol. 2004, 92, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Wang, J.; Lu, J. Extraction, distribution and characterisation of phenolic compounds and oil in grapeseeds. Food Chem. 2010, 122, 688–694. [Google Scholar] [CrossRef]
- Kabir, M.J.; Chowdhury, A.A.; Rasul, M.G. Pyrolysis of Municipal Green Waste: A Modelling, Simulation and Experimental Analysis. Energies 2015, 8, 7522–7541. [Google Scholar] [CrossRef]
- Villaescusa, I.; Fiol, N.; Martinez, M.; Miralles, N.; Poch, J.; Serarols, J. Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res. 2004, 38, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Bertran, E.; Sort, X.; Soliva, M.; Trillas, I. Composting winery waste: Sludges and grape stalks. Bioresour. Technol. 2004, 95, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Pérez-Murcia, M.D.; Paredes, C.; Moral, R.; Pérez-Espinosa, A.; Moreno-Caselles, J. Short-term carbon and nitrogen mineralisation in soil amended with winery and distillery organic wastes. Biores. Technol. 2007, 98, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.T.; Arranz, J.I.; Román, S.; Rojas, S.; Montero, I.; López, M. Characterization of grape pomace and pyrenean oak pellets. Fuel Process Technol. 2011, 92, 278–283. [Google Scholar] [CrossRef]
- Agence Nationale de Gestion des Déchets—ANGED. Strategic Options for the Promotion of Value of Organic Waste VDO Tunisia; Agence Nationale de Gestion des Déchets: Tunis, Tunisie, 2009. [Google Scholar]
- Kraiem, N.; Lajili, M.; Limousy, L.; Said, R.; Jeguirim, M. Energy recovery from Tunisian agri-food wastes: Evaluation of combustion performance and emissions characteristics of green pellets prepared from tomato residues and grape marc. Energy 2016, 107, 409–418. [Google Scholar] [CrossRef]
- Jeguirim, M.; Kraiem, N.; Lajili, M.; Guizani, C.; Zorpas, A.; Leva, Y.; Michelin, L.; Josien, L.; Limousy, L. The relationship between mineral contents, particle matter and bottom ash distribution during pellet combustion: Molar balance and chemometric analysis. Environ. Sci. Pollut. Res. Int. 2017, 24, 9927–9939. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.H.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Strong, P.J.; Xu, S.; Liu, S.; Lu, K.; Sheng, K.; Guo, J.; Che, L.; He, L.; et al. Thermal Properties of Biochars Derived from Waste Biomass Generated by Agricultural and Forestry Sectors. Energies 2017, 10, 469. [Google Scholar] [CrossRef]
- Jeguirim, M.; Elmay, Y.; Limousy, L.; Lajili, M.; Said, R. Devolatilization behavior and pyrolysis kinetics of potential Tunisian biomass fuels. Environ. Prog. Sustain. Energy 2014, 33, 1452–1458. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomassusing thermogravimetric analysis. Biores. Tech. 2018, 25, 63–74. [Google Scholar] [CrossRef] [PubMed]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrol. 2011, 9, 11–33. [Google Scholar] [CrossRef]
- Cepeliogullar, O.; Haykiri Acma, H.; Yaman, S. Kinetic modelling of RDF pyrolysis: Model-fitting and model-free approaches. Waste Manag. 2016, 48, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bartocci, P.; Anca Couce, A.; Slopiecka, K.; Nefkens, S.; Evic, N.; Retschitzegger, S.; Barbanera, M.; Buratti, C.; Cotana, F.; Bidini, G.; et al. Pyrolysis of pellets made with biomass and glycerol: Kinetic analysis and evolved gas analysis. Biomass Bioenergy 2017, 97, 11–19. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of thermogravimetry of polymers. J. Res. NBS A Phys. Chem. 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; Garcia, R.; Pevida, C. Optimization of a Bubbling Fluidized Bed Plant for Low-Temperature Gasification of Biomass. Energies 2017, 10, 306. [Google Scholar] [CrossRef]
