Liquid Lipase-Catalyzed Esterification of Oleic Acid with Methanol for Biodiesel Production in the Presence of Superabsorbent Polymer: Optimization by Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Effect of SAP on the Esterification
2.3. Optimization of Esterification Using RSM
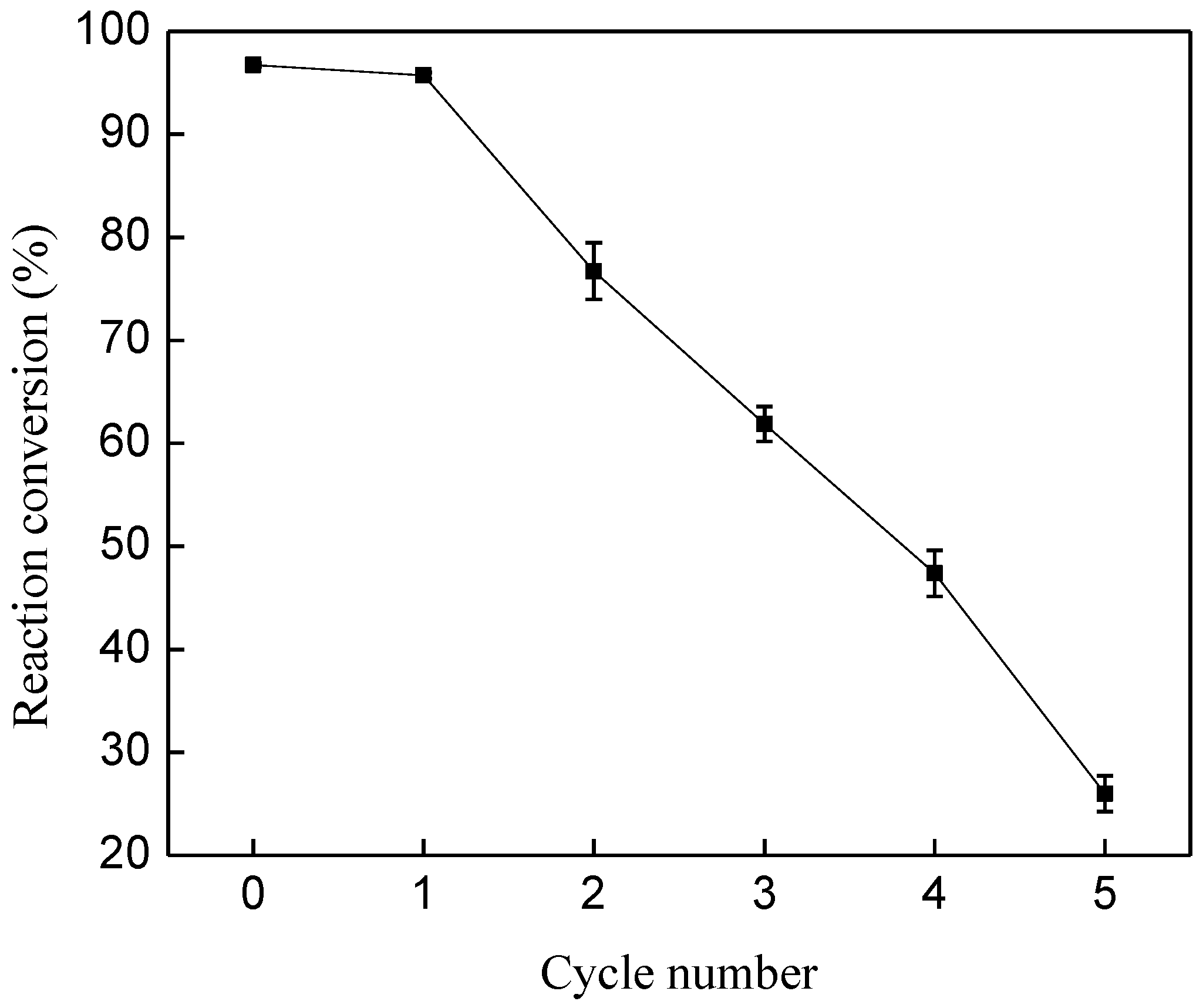
2.4. Enzyme Reuse
3. Results and Discussion
3.1. Effect of SAP on the Reaction Conversion
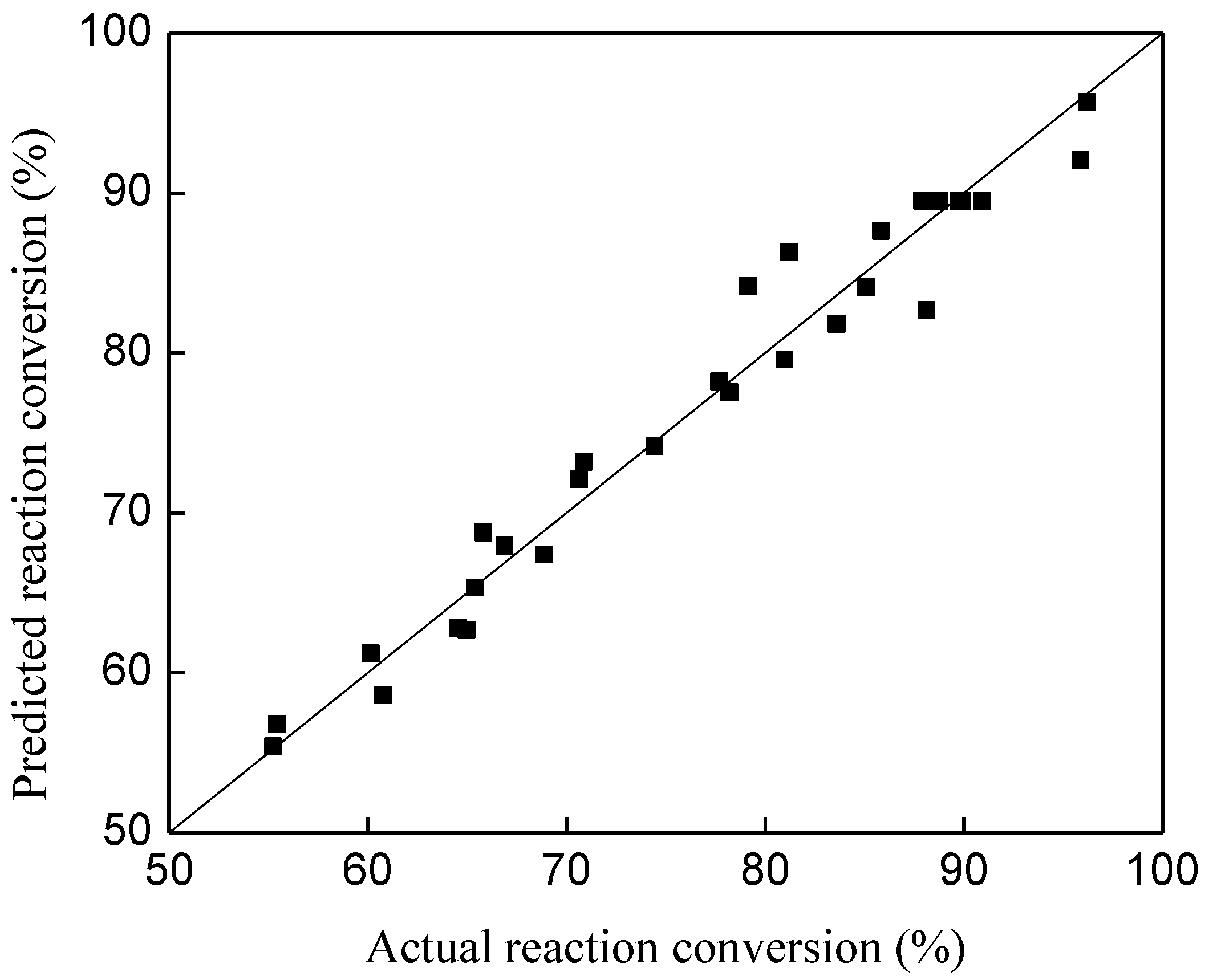
3.2. The RSM Model Development
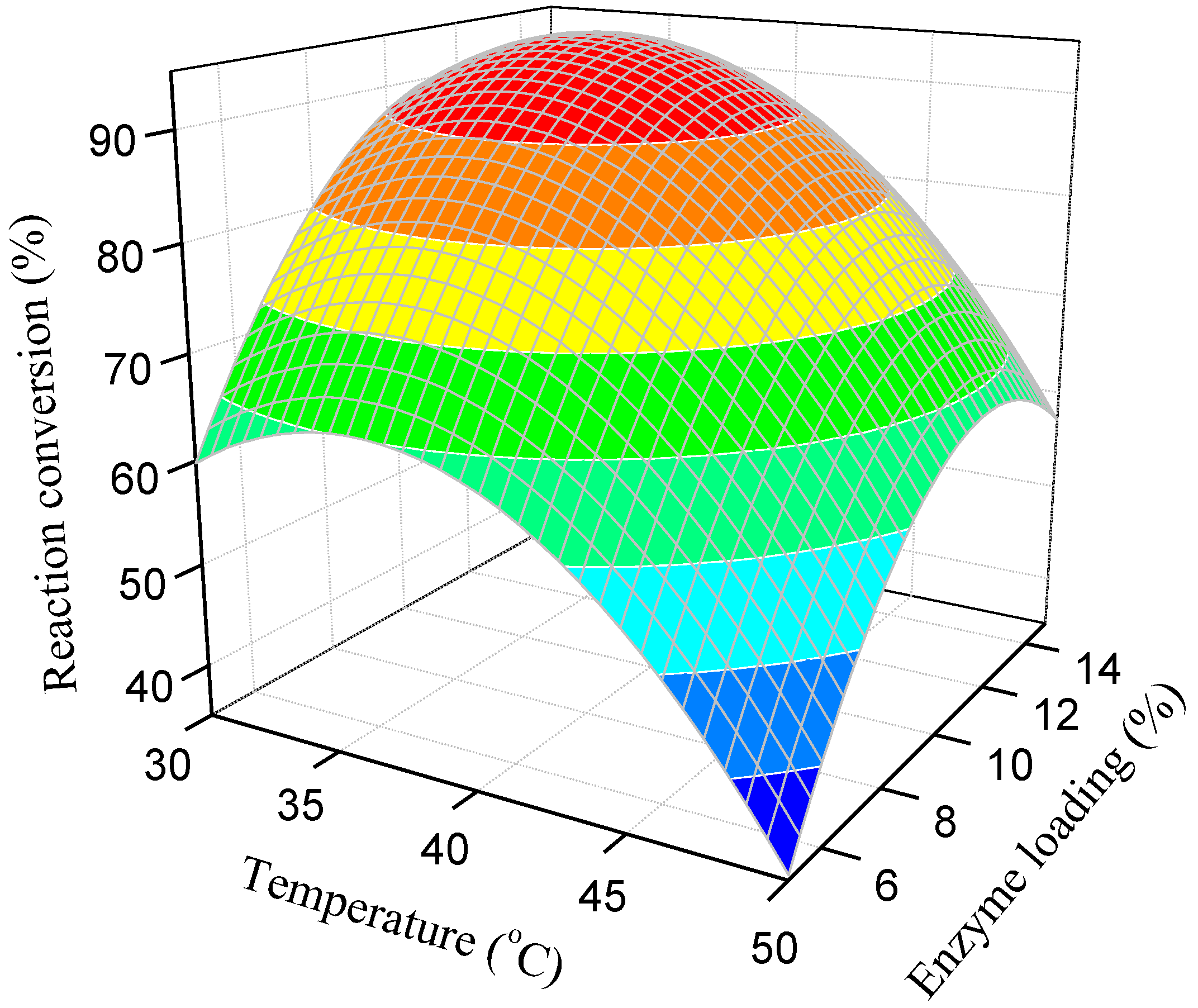
3.3. Effect of Reaction Factors on Reaction Conversion
3.4. Obtaining Optimal Reaction Conditions
3.5. Reusability of Liquid Lipase
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Da Silva Araújo, F.D.; do Nascimento Cavalcante, A.; Sousa, M.D.D.B.; de Moura, C.V.R.; Chaves, M.H.; Aued-Pimentel, S.; Fernandes Caruso, M.S.; Tozetto, L.J.; Kaline Morais Chaves, S. Biodiesel production from Bombacopsis glabra oil by methyl transesterification method. Energies 2017, 10, 1360. [Google Scholar] [CrossRef]
- Kakati, J.; Gogoi, T. Biodiesel production from Kutkura (Meyna spinosa Roxb. Ex.) fruit seed oil: Its characterization and engine performance evaluation with 10% and 20% blends. Energy Convers. Manag. 2016, 121, 152–161. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N. Production optimization and quality assessment of papaya (Carica papaya) biodiesel with response surface methodology. Energy Convers. Manag. 2018, 156, 103–112. [Google Scholar] [CrossRef]
- Mosarof, M.; Kalam, M.; Masjuki, H.; Alabdulkarem, A.; Ashraful, A.; Arslan, A.; Rashedul, H.; Monirul, I. Optimization of performance, emission, friction and wear characteristics of palm and Calophyllum inophyllum biodiesel blends. Energy Convers. Manag. 2016, 118, 119–134. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, E.Y. Simultaneous production of transformer insulating oil and value-added glycerol carbonates from soybean oil by lipase-catalyzed transesterification in dimethyl carbonate. Energies 2017, 11, 82. [Google Scholar] [CrossRef]
