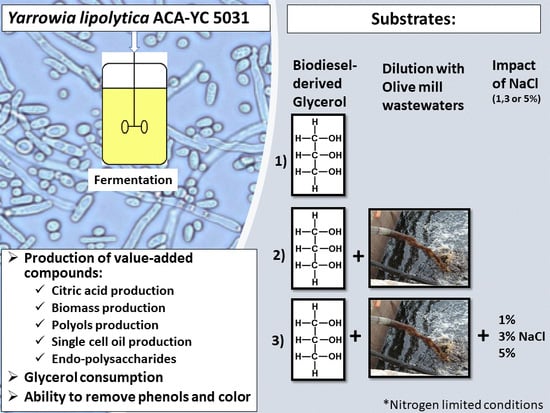
Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters
Abstract
:1. Introduction
2. Results
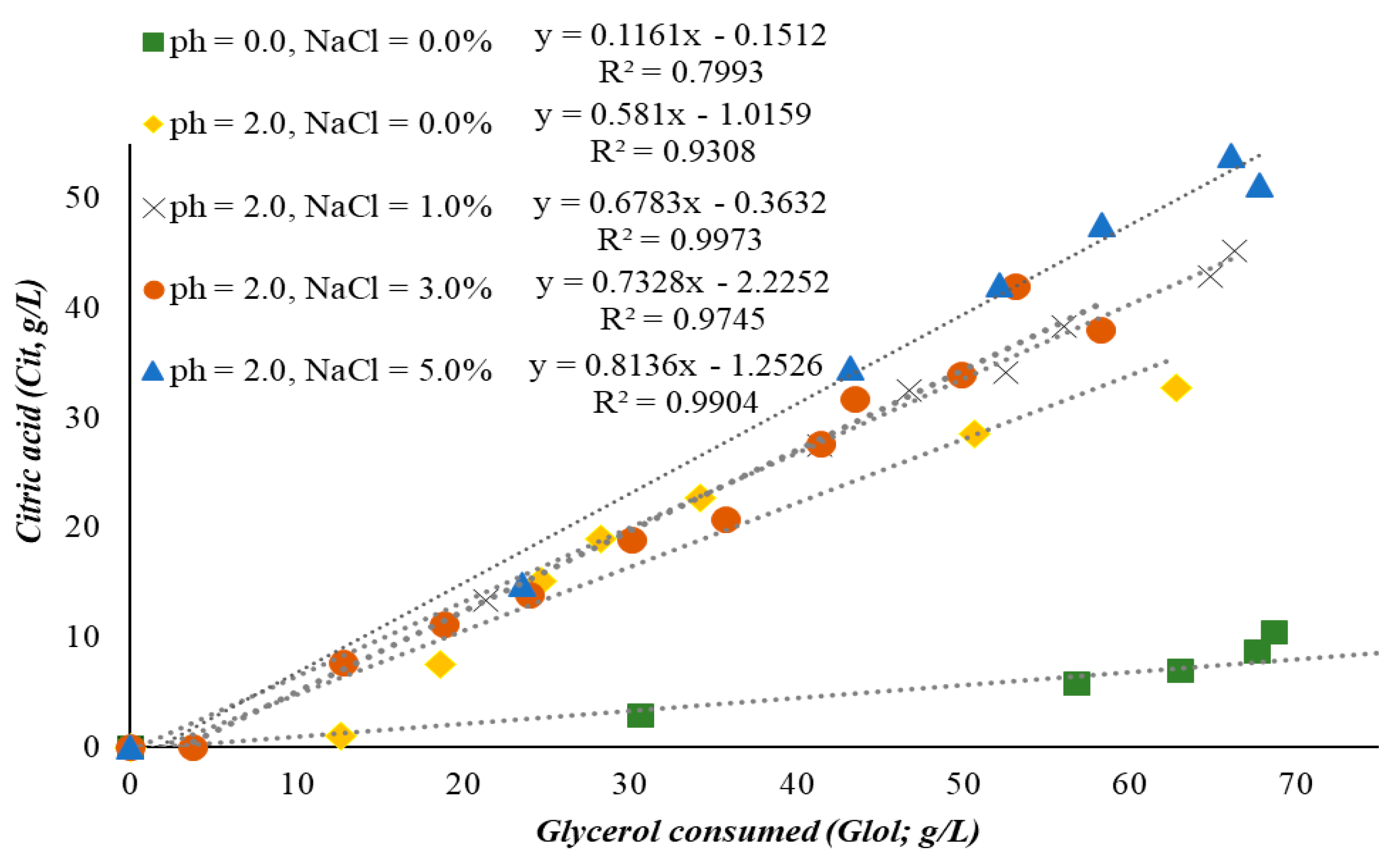
2.1. Batch Fermentations and Production of Value-Added Compounds by Yarrowia lipolytica Growing on OMW/Glycerol Blends Supplemented with NaCl
2.2. Intra-Cellular Lipid Concentration and Fatty Acid Composition
2.3. Color and Phenolic Compounds Removal
3. Discussion
4. Materials and Methods
4.1. Microorganism, Media, and Culture Conditions
4.2. Dry Weight Determination
4.3. Determination of Total Intra-Cellular Polysaccharides (IPS)
4.4. Determination of Glycerol, Polyols, and Citric Acid
4.5. Quantitative Determination of the Cellular Lipid and Fatty Acid (FA) Composition Analysis
4.6. Phenolic Compounds Determination
4.7. Decolorization
4.8. Determination of Extra-Cellular Nitrogen Into the Fermentation Medium
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents, Part I. Organic matter degradation by chemical and biological processes-an overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive oil mill wastewater valorization by fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowialipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M. Toxicity and biodegradability of olive mill wastewaters in batch anaerobic digestion. Appl. Biochem. Biotechnol. 1992, 37, 155–163. [Google Scholar] [CrossRef]
- Zervakis, G.; Balis, C. Bioremediation of olive mill wastes water through the production of fungal biomass. In Proceedings of the Second International Conference on Mushrooms Biology and Mushrooms Products, University Park, PA, USA, 9–12 June 1996; pp. 311–323. [Google Scholar]
- Tsioulpas, A.; Dimou, D.; Iconomou, D.; Aggelis, G. Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour. Technol. 2002, 84, 251–257. [Google Scholar] [CrossRef]
- Aggelis, G.; Iconomou, D.; Christou, M.; Bokas, D.; Kotzailias, S.; Christou, G.; Tsagou, V.; Papanikolaou, S. Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 2003, 37, 3897–3904. [Google Scholar] [CrossRef]
- Crognale, S.; Federici, F.; Petruccioli, M. β-Glucan production by Botryosphaeria rhodina on undiluted olive-mill waste waters. Biotechnol. Lett. 2003, 25, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- D’Annibale, A.; Sermani, G.G.; Federici, F.; Petruccioli, M. Olive-mill wastewaters: A promising substrate for microbial lipase production. Bioresour. Technol. 2006, 97, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Sarris, D.; Papanikolaou, S. Biotechnological production of ethanol: biochemistry, processes and technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar] [CrossRef]
- Rivaldi, J.D.; Sarrouh, B.F.; da Silva, S.S. Development of biotechnological processes using glycerol from biodiesel production. In Current Research Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; World Scientific Publishing Co. Formatex Research Center: Madrid, Spain, 2009; pp. 429–433. [Google Scholar]
