Reaction Mechanism Reduction for Ozone-Enhanced CH4/Air Combustion by a Combination of Directed Relation Graph with Error Propagation, Sensitivity Analysis and Quasi-Steady State Assumption
Abstract
:1. Introduction
2. Methods for the Development of Reduced Global Mechanisms
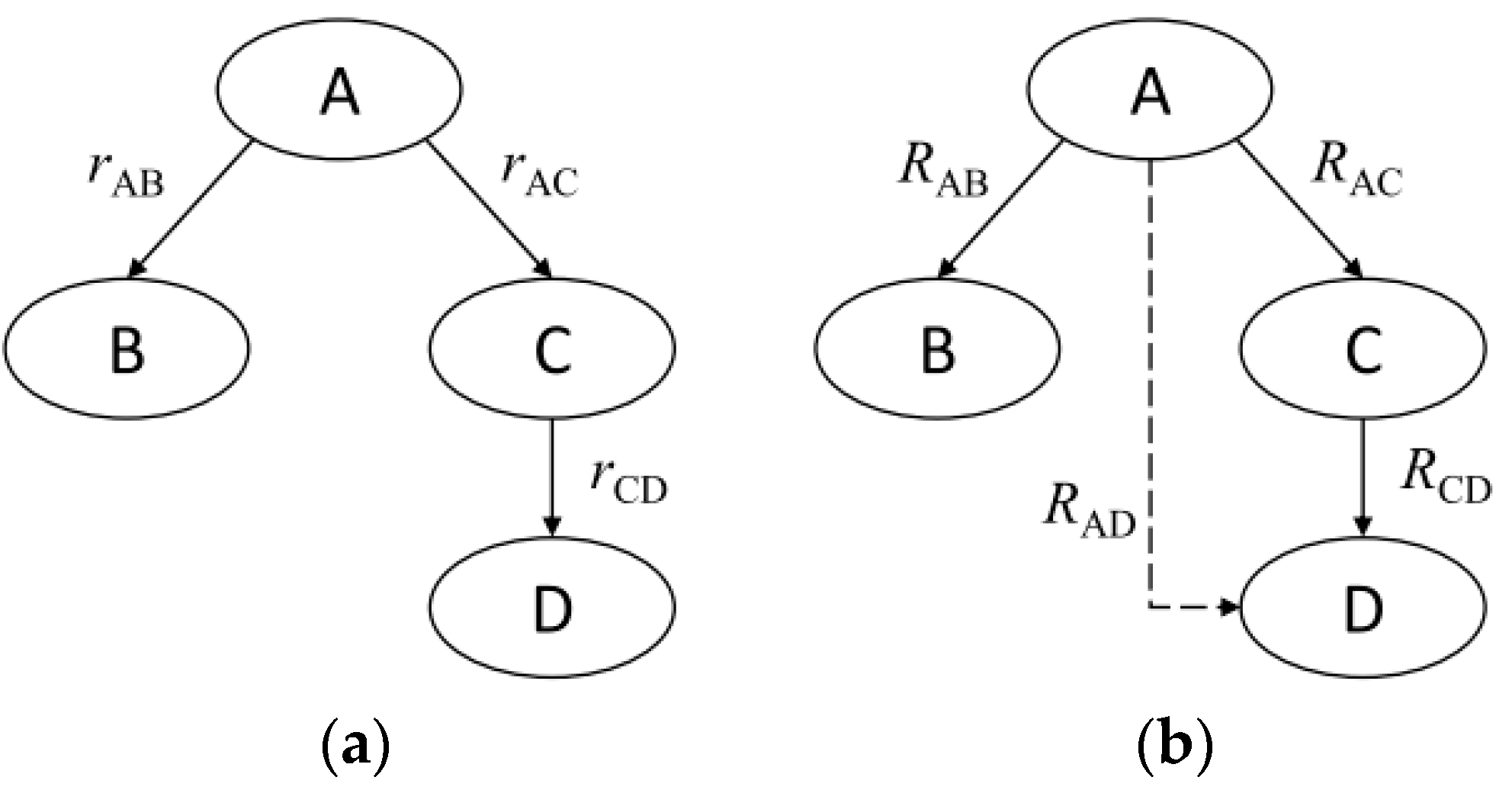
2.1. Preprocessing by DRGEP Method