- Jin, H.; Kolar, P.; Peretti, S.W.; Osborne, J.A.; Cheng, J.J. Kinetics and mechanism of NaOH-impregnated calcined oyster shell-catalyzed transesterification of soybean oil. Energies 2017, 10, 1920. [Google Scholar] [CrossRef]
- Vahid, B.R.; Haghighi, M. Biodiesel production from sunflower oil over MgO/MgAl2O4 nanocatalyst: Effect of fuel type on catalyst nanostructure and performance. Energy Convers. Manag. 2017, 134, 290–300. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Poudel, J.; Shah, M.; Karki, S.; Oh, S.C. Qualitative analysis of transesterification of waste pig fat in supercritical alcohols. Energies 2017, 10, 265. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Doan, T.T.; Su, C.H.; Yang, P.C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Martindale, W.; Trewavas, A. Fuelling the 9 billion. Nat. Biotechnol. 2008, 26, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Santos, M.A.D.; Freitas, M.A.; Mata, T.M.; Martins, A.A.; Caetano, N.S. Potential of Phaeodactylum tricornutum for biodiesel production under natural conditions in Chile. Energies 2017, 11, 54. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Sanjel, N.; Shah, M.; Oh, S.C. Comparison of biodiesel obtained from virgin cooking oil and waste cooking oil using supercritical and catalytic transesterification. Energies 2017, 10, 546. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Chen, S.S.; Su, C.H.; Lin, J.H.; Chien, C.C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Li, S.Y.; Su, C.H.; Chien, C.C.; Chen, Y.J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Yang, S.; Li, Q.; Gao, Y.; Zheng, L.; Liu, Z. Biodiesel production from swine manure via housefly larvae (Musca domestica L.). Renew. Energy 2014, 66, 222–227. [Google Scholar] [CrossRef]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Anguebes-Franseschi, F.; Abatal, M.; Bassam, A.; Escalante Soberanis, M.A.; May Tzuc, O.; Bucio-Galindo, L.; Cordova Quiroz, A.V.; Aguilar Ucan, C.A.; Ramirez-Elias, M.A. Esterification optimization of crude African palm olein using response surface methodology and heterogeneous acid catalysis. Energies 2018, 11, 157. [Google Scholar] [CrossRef]