- Wen, Z.; Pyle, D.J.; Athalye, S.K. Glycerol waste from biodiesel manufacturing. In Microbial Conversions of Raw Glycerol; Aggelis, G., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 1–7. [Google Scholar]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Ren. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Bellou, S.; Moustogianni, A.; Makri, A.; Aggelis, G. Lipids containing polyunsaturated fatty acids synthesized by Zygomycetes grown on glycerol. Appl. Biochem. Biotechnol. 2012, 166, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Dedyukhina, E.G.; Chistyakova, T.I.; Kamzolova, S.V.; Vinter, M.V.; Vainshtein, M.B. Arachidonic acid synthesis by glycerol-grown Mortierella alpina. Eur. J. Lipid Sci. Technol. 2012, 114, 833–841. [Google Scholar] [CrossRef]
- Dedyukhina, E.G.; Chistyakova, T.I.; Mironov, A.A.; Kamzolova, S.V.; Morgunov, I.G.; Vainshtein, M.B. Arachidonic acid synthesis from biodiesel-derived waste by Mortierella alpina. Eur. J. Lipid Sci. Technol. 2014, 116, 429–437. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, X.; Wang, W.; Du, W.; Liu, D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36. [Google Scholar] [CrossRef]
- Chang, G.; Luo, Z.; Gu, S.; Wu, Q.; Chang, M.; Wang, X. Fatty acid shifts and metabolic activity changes of Schizochyrium sp. S31 cultured on glycerol. Bioresour. Technol. 2013, 142, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Blackburn, J.W.; Liang, Y. Fermentation optimization for the production of lipid by Cryptococcus curvatus: Use of response surface methodology. Biomass Bioenergy 2012, 47, 410–417. [Google Scholar] [CrossRef]
- Yang, X.; Jin, G.; Gong, Z.; Shen Bai, F.; Zhao, Z.K. Recycling biodiesel-derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two-stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91. [Google Scholar] [CrossRef]
- Bommareddy, R.R.; Sabra, W.; Maheshwari, G.; Zeng, A.P. Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb. Cell Fact. 2015, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Tchakouteu, S.S.; Kalantzi, O.; Gardeli, C.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production by yeasts growing on biodiesel-derived crude glycerol: Strain selection and impact of substrate concentration on the fermentation efficiency. J. Appl. Microbiol. 2015, 118, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Rymowicz, W.; Rywińska, A.; Źarowska, B.; Juszczyk, P. Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem. Pap. 2006, 60, 391–394. [Google Scholar] [CrossRef]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywinska, A.; Morgunov, I.G. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Beopoulos, A.; Koletti, A.; Thevenieau, F.; Koutinas, A.A.; Nikaud, J.M.; Aggelis, G. Importance of the methyl-citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica. J. Biotechnol. 2013, 168, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Rymowicz, W. High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Rymowicz, W.; Zarowska, B.; Skrzypiński, A. Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Rymowicz, W.; Marcinkiewicz, M. Valorization of raw glycerol for citric acid production by Yarrowia lipolytica yeast. Electron. J. Biotechnol. 2010, 13, 9–10. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Fatykhova, A.R.; Dedyukhina, E.G.; Anastassiadis, S.G.; Golovchenko, N.P.; Morgunov, I.G. Citric acid production by yeast grown on glycerol-containing waste from biodiesel industry. Food Technol. Biotechnol. 2011, 49, 65–74. [Google Scholar]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef]
- Rywińska, A.; Tomaszewska, L.; Rymowicz, W. Erythritol biosynthesis by Yarrowia lipolytica yeast under various culture conditions. Afr. J. Microbiol. Res. 2013, 7, 3511–3516. [Google Scholar]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Abghari, A.; Chen, S. Yarrowia lipolytica as an oleaginous cell factory platform for production of fatty acid-based biofuel and bioproducts. Front. Energy Res. 2014, 2, 21. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Marc, I.; Aggelis, G. Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl. Microbiol. Biotechnol. 2002, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Rymowicz, W.; Rywinska, A.; Gladowski, W. Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chem. Pap. 2008, 62, 239–246. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywinska, A.; Marcinkiewicz, M. High yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009, 31, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Rymowicz, W. Chemostat study of citric acid production from glycerol by Yarrowia lipolytica. J. Biotechnol. 2011, 152, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Petrou, I.; Gardeli, C.; Komaitis, M.; Papanikolaou, S. Effect of Origanum vulgare L. essential oil on growth and lipid profile of Yarrowia lipolytica cultivated on glycerol-based media. J. Am. Oil Chem. Soc. 2011, 88, 1955–1964. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.; Xiong, L.; Chen, X.; Ma, L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Hasan, Y.N.; Al-Farraj, A.S. Olive mill wastewater treatment using a simple zeolite-based low-cost method. J. Environ. Manag. 2014, 145, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Aggelis, G.; Marc, I. Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica apable of producing a cocoa-butter substitute from industrial fats. Antonie Leeuwenhoek 2001, 80, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Muniglia, L.; Chevalot IAggelis, G.; Marc, I. Accumulation of cocoa-butter-like lipid by Yarrowia lipolytica cultivated on agro-industrial residues. Curr. Microbiol. 2003, 46, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Zhang, H.; Xie, Y.; Tang, N.; Berenjian, A.; Song, Y. Conversion of mutton fat to cocoa butter equivalent by increasing the unsaturated fatty acids at the sn-2 position of triacylglycerol through fermentation by Yarrowia lipolytica. Am. J. Biochem. Biotechnol. 2015, 11, 57–65. [Google Scholar] [CrossRef]