2.2. Advanced Processing by SA
2.3. Global Mechanism Formation by QSSA Method
3. Detailed Mechanism for Ozone-Enhanced CH4/Air Combustion Process
4. Results and Discussion
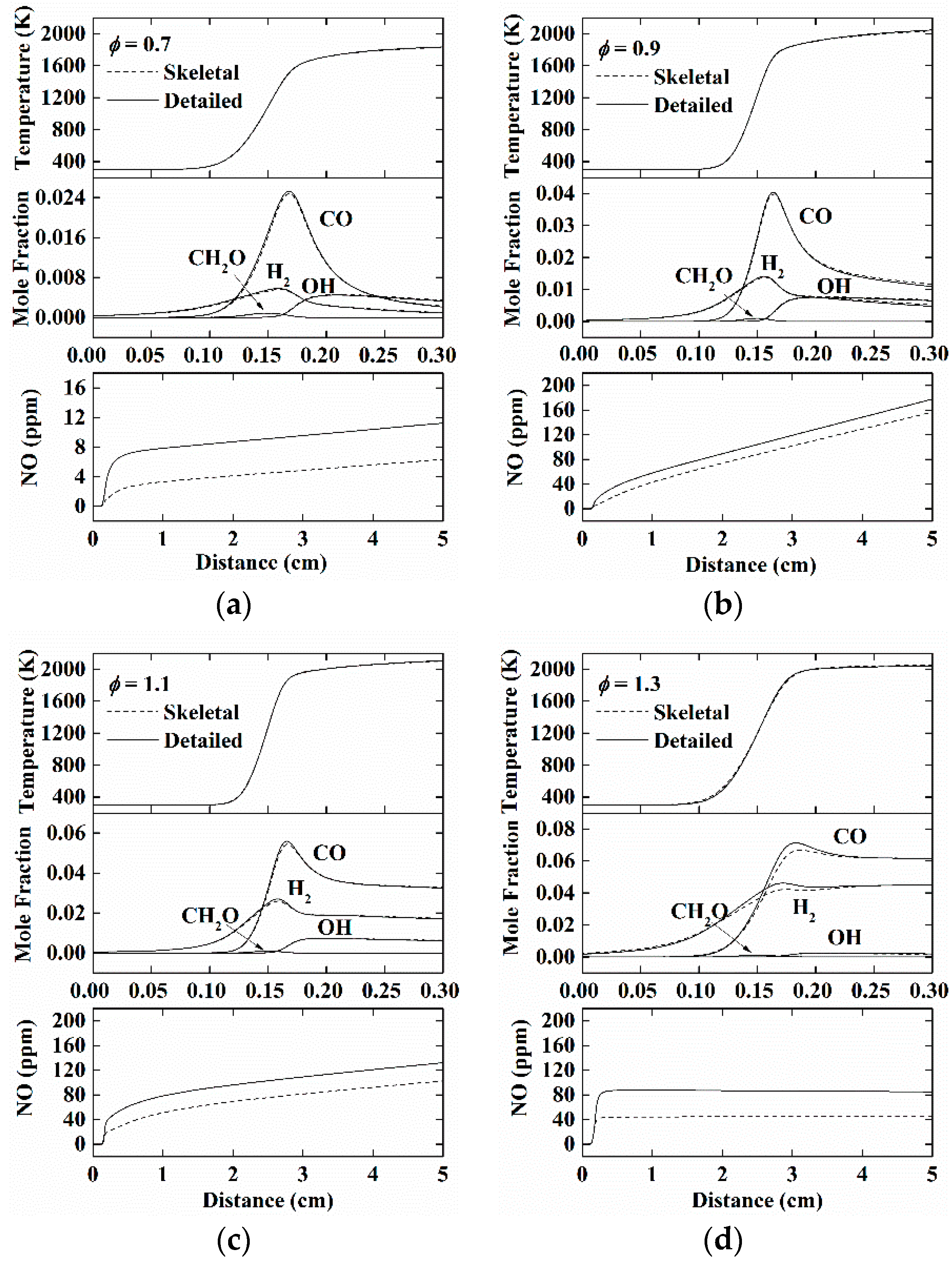
4.1. Skeletal Mechanism
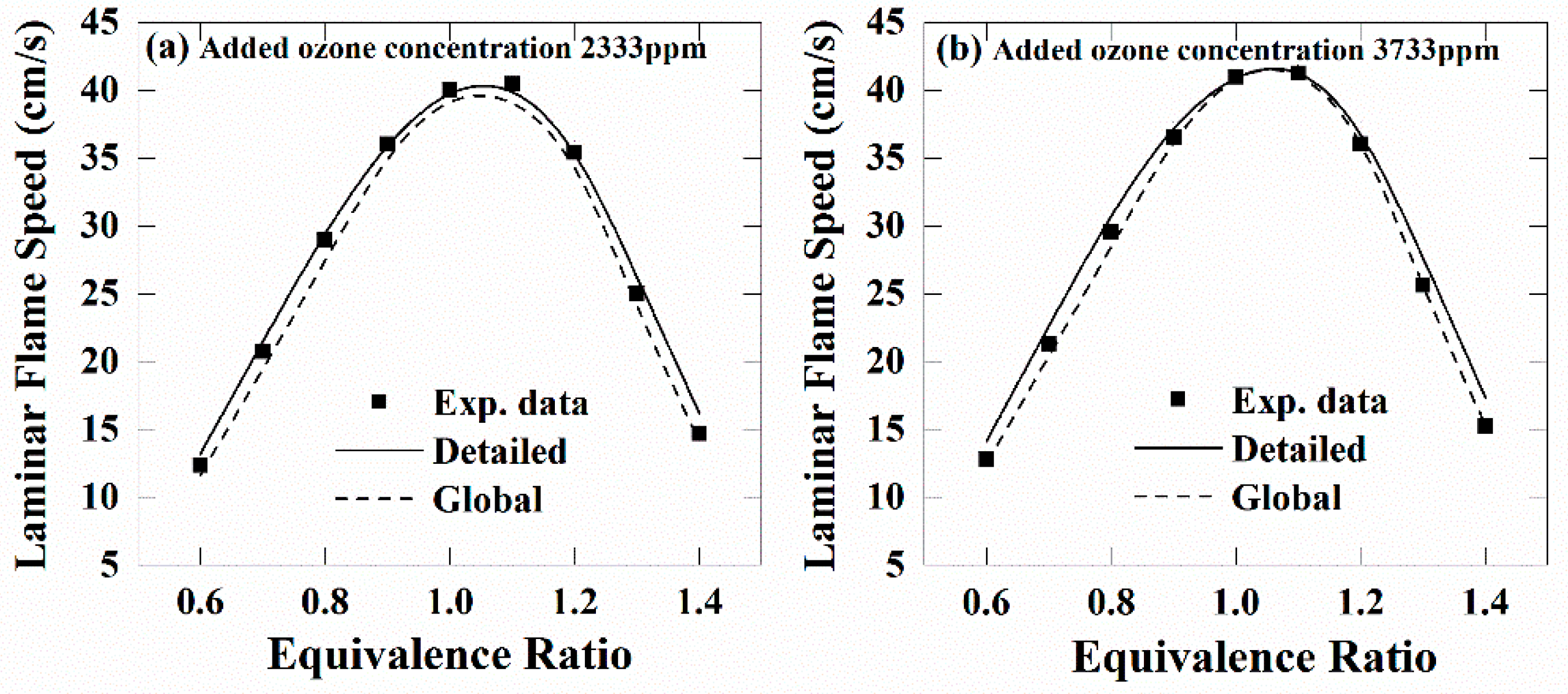
4.2. Global Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Cai, J.; Zhang, L.; Yang, J.; Wang, Z.; Qi, F. Experimental and modeling investigation on premixed ethylbenzene flames at low pressure. Proc. Combust. Inst. 2011, 33, 617–624. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr. GRI-Mech 3.0. 1999. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 1 June 2011).