- Bhuyan, M.S.U.S.; Alam, A.H.M.A.; Chu, Y.; Seo, Y.C. Biodiesel production potential from littered edible oil fraction using directly synthesized S-TiO2/MCM-41 catalyst in esterification process via non-catalytic subcritical hydrolysis. Energies 2017, 10, 1290. [Google Scholar] [CrossRef]
- Chongkhong, S.; Tongurai, C.; Chetpattananondh, P.; Bunyakan, C. Biodiesel production by esterification of palm fatty acid distillate. Biomass Bioenergy 2007, 31, 563–568. [Google Scholar] [CrossRef]
- Hayyan, A.; Alam, M.Z.; Mirghani, M.E.; Kabbashi, N.A.; Hakimi, N.I.N.M.; Siran, Y.M.; Tahiruddin, S. Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Process. Technol. 2011, 92, 920–924. [Google Scholar] [CrossRef]
- Campanelli, P.; Banchero, M.; Manna, L. Synthesis of biodiesel from edible, non-edible and waste cooking oils via supercritical methyl acetate transesterification. Fuel 2010, 89, 3675–3682. [Google Scholar] [CrossRef]
- Minami, E.; Saka, S. Kinetics of hydrolysis and methyl esterification for biodiesel production in two-step supercritical methanol process. Fuel 2006, 85, 2479–2483. [Google Scholar] [CrossRef]
- Voulgaris, S.; Papadopoulou, A.A.; Alevizou, E.; Stamatis, H.; Voutsas, E. Measurement and prediction of solvent effect on enzymatic esterification reactions. Fluid Phase Equilib. 2015, 398, 51–62. [Google Scholar] [CrossRef]
- Rosset, I.G.; Cavalheiro, M.C.H.; Assaf, E.M.; Porto, A.L. Enzymatic esterification of oleic acid with aliphatic alcohols for the biodiesel production by Candida antarctica lipase. Catal. Lett. 2013, 143, 863–872. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Evaluation of reaction mechanisms and kinetic parameters for the transesterification of castor oil by liquid enzymes. Ind. Eng. Chem. Res. 2017, 56, 9478–9488. