The Future Agricultural Biogas Plant in Germany: A Vision
Abstract
:1. The Controversial Development of Agricultural Biogas Production in Germany in the Last Two Decades
2. The Vision of the Future Agricultural Biogas Plant
- Future agricultural biogas production is an integral part of the circular bioeconomy. Residues are received from other production systems and returned to the biomass cycle. Other production systems are supplied with energy.
- Hence, the future agricultural biogas plant is primarily based on residues.
- The future agricultural biogas plant is flexible with regard to feedstocks, digester operation, microbial communities and biogas output. The digestion process is stable, susceptibility to disturbances is low.
- The future agricultural biogas plant is modular in design. Plant components are constructed separately and coupled on demand.
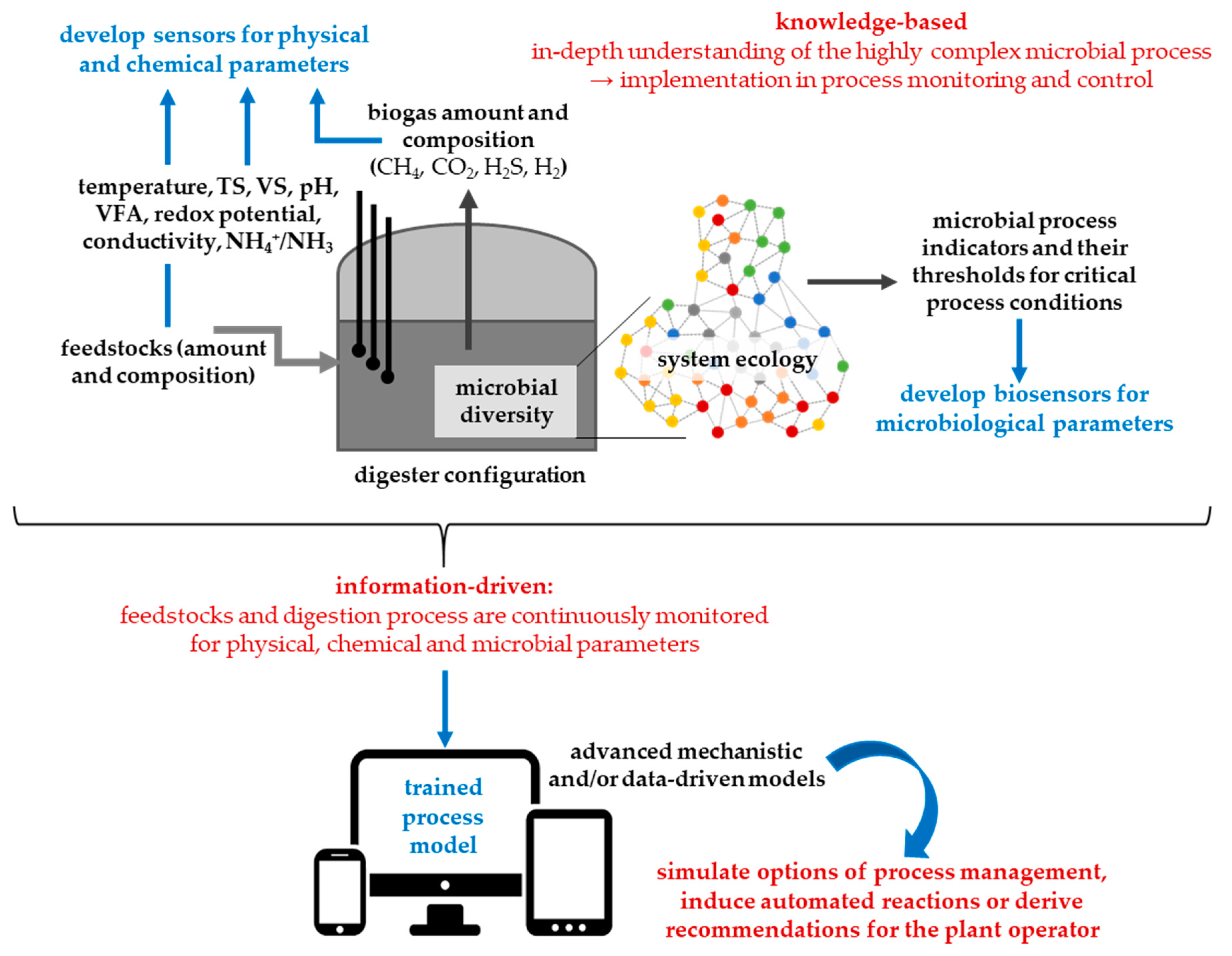
- The future biogas plant operates knowledge-based, information-driven and largely automated.
- Future agricultural biogas production becomes more efficient, achieves decreasing costs of production and creates supportive business environments.