- Li, J.; Huang, H.; Osaka, Y.; Bai, Y.; Kobayashi, N.; Chen, Y. Combustion and Heat Release Characteristics of Biogas under Hydrogen-and Oxygen-Enriched Condition. Energies 2017, 10, 1200. [Google Scholar] [CrossRef]
- Liu, T.; Bai, F.; Zhao, Z.; Lin, Y.; Du, Q.; Peng, Z. Large Eddy Simulation Analysis on Confined Swirling Flows in a Gas Turbine Swirl Burner. Energies 2017, 10, 2081. [Google Scholar] [CrossRef]
- Contino, F.; Lucchini, T.; D’Errico, G.; Duynslaegher, C.; Dias, V.; Jeanmart, H. Simulations of advanced combustion modes using detailed chemistry combined with tabulation and mechanism reduction techniques. SAE Int. J. Engines 2012, 5, 185–196. [Google Scholar] [CrossRef]
- Minotti, A.; Sciubba, E. LES of a meso combustion chamber with a detailed chemistry model: Comparison between the flamelet and EDC models. Energies 2010, 3, 1943–1959. [Google Scholar] [CrossRef]
- Peters, N.; Rogg, B. Reduced Kinetic Mechanisms for Applications in Combustion Systems, 1st ed.; Springer Science & Business Media: New York, NY, USA, 1993. [Google Scholar]
- Lv, Y.; Wang, Z.; Zhou, J.; Cen, K. Reduced mechanism for hybrid NOx control process. Energy Fuels 2009, 23, 5920–5928. [Google Scholar] [CrossRef]
- Gokulakrishnan, P.; Lawrence, A.; McLellan, P.; Grandmaison, E. A functional-PCA approach for analyzing and reducing complex chemical mechanisms. Comput. Chem. Eng. 2006, 30, 1093–1101. [Google Scholar] [CrossRef]
- Lu, T.; Law, C.K. On the applicability of directed relation graphs to the reduction of reaction mechanisms. Combust. Flame 2006, 146, 472–483. [Google Scholar] [CrossRef]
- Liang, L.; Stevens, J.G.; Farrell, J.T. A dynamic adaptive chemistry scheme for reactive flow computations. Proc. Combust. Inst. 2009, 32, 527–534. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Z.; Gou, X.; Ju, Y. A path flux analysis method for the reduction of detailed chemical kinetic mechanisms. Combust. Flame 2010, 157, 1298–1307. [Google Scholar] [CrossRef]
- Zsély, I.G.; Nagy, T.; Simmie, J.; Curran, H. Reduction of a detailed kinetic model for the ignition of methane/propane mixtures at gas turbine conditions using simulation error minimization methods. Combust. Flame 2011, 158, 1469–1479. [Google Scholar] [CrossRef]
- Turányi, T. Sensitivity analysis of complex kinetic systems. Tools and applications. J. Math. Chem. 1990, 5, 203–248. [Google Scholar] [CrossRef]
- Zhong, B.-J.; Yao, T.; Wen, F. Skeletal and Reduced Mechanisms of n-Decane Simplified with Eigenvalue Analysis. Acta Phys.-Chim. Sin. 2014, 30, 210–216. [Google Scholar]
- Lu, T.; Ju, Y.; Law, C.K. Complex CSP for chemistry reduction and analysis. Combust. Flame 2001, 126, 1445–1455. [Google Scholar] [CrossRef]
- Correa, C.; Niemann, H.; Schramm, B.; Warnatz, J. Reaction mechanism reduction for higher hydrocarbons by the ILDM method. Proc. Combust. Inst. 2000, 28, 1607–1614. [Google Scholar] [CrossRef]