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Kodali, S.; Balle, T.; Chen, B.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-lipase for heterogeneous substrates: A new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef]
- Ren, H.; Li, Y.; Du, W.; Liu, D. Free lipase-catalyzed esterification of oleic acid for fatty acid ethyl ester preparation with response surface optimization. J. Am. Oil Chem. Soc. 2013, 90, 73–79. [Google Scholar] [CrossRef]
- Cesarini, S.; Diaz, P.; Nielsen, P.M. Exploring a new, soluble lipase for FAMEs production in water-containing systems using crude soybean oil as a feedstock. Process Biochem. 2013, 48, 484–487. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Effect of water on lipase NS81006-catalyzed alcoholysis for biodiesel production. Process Biochem. 2017, 58, 239–244. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Influence of the reaction conditions on the enzyme catalyzed transesterification of castor oil: A possible step in biodiesel production. Bioresour. Technol. 2017, 243, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Adewale, P.; Vithanage, L.N.; Christopher, L. Optimization of enzyme-catalyzed biodiesel production from crude tall oil using Taguchi method. Energy Convers. Manag. 2017, 154, 81–91. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M.A.; Wang, Z.; Wu, J.; Zhang, Y.; Yuan, Z. Enhanced esterification of oleic acid and methanol by deep eutectic solvent assisted Amberlyst heterogeneous catalyst. Bioresour. Technol. 2016, 220, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Giacometti, F.; Milin, Č.; Vasić-Rački, Đ.A. Kinetic characterisation of enzymatic esterification in a solvent system: Adsorptive control of water with molecular sieves. J. Mol. Catal. B Enzym. 2001, 11, 921–928. [Google Scholar] [CrossRef]