- Zhao, L.; Li, B.; Xiong, D.; Zhang, H.; Song, Y.; Yang, S. Cocoa-butter equivalent production from Yarrowia lipolytica by optimization of fermentation technology. Am. J. Biochem. Biotechnol. 2016, 12, 196–205. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Yarrowia lipolytica: A model microorganism used for the production of tailor-made lipids. Eur. J. Lipid Sci. Technol. 2010, 112, 639–654. [Google Scholar] [CrossRef]
- Poli, J.S.; da Silva, M.A.N.; Siqueira, E.P.; Pasa, V.M.D.; Rosa, C.A.; Valente, P. Microbial lipid produced by Yarrowia lipolytica QU21 using industrial waste: A potential feedstock for biodiesel production. Bioresour. Technol. 2014, 161, 320–326. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chatzifragkou, A.; Fakas, S.; Galiotou–Panayotou, M.; Komaitis, M.; Nicaud, J.M.; Aggelis, G. Biosynthesis of lipids and organic acids by Yarrowia lipolytica strains cultivated on glucose. Eur. J. Lipid Sci. Technol. 2009, 111, 1221–1232. [Google Scholar] [CrossRef]
- Juszczyk, P.; Rymowicz, W. Characterization of microbial biomass production from glycerin waste by various yeast strains. In Microbial Conversions of Raw Glycerol; Aggelis, G., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 125–135. [Google Scholar]
- Celińska, E.; Grajek, W. A novel multigene expression construct for modification of glycerol metabolism in Yarrowia lipolytica. Microb. Cell Fact. 2013, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Rakicka, M.; Kieron, A.; Hapeta, P.; Neuvéglise, C.; Lazar, Z. Sweet and sour potential of yeast from the Yarrowia clade. Biomass Bioenergy 2016, 92, 48–54. [Google Scholar] [CrossRef]
- Rakicka, M.; Rywinska, A.; Cybulski, K.; Rymowicz, W. Enhanced production of erythritol and mannitol by Yarrowia lipolytica in media containing surfactants. Braz. J. Microbiol. 2016, 47, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of olive mill wastewater into high-added value products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1, 3-propanediol, citric acid and single cell oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater-based media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef] [PubMed]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Yeasts, moulds, algae and bacteria as sources of lipids. In Technological Advances in Improved and Alternative Sources of Lipids; Kamel, B.S., Kakuda, Y., Eds.; Blackie Academic and Professional: London, UK, 1994; pp. 235–291. [Google Scholar]
- Paramithiotis, S.; Muller, M.R.A.; Ehrmann, M.A.; Tsakalidou, E.; Seiler, H.; Vogel, R.; Kalantzopoulos, G. Polyphasic identification of wild yeast strains isolated from Greek sourdoughs. Syst. Appl. Microbiol. 2000, 23, 156–164. [Google Scholar] [CrossRef]
- Liang, Y.N.; Sarkany, N.; Cui, Y.; Blackburn, J.W. Batch stage study of lipid production from crude glycerol derived from yellow grease or animal fats through microagal fermentation. Bioresour. Technol. 2010, 101, 6745–6750. [Google Scholar] [CrossRef]