- Sung, C.; Law, C.; Chen, J.-Y. Augmented reduced mechanisms for NO emission in methane oxidation. Combust. Flame 2001, 125, 906–919. [Google Scholar] [CrossRef]
- Dec, J.E. Advanced compression-ignition engines—Understanding the in-cylinder processes. Proc. Combust. Inst. 2009, 32, 2727–2742. [Google Scholar] [CrossRef]
- Yao, M.; Zheng, Z.; Liu, H. Progress and recent trends in homogeneous charge compression ignition (HCCI) engines. Prog. Energy Combust. Sci. 2009, 35, 398–437. [Google Scholar] [CrossRef]
- Fru, G.; Thévenin, D.; Janiga, G. Impact of turbulence intensity and equivalence ratio on the burning rate of premixed methane–air flames. Energies 2011, 4, 878–893. [Google Scholar] [CrossRef]
- Starikovskaia, S.M. Plasma assisted ignition and combustion. J. Phys. D Appl. Phys. 2006, 39, R265. [Google Scholar] [CrossRef]
- Tachibana, T.; Hirata, K.; Nishida, H.; Osada, H. Effect of ozone on combustion of compression ignition engines. Combust. Flame 1991, 85, 515–519. [Google Scholar] [CrossRef]
- Masurier, J.B.; Foucher, F.; Dayma, G.; Dagaut, P. Homogeneous Charge Compression Ignition Combustion of Primary Reference Fuels Influenced by Ozone Addition. Energy Fuels 2013, 27, 5495–5505. [Google Scholar] [CrossRef]
- Ombrello, T.; Won, S.H.; Ju, Y.; Williams, S. Flame propagation enhancement by plasma excitation of oxygen. Part I: Effects of O3. Combust. Flame 2010, 157, 1906–1915. [Google Scholar] [CrossRef]
- Lu, T.; Law, C.K. A directed relation graph method for mechanism reduction. Proc. Combust. Inst. 2005, 30, 1333–1341. [Google Scholar] [CrossRef]
- Pepiot-Desjardins, P.; Pitsch, H. An efficient error-propagation-based reduction method for large chemical kinetic mechanisms. Combust. Flame 2008, 154, 67–81. [Google Scholar] [CrossRef]
- Giral, I.; Alzueta, M.U. An augmented reduced mechanism for the reburning process. Fuel 2002, 81, 2263–2275. [Google Scholar] [CrossRef]
- Weng, W.; Nilsson, E.; Ehn, A.; Zhu, J.; Zhou, Y.; Wang, Z.; Li, Z.; Aldén, M.; Cen, K. Investigation of formaldehyde enhancement by ozone addition in CH4/air premixed flames. Combust. Flame 2015, 162, 1284–1293. [Google Scholar] [CrossRef]
- Halter, F.; Higelin, P.; Dagaut, P. Experimental and detailed kinetic modeling study of the effect of ozone on the combustion of methane. Energy Fuels 2011, 25, 2909–2916. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Li, B.; Li, Z.; Sun, Z.; Aldén, M.; Cen, K.; Konnov, A. Investigation of combustion enhancement by ozone additive in CH4/air flames using direct laminar burning velocity measurements and kinetic simulations. Combust. Flame 2012, 159, 120–129. [Google Scholar] [CrossRef]
- Konnov, A. Modeling ozone decomposition flames. Energy Fuels 2012, 27, 501–506. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Z.; Weng, W.; Zhou, Z.; Huang, Z.; Zhou, J.; Cen, K. Study of ozone-enhanced combustion in H2/CO/N2/air premixed flames by laminar burning velocity measurements and kinetic modeling. Int. J. Hydrogen Energy 2013, 38, 1177–1188. [Google Scholar] [CrossRef]