- Duan, Y.; Du, Z.; Yao, Y.; Li, R.; Wu, D. Effect of molecular sieves on lipase-catalyzed esterification of rutin with stearic acid. J. Agric. Food Chem. 2006, 54, 6219–6225. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xin, Z.; Meng, X.; Sun, S.; Qiao, Q.; Deng, H. Studies on biodiesel production from DDGS-extracted corn oil at the catalysis of Novozym 435/super absorbent polymer. Fuel 2015, 146, 33–40. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H. Recoverable and reusable hydrochloric acid used as a homogeneous catalyst for biodiesel production. Appl. Energy 2013, 104, 503–509. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Arteaga, G.; Li-Chan, E.; Vazquez-Arteaga, M.; Nakai, S. Systematic experimental designs for product formula optimization. Trends Food Sci. Technol. 1994, 5, 243–254. [Google Scholar] [CrossRef]
- Li, S.F.; Wu, W.T. Lipase-immobilized electrospun PAN nanofibrous membranes for soybean oil hydrolysis. Biochem. Eng. J. 2009, 45, 48–53. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, T.; Li, T.; Zhou, J.; Cen, K. Using wet microalgae for direct biodiesel production via microwave irradiation. Bioresour. Technol. 2013, 131, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Kusdiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. Rapid biodiesel production using wet microalgae via microwave irradiation. Energy Convers. Manag. 2014, 84, 227–233. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Veillette, M.; Giroir-Fendler, A.; Faucheux, N.; Heitz, M. Esterification of free fatty acids with methanol to biodiesel using heterogeneous catalysts: From model acid oil to microalgae lipids. Chem. Eng. J. 2017, 308, 101–109. [Google Scholar] [CrossRef]
- Berrios, M.; Siles, J.; Martin, M.; Martin, A. A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel 2007, 86, 2383–2388. [Google Scholar] [CrossRef]
- Köse, Ö.; Tüter, M.; Aksoy, H.A. Immobilized Candida antarctica lipase-catalyzed alcoholysis of cotton seed oil in a solvent-free medium. Bioresour. Technol. 2002, 83, 125–129. [Google Scholar] [CrossRef]