- Miller, G. Determination of reducing sugar by DNS method. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.K.; Koutinas, A.A. Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valor. 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Zikou, E.; Chatzifragkou, A.; Koutinas, A.A.; Papanikolaou, S. Evaluating glucose and xylose as cosubstrates for lipid accumulation and γ-linolenic acid biosynthesis of Thamnidium elegans. J. Appl. Microbiol. 2013, 114, 1020–1032. [Google Scholar] [CrossRef]
- Sayadi, S.; Ellouz, R. Decolourization of olive mill waste-waters by the white-rot fungus Phanerochaete chrysosporium: Involvement of the lignin-degrading system. Appl. Microbiol. Biotechnol. 1992, 37, 813–817. [Google Scholar] [CrossRef]



| Culture medium | Time (h) | X (g/L) | L (g/L) | Glolcons (g/L) | Cit (g/L) | Man (g/L) | Ery (g/L) | YX/Glol (g/g) | YL/X % (w/w) | YCit/Glol (g/g) | YMan/Glol (g/g) | YEry/Glol (g/g) | IPS (g/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| blank | 164 | 10.2 | 2.10 | 67.7 | 8.7 | 11.6 | 8.8 | 0.15 | 20.5 | 0.13 | 0.17 | 0.13 | 0.16 | |
| 188 | 10.0 | 2.39 | 68.7 | 10.5 | 13.4 | 5.6 | 0.15 | 23.9 | 0.15 | 0.20 | 0.08 | 0.33 | ||
| 260 | 10.8 | 2.33 | 75.5 | 6.2 | 7.5 | 3.7 | 0.14 | 21.6 | 0.08 | 0.10 | 0.05 | 0.88 | ||
| 2.0 g/L phenols and x% NaCl (w/v) | 0.0% | 140 | 6.9 | 1.69 | 50.7 | 28.6 | 8.0 | 4.9 | 0.14 | 24.5 | 0.56 | 0.16 | 0.10 | 1.41 |
| 188 | 8.7 | 2.47 | 62.8 | 32.7 | 4.5 | 3.7 | 0.14 | 28.3 | 0.52 | 0.07 | 0.06 | 1.52 | ||
| 1.0% | 144 | 7.6 | 1.83 | 52.5 | 34.2 | 5.8 | 2.5 | 0.14 | 24.2 | 0.65 | 0.11 | 0.05 | 1.64 | |
| 224 | 8.1 | 2.07 | 66.3 | 45.2 | 6.0 | 0.0 | 0.12 | 25.7 | 0.68 | 0.09 | 0.00 | 2.59 | ||
| 242 | 8.4 | 2.52 | 69.0 | 21.6 | 5.2 | 0.0 | 0.12 | 29.9 | 0.31 | 0.08 | 0.00 | 2.40 | ||
| 3.0% | 140 | 5.6 | 0.91 | 35.8 | 20.8 | 2.9 | 4.9 | 0.16 | 16.2 | 0.58 | 0.08 | 0.14 | 0.46 | |
| 280 | 4.6 | 1.48 | 53.2 | 42.0 | 3.6 | 0.0 | 0.08 | 31.5 | 0.79 | 0.07 | 0.00 | 0.72 | ||
| 5.0% | 210 | 6.5 | 1.63 | 58.3 | 45.7 | 3.1 | 1.9 | 0.12 | 25.2 | 0.88 | 0.06 | 0.04 | 1.72 | |
| 233 | 6.8 | 2.16 | 66.1 | 54.0 | 1.0 | 0.0 | 0.10 | 31.7 | 0.82 | 0.01 | 0.00 | 0.87 | ||
| 308 | 6.4 | 2.25 | 67.8 | 51.2 | 2.4 | 3.2 | 0.09 | 35.1 | 0.75 | 0.04 | 0.01 | 0.69 | ||
| Phenolic compounds (g/L) | 0.0 g/L | 2.0 g/L | |||
|---|---|---|---|---|---|
| NaCl% (w/v) | 0.0% | 0.0% | 1.0% | 3.0% | 5.0% |
| Glycerol consumption (% w/w) | 99.6% | 94% | 89.1% | 76.4% | 90.4% |
| Glycerol consumption rate, g/(L·h) | 0.40 | 0.32 | 0.32 | 0.23 | 0.27 |
| Citric acid volumetric productivity, g/(L·h) | 0.05 | 0.18 | 0.21 | 0.16 | 0.24 |
| Citric acid yield on glycerol consumed, (g/g) | 0.12 | 0.58 | 0.68 | 0.73 | 0.81 |
| pHØ | ΝaCl (% w/v) | Time (h) | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 |
|---|---|---|---|---|---|---|---|
| 0.0 | 0.0 | 116 | 10.4 | 10.4 | 6.5 | 66.4 | 6.3 |
| 212 | 9.2 | 11.6 | 6.3 | 66.5 | 6.4 | ||