- Slavinskaya, N.; Braun-Unkhoff, M.; Frank, P. Reduced reaction mechanisms for methane and syngas combustion in gas turbines. J. Eng. Gas Turbines Power 2008, 130, 021504. [Google Scholar] [CrossRef]





| NO. | Reaction | A | n | E |
|---|---|---|---|---|
| 1 | O3 + O2 → O2 + O + O2 | 1.54 × 1014 | 0 | 23,064 |
| 2 | O2 + O + O2 → O3 + O2 | 3.26 × 1019 | −2.1 | 0 |
| 3 | O3 + N2 → O2 + O + N2 | 4.00 × 1014 | 0 | 22,667 |
| 4 | O2 + O + N2 → O3 + N2 | 1.60 × 1014 | −0.4 | −1391 |
| 5 | O3 + O → O2 + O + O | 2.48 × 1015 | 0 | 22,727 |
| 6 | O2 + O + O → O3 + O | 2.28 × 1015 | −0.5 | −1391 |
| 7 | O3 + O3 → O2 + O + O3 | 4.40 × 1014 | 0 | 23,064 |
| 8 | O2 + O + O3 → O3 + O3 | 1.67 × 1015 | −0.5 | −1391 |
| 9 | O3 + H ↔ O2 + OH | 8.43 × 1013 | 0 | 934 |
| 10 | O3 + H ↔ O + HO2 | 4.52 × 1011 | 0 | 0 |
| 11 | O3 + OH ↔ O3 + HO2 | 1.85 × 1011 | 0 | 831 |
| 12 | O3 + H2O ↔ O2 + H2O2 | 6.62 × 101 | 0 | 0 |
| 13 | O3 + HO2 ↔ OH + O2 + O2 | 6.62 × 109 | 0 | 994 |
| 14 | O3 + O ↔ O2 + O2 | 4.82 × 1012 | 0 | 4094 |
| 15 | O3 + NO ↔ O2 + NO2 | 8.43 × 1011 | 0 | 2603 |
| 16 | O3 + CH3 ↔ CH3O + O2 | 5.83 × 1010 | 0 | 0 |
| Reference Components/Parameter | Relative Tolerance | Absolute Tolerance |
|---|---|---|
| CH4, O2, O3, CO2, H2O | 5% | 0 |
| CO, H2 | 10% | 1.0 × 10−4 |
| OH | 10% | 1.0 × 10−4 |
| CH2O | 10% | 1.0 × 10−4 |
| NO | 10% | 1.0 × 10−4 |
| Laminar flame speed | 5% | 2 cm/s |
| Entry | Overall Reactions | Entry | Overall Reactions |
|---|---|---|---|
| 1 | H2 + O = OH + H | 10 | CH + NO = HCN + O |
| 2 | OH + OH + OH = H2O + HO2 | 11 | O3 = O + O2 |
| 3 | CH + H = CH2(S) | 12 | H2O + HO2 = O3 + H2 + H |
| 4 | CH2 + H2 = CH4 | 13 | O2 = O + O |
| 5 | C2H4 = CH2 + CH2(S) | 14 | CH4 + CH + H = C2H6 |
| 6 | C2H4 = CH3 + CH | 15 | H + CO = HCO |
| 7 | HCO + H = CH2O | 16 | CO2 = CO + O |
| 8 | CH2O + CH2O = C2H4 + O2 | 17 | NO + HCN = H = N2 + CH2O |
| 9 | O2 + CH2(S) = HCO + OH | 18 | O + O2 + C2H6 = CO2 + H2O + CH + H + H2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Z.; Li, L.; Wan, K.; Cen, K. Reaction Mechanism Reduction for Ozone-Enhanced CH4/Air Combustion by a Combination of Directed Relation Graph with Error Propagation, Sensitivity Analysis and Quasi-Steady State Assumption. Energies 2018, 11, 1470. https://doi.org/10.3390/en11061470
Liu Y, Wang Z, Li L, Wan K, Cen K. Reaction Mechanism Reduction for Ozone-Enhanced CH4/Air Combustion by a Combination of Directed Relation Graph with Error Propagation, Sensitivity Analysis and Quasi-Steady State Assumption. Energies. 2018; 11(6):1470. https://doi.org/10.3390/en11061470
Chicago/Turabian StyleLiu, Yingzu, Zhihua Wang, Liang Li, Kaidi Wan, and Kefa Cen. 2018. "Reaction Mechanism Reduction for Ozone-Enhanced CH4/Air Combustion by a Combination of Directed Relation Graph with Error Propagation, Sensitivity Analysis and Quasi-Steady State Assumption" Energies 11, no. 6: 1470. https://doi.org/10.3390/en11061470