- Makela, M.; Kwong, C.W.; Bostrom, M.; Yoshikawa, K. Hydrothermal treatment of grape marc for solid fuel applications. Energy Convers. Manag. 2017, 145, 371–377. [Google Scholar] [CrossRef]
- Arvelakis, S.; Koukios, E.G. Physicochemical upgrading of agroresidues as feedstocks for energy production via thermochemical conversion methods. Biomass Bioenergy 2002, 22, 331–348. [Google Scholar] [CrossRef]
- Limousy, L.; Jeguirim, M.; Dutournié, P.; Kraiem, N.; Lajili, M.; Said, R. Gaseous products and particulate matter emissions of biomass residential boiler fired with spent coffee grounds pellets. Fuel 2013, 107, 323–329. [Google Scholar] [CrossRef]
- Gamzenur, O.; Ayse Eren, P. Kinetics and evolved gas analysis for pyrolysis of food processing wastes using TGA/MS/FT-IR. Waste Manag. 2017. [Google Scholar] [CrossRef]
- ElSayed, S.A.; Khairy, M. Effect of heating rate on the chemical kinetics of different biomass pyrolysis materials. Biofuels 2015, 6, 157–170. [Google Scholar] [CrossRef]
- Orfao, J.J.M.; Antunes, F.J.A.; Figueiredo, J.L. Pyrolysis kinetics of lignocellulosic materials of three independent reactions model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- ElSayed, S.A.; Mostafa, M.E. Kinetic Parameters Determination of Biomass Pyrolysis Fuels Using TGA and DTA Techniques. Waste Biomass Valoriz. 2015, 6, 401–415. [Google Scholar] [CrossRef]
- Jeguirim, M.; Bikai, J.; Elmay, Y.; Limousy, L.; Njeugna, E. Thermal characterization and pyrolysis kinetics of tropical biomass feedstocks for energy recovery. Energy Sustain. Dev. 2014, 23, 188–193. [Google Scholar] [CrossRef]
- Haddad, K.; Jeguirim, M.; Jellali, S.; Guizani, C.; Delmottea, L.; Bennicia, S.; Limousy, L. Combined NMR structural characterization and thermogravimetric analyses for the assessment of the AAEM effect during lignocellulosic biomass pyrolysis. Energy 2017, 134, 10–23. [Google Scholar] [CrossRef]
- El may, Y.; Jeguirim, M.; Dorge, S.; Trouvé, G.; Said, R. Study on the thermal behavior of different date palm residues: Characterization and devolatilization kinetics under inert and oxidative atmospheres. Energy 2012, 44, 702–709. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, S.; Huhetaoli, L.T.; Chen, Y. Influences of copper on the pyrolysis process of demineralized wood dust through thermogravimetric and PyeGC/ MS analysis. J. Anal. Appl. Pyrolysis 2015, 112, 325–332. [Google Scholar] [CrossRef]
- Jeguirim, M.; Dorge, S.; Trouvé, G. Thermogravimetric analysis and emission characteristics of two energy crops in air atmosphere: Arundo donax and Miscanthus giganthus. Bioresour. Technol. 2010, 101, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Skreiberg, A.; Skreiberg, O.; Sandquist, J.; Sorum, L. TGA and macro-TGA characterisation of biomass fuels and fuel mixtures. Fuel 2011, 90, 2182–2197. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguirim, M.; Khiari, B.; Zagrouba, F.; Trouvé, G. Thermal degradation of olive solid waste: Influence of particle size and oxygen concentration. Resour. Conserv. Recycl. 2010, 54, 271–277. [Google Scholar] [CrossRef]
- Rhén, C.; Ohman, M.; Gref, R.; Wasterlund, I. Effect of raw material composition in woody biomass pellets on combustion characteristics. Biomass Bioenergy 2007, 1, 66–72. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguirim, M.; Trouvé, G.; Favre-Reguillon, A.; Le Buzit, G. Combined process for the treatment of olive oil mill wastewater: Absorption on sawdust and combustion of the impregnated sawdust. Bioresour. Technol. 2010, 101, 6962–6971. [Google Scholar] [CrossRef] [PubMed]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis —A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Guizani, C.; Ouederni, A.; Limousy, L. Pyrolysis of Olive Pomace: Degradation Kinetics, Gaseous Analysis and Char Characterization. Waste Biom. Valoriz. 2017, 8, 1689–1697. [Google Scholar] [CrossRef]