| 2.0 | 0.0 | 50 | 13.0 | 3.5 | 6.6 | 65.3 | 11.6 |
| 212 | 11.7 | 10.6 | 6.5 | 62.9 | 8.4 | ||
| 2.0 | 1.0 | 46 | 12.6 | 6.9 | 9.3 | 59.8 | 11.4 |
| 116 | 12.3 | 10.9 | 9.1 | 61.6 | 6.1 | ||
| 2.0 | 3.0 | 95 | 13.1 | 12.1 | 8.4 | 57.0 | 9.4 |
| 348 | 9.3 | 15.7 | 4.9 | 62.9 | 7.1 | ||
| 2.0 | 5.0 | 48 | 16.0 | 2.9 | 5.2 | 54.7 | 21.1 |
| 250 | 10.6 | 11.6 | 4.3 | 59.5 | 14.1 |
| Strain | Citric acid (g/L) | Substrate | Yield (g/g) | Fermentation Type | Reference |
|---|---|---|---|---|---|
| ACA-DC 50109 | 33.6 | Crude glycerol | 0.44 | Shake flasks | [38] |
| Wratislavia 1.31 | 124.5 | *» | 0.62 | Batch bioreactor | [26] |
| Wratislavia AWG7 | 88.1 | » | 0.46 | » | » |
| Wratislavia K1 | 75.7 | » | 0.40 | » | » |
| ACA-DC 50109 | 28.9 | Glucose/OMWs | 0.53 | Shake flasks | [11] |
| » | 62.5 | Crude glycerol | 0.56 | » | [61] |
| A-101-1.22 | 112.0 | » | 0.60 | Batch bioreactor | [27] |
| ACA-YC 5033 | 50.1 | » | 0.44 | Shake flasks | [20] |
| A-101 | 66.5 | Pure glycerol | 0.44 | Batch bioreactor | [30] |
| » | 66.8 | Crude glycerol | 0.43 | » | » |
| Wratislavia K1 | 53.3 | Pure glycerol | 0.34 | » | » |
| » | 36.8 | Crude glycerol | 0.25 | » | » |
| Wratislavia 1.31 | 126.0 | » | 0.63 | Fed-batch bioreactor | [31] |
| Wratislavia AWG7 | 157.5 | » | 0.58 | » | » |
| Wratislavia 1.31 | 155.2 | » | 0.55 | » | » |
| Wratislavia AWG7 | 154.0 | » | 0.78 | Repeated batch | [29] |
| N15 | 19.08 | Pure glycerol | 0.55 | Shake flasks | [32] |
| » | 98.0 | » | 0.70 | Fed-batch bioreactor | » |
| Wratislavia AWG7 | 86.5 | » | 0.59 | Continuous bioreactora | [41] |
| » | 63.3 | » | 0.67 | Continuous bioreactorb | » |
| W29 | 15.8 | Glucose/OMWs | 0.46 | Shake flasks | [62] |
| ACA-YC 5033 | 18.1 | » | 0.51 | » | » |
| NG40/UV7 | 115.0 | Pure glycerol | 0.64 | Fed-batch bioreactor | [44] |
| » | 112.0 | Crude glycerol | 0.90 | » | » |
| JMY1203 | 57.7 | » | 0.92 | Shake flasks | [28] |
| ACA-YC 5029 | 39.0 | » | 0.42 | Batch-bioreactor | [63] |
| ACA-YC 5033 | 15.2 | Glucose/OMWs | 0.61 | Batch-bioreactor | [3] |
| » | 13.9 | » | 0.58 | Batch-bioreactorc | » |
| » | 51.9 | » | 0.64 | Shake flasks | » |
| ACA-DC 5029 | 37.4 | Glycerol/OMWs | 0.55 | » | [4] |
| » | 79.0 | Crude glycerol | 0.46 | » | » |
| ACA-YC 5031 | 54.0d | Glycerol/OMWs | 0.82 | Shake flasks | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzirita, M.; Kremmyda, M.; Sarris, D.; Koutinas, A.A.; Papanikolaou, S. Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters. Energies 2019, 12, 3649. https://doi.org/10.3390/en12193649
Tzirita M, Kremmyda M, Sarris D, Koutinas AA, Papanikolaou S. Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters. Energies. 2019; 12(19):3649. https://doi.org/10.3390/en12193649
Chicago/Turabian StyleTzirita, Markella, Maria Kremmyda, Dimitris Sarris, Apostolis A. Koutinas, and Seraphim Papanikolaou. 2019. "Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters" Energies 12, no. 19: 3649. https://doi.org/10.3390/en12193649