- Waghmare, G.V.; Rathod, V.K. Ultrasound assisted enzyme catalyzed hydrolysis of waste cooking oil under solvent free condition. Ultrason. Sonochem. 2016, 32, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rotková, J.; Šuláková, R.; Korecká, L.; Zdražilová, P.; Jandová, M.; Lenfeld, J.; Horák, D.; Bílková, Z. Laccase immobilized on magnetic carriers for biotechnology applications. J. Magn. Magn. Mater. 2009, 321, 1335–1340. [Google Scholar] [CrossRef]
- Nielsen, P.; Rancke-Madsen, A.; Holm, H.; Burton, R. Production of biodiesel using liquid lipase formulations. J. Am. Oil Chem. Soc. 2016, 93, 905–910. [Google Scholar] [CrossRef]



| Variables | Symbols | Variable Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Temperature (°C) | X1 | 30 | 35 | 40 | 45 | 50 |
| Methanol:oleic acid molar ratio | X2 | 1 | 3 | 5 | 7 | 9 |
| SAP loading (%) | X3 | 5 | 7.5 | 10 | 12.5 | 15 |
| Enzyme loading (%) | X4 | 5 | 7.5 | 10 | 12.5 | 15 |
| Run | Variable | Response, Y | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||
| 1 | 1 | 1 | 1 | 1 | 66.87 |
| 2 | 1 | −1 | 1 | 1 | 88.13 |
| 3 | −2 | 0 | 0 | 0 | 85.83 |
| 4 | 1 | 1 | −1 | 1 | 68.91 |
| 5 | 0 | 0 | 2 | 0 | 77.68 |
| 6 | 0 | 2 | 0 | 0 | 60.76 |
| 7 | −1 | −1 | 1 | 1 | 96.20 |
| 8 | 1 | −1 | −1 | 1 | 74.42 |
| 9 | −1 | −1 | −1 | 1 | 95.88 |
| 10 | 0 | 0 | 0 | 2 | 81.21 |
| 11 | 1 | 1 | 1 | −1 | 55.43 |
| 12 | 1 | −1 | 1 | −1 | 70.65 |
| 13 | −1 | 1 | −1 | −1 | 65.83 |
| 14 | −1 | −1 | −1 | −1 | 80.98 |
| 15 | −1 | −1 | 1 | −1 | 85.09 |
| 16 | 1 | 1 | −1 | −1 | 55.23 |
| 17 | 1 | −1 | −1 | −1 | 64.56 |
| 18 | 0 | −2 | 0 | 0 | 79.16 |
| 19 | 2 | 0 | 0 | 0 | 60.15 |
| 20 | −1 | 1 | 1 | 1 | 78.20 |
| 21 | 0 | 0 | 0 | −2 | 64.98 |
| 22 | 0 | 0 | −2 | 0 | 70.88 |
| 23 | −1 | 1 | −1 | 1 | 83.59 |
| 24 | −1 | 1 | 1 | −1 | 65.38 |
| 25 | 0 | 0 | 0 | 0 | 90.92 |
| 26 | 0 | 0 | 0 | 0 | 89.90 |
| 27 | 0 | 0 | 0 | 0 | 88.79 |
| 28 | 0 | 0 | 0 | 0 | 90.92 |
| 29 | 0 | 0 | 0 | 0 | 87.92 |
| 30 | 0 | 0 | 0 | 0 | 88.36 |