- Jun-Ho, J.; Seung-Soo, K.; Ye-Eun, L.; Yeong-Seok, Y. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies 2017, 10, 1191. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Ghetti, P.; Ricca, L.; Angelini, L. Thermal analysis of biomass and corresponding pyrolysis products. Fuel 1996, 75, 565–573. [Google Scholar] [CrossRef]
- Fiori, L.; Valbusa, M.; Lorenzi, D.; Fambri, L. Modeling of the devolatilization kinetics during pyrolysis of grape residues. Biores. Tech. 2012, 103, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Marculescu, C.; Ciuta, S. Wine industry waste thermal processing for derived fuel properties improvement. Renew. Energy 2013, 57, 645–652. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Wang, P.; Bret, H.; Howard, B.H. Impact of Thermal Pretreatment Temperatures on Woody Biomass Chemical Composition, Physical Properties and Microstructure. Energies 2018, 11, 25. [Google Scholar] [CrossRef]


| Method | Expression | Plots | Reference |
|---|---|---|---|
| Friedman | vs. | [26] | |
| FWO | vs. | [27] | |
| KAS | vs. | [28] |
| This Study | Miranda et al. [15] | Gonzalez-Vazquez et al. [30] | Makela et al. [31] | |
|---|---|---|---|---|
| Proximate Analysis | % | |||
| Moisture wb | 10 | 7.49 | - | 1.4 |
| Fixed Carbon db | 31.1 | 24.73 | 19.7 | - |
| Volatile matter db | 55.6 | 67.80 | 67.6 | - |
| Ash db | 13.3 | 7.47 | 12.7 | 8.23 |
| LHV db (MJ·kg−1) | 18.02 | 19.54 | 18.7 | 19.6 |
| Elemental Analysis db | % | |||
| C | 42.2 | 42.97 | 45.5 | 48.7 |
| H | 3.5 | 9.28 | 5.1 | 5.57 |
| O | 37.7 | - | 34.7 | 35.9 |
| N | 3.0 | 2.05 | 1.8 | 1.66 |
| S | 0.3 | 0.17 | 0.17 | - |
| Pyrolysis Zone | T (°C) | 166–750 | |
| X (%) | 94.5–41.3 | ||
| Active Pyrolsis | Zone 1 | T (°C) | 166–362 |
| X (%) | 94.5–61.2 | ||
| Tpeak 1 (°C) | 288 | ||
| R1 (%·s−1) | 0.032 | ||
| Zone 2 | T (°C) | 362–500 | |
| X (%) | 64.81–46.4 | ||
| Tpeak 2 (°C) | 423 | ||
| R2 (%·s−1) | 0.021 | ||
| RM × 103 (%·s−1·°C−1) | 0.14 | ||
| Passive Pyrolysis | T (°C) | 500–750 | |
| X (%) | 46.4–41.3 |
| X | Friedman | FWO | KAS | |||
|---|---|---|---|---|---|---|
| Ea | R2 | Ea | R2 | Ea | R2 | |
| 0.20 | 295.9 | 0.999 | 323.3 | 0.999 | 333.3 | 0.999 |
| 0.25 | 295.0 | 0.995 | 316.9 | 0.979 | 324.4 | 0.978 |
| 0.30 | 259.2 | 0.995 | 282.0 | 0.999 | 287.4 | 0.999 |
| 0.35 | 280.5 | 0.991 | 244.4 | 0.947 | 247.7 | 0.943 |
| 0.40 | 267.5 | 0.955 | 217.0 | 0.964 | 218.8 | 0.961 |
| 0.45 | 111.4 | 0.947 | 172.1 | 0.959 | 171.3 | 0.954 |
| 0.50 | 163.8 | 0.911 | 154.5 | 0.948 | 157.6 | 0.941 |
| 0.55 | 148.5 | 0.936 | 142.9 | 0.939 | 140.0 | 0.930 |
| 0.60 | 212.3 | 0.965 | 176.9 | 0.962 | 175.3 | 0.958 |
| 0.65 | 262.2 | 0.959 | 212.1 | 0.941 | 212.1 | 0.935 |
| Mean | 229.5 | 226.8 | 224.2 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khiari, B.; Jeguirim, M. Pyrolysis of Grape Marc from Tunisian Wine Industry: Feedstock Characterization, Thermal Degradation and Kinetic Analysis. Energies 2018, 11, 730. https://doi.org/10.3390/en11040730
Khiari B, Jeguirim M. Pyrolysis of Grape Marc from Tunisian Wine Industry: Feedstock Characterization, Thermal Degradation and Kinetic Analysis. Energies. 2018; 11(4):730. https://doi.org/10.3390/en11040730
Chicago/Turabian StyleKhiari, Besma, and Mejdi Jeguirim. 2018. "Pyrolysis of Grape Marc from Tunisian Wine Industry: Feedstock Characterization, Thermal Degradation and Kinetic Analysis" Energies 11, no. 4: 730. https://doi.org/10.3390/en11040730