3. How to Get There—the Required Contribution of Research
3.1. Systemic Multidisciplinary Research Approach
- to better understand the digestion process,
- to develop new tools for monitoring the digestion process,
- to advance with modelling the digestion process,
- to further explore the characteristics of residues and their effects on the digestion process,
- to identify and investigate new types of feedstocks,
- to further develop digester technologies and operation strategies,
- to identify and develop diverse links of biogas production with the supply of food, feed and biomaterials,
- to explore and reduce potential risks of cycling biomass via biogas plants,
- to assess environmental and economic performance of biogas production systems.
3.2. Knowledge-Based, Information-Driven and Largely Automated Biogas Production
3.2.1. Requirements
3.2.2. Understanding the Digestion Process
- Single species: Efforts to isolate, cultivate and characterize known and yet unknown species need to be expanded. To explore the response of the microorganisms to varying environmental conditions, special emphasis has to be given on determining growth kinetics by up and down regulation of their metabolism through altering physico-chemical parameters such as temperature and nutrient supply. To capture the vast majority of not yet cultivable microorganisms [42], it is indispensable to develop new and more complex cultivation media that correspond with the natural living conditions of the microorganisms.
- Microbiome: New sequencing technologies such as Nanopore-sequencing [43] will allow for deeper exploration of the entire biogas microbiome. Nanopore-sequencing is supposed to elucidate the microbial diversity down to the species level by full-length sequencing of the 16S rRNA gene or the whole rrn-operon in a high temporal resolution [44,45,46].
- System ecology: To obtain an integrated view of all the existing biotic and abiotic relationships and interactions it is necessary to describe and understand the γ-diversity level of the biogas microbiome by using, for instance, co-occurrence network [38,47,48] or artificial neural network analyses [37]. This provides the opportunity to reveal if and how members of the microbiome are affected in the case of disturbances and can possibly be used for process monitoring (Section 3.2.3). Microbial communities can adapt to potentially unfavorable process conditions [49,50,51]. The question is how fast microbiomes acclimatize to changing environmental conditions or what kind of diversity level (high, medium or low regarding structure, function and ecology) is needed to withstand unfavorable process conditions. A certain level of functional redundancy (inhibited community members can be replaced by others with similar function) at any process stage seems to play a crucial role in ensuring a stable process [52].
- Assessment: develop new methods that assess the adaptability and resilience of microbial populations to specific environmental conditions delivering opportunities to derive microbial performance indicators.
3.2.3. Monitoring the Digestion Process
3.2.4. Modelling the Digestion Process
- to online measure process parameters, nutrient content, biogas production;
- to investigate growth rates and growth kinetics, gene expression and metabolic profiling, substrate utilization kinetics as well as the formed products of cultivable microorganisms;
- to elucidate the entire microbiome at the species level using the most modern sequencing approaches in order to quantitatively describe and understand the biogas microbiome in its complexity and to predict its response to external and internal influences by using co-occurrence network analyses which provide an integrated view of all ecologic relationships between the occurring microorganisms in a given environmental matrix [37,38,47,48].
3.3. Diverse Feedstock Spectrum, Mainly Based on Residues
3.3.1. Requirements
3.3.2. Unlocking Residues
- often have feedstock characteristics that make them difficult to handle in anaerobic digestion, (e.g., high contents of lignocellulose and substances that inhibit the digestion process);
- are of heterogeneous and fluctuating composition;
- may harbor risks such as possible contamination with pathogens, antibiotics, heavy metals, organic compounds;
- accrue decentrally in small amounts;
- are often difficult to collect and to store.
3.3.3. Aquatic Biomass
3.3.4. Crops
3.3.5. Gases
3.4. Flexible and Modular Biogas Plants
3.4.1. Requirements
3.4.2. Engineering for an Increased Use of Residues and Novel Feedstocks
3.4.3. Biogas Supply on Demand
- Enlargement of on-site biogas storage capacities allows the storage of surplus biogas during periods of negative balancing power demand and additional biogas utilization in combined heat and power (CHP) units during periods of positive balancing power demand. No changes in digester operation are required. Although this option is comparatively easy to realize in existing biogas plants, additional gas storage installations are expensive and capacities might be limited by legal regulations [175,176]. Expansion of on-site storage capacity is most suitable for small biogas plants and balancing of short periods without biogas utilization [176].
- Increased feeding-in of biomethane into the natural gas grid instead of on-site combustion with combined heat and power units. The natural gas grid already features large storage capacities [177]. As the feeding-in of biomethane into the natural gas net allows for a conversion into electricity at larger scale, combined cycle gas turbines with conversion efficiencies of above 60%, electric efficiencies may increase compared to the local, small scale power generation units that are currently common [149].
- Variable feeding (feedstock amount and composition) to the digester or an adapted temperature regime can regulate the amount of methane produced within the biogas plant [49,51,178,179,180,181]. This option reduces necessary