| 31 | 0 | 0 | 0 | 0 | 89.72 |
| Source | DF b | SS b | MS b | F Value | Probability (P) > F |
|---|---|---|---|---|---|
| Model a | 14 | 4319.58 | 308.54 | 32.91 | <0.0001 |
| Residual (error) | 16 | 150.01 | 9.38 | - | - |
| Total | 30 | 4469.59 | - | - | - |
| Model Term | Parameter Estimate | Standard Error | t Value a | p Value |
|---|---|---|---|---|
| β0 | 89.50 | 1.16 | 77.34 | 0.000 b |
| β1 | −6.60 | 0.63 | −10.55 | 0.000 b |
| β2 | −6.39 | 0.63 | −10.22 | 0.000 b |
| β3 | 1.26 | 0.63 | 2.01 | 0.062 |
| β4 | 5.90 | 0.63 | 9.43 | 0.000 b |
| β11 | −3.77 | 0.57 | −6.59 | 0.000 b |
| β22 | −4.53 | 0.57 | −7.91 | 0.000 b |
| β33 | −3.45 | 0.57 | −6.03 | 0.000 b |
| β44 | −3.75 | 0.57 | −6.54 | 0.000 b |
| β12 | 0.86 | 0.77 | 1.13 | 0.275 |
| β13 | 1.21 | 0.77 | 1.58 | 0.133 |
| β14 | −0.26 | 0.77 | −0.34 | 0.740 |
| β23 | −1.99 | 0.77 | −2.61 | 0.019 b |
| β24 | 0.15 | 0.77 | 0.19 | 0.850 |
| β34 | −0.21 | 0.77 | −0.27 | 0.788 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.C.; Huong, D.T.M.; Juan, H.-Y.; Su, C.-H.; Chien, C.-C. Liquid Lipase-Catalyzed Esterification of Oleic Acid with Methanol for Biodiesel Production in the Presence of Superabsorbent Polymer: Optimization by Using Response Surface Methodology. Energies 2018, 11, 1085. https://doi.org/10.3390/en11051085
Nguyen HC, Huong DTM, Juan H-Y, Su C-H, Chien C-C. Liquid Lipase-Catalyzed Esterification of Oleic Acid with Methanol for Biodiesel Production in the Presence of Superabsorbent Polymer: Optimization by Using Response Surface Methodology. Energies. 2018; 11(5):1085. https://doi.org/10.3390/en11051085
Chicago/Turabian StyleNguyen, Hoang Chinh, Dinh Thi My Huong, Horng-Yi Juan, Chia-Hung Su, and Chien-Chung Chien. 2018. "Liquid Lipase-Catalyzed Esterification of Oleic Acid with Methanol for Biodiesel Production in the Presence of Superabsorbent Polymer: Optimization by Using Response Surface Methodology" Energies 11, no. 5: 1085. https://doi.org/10.3390/en11051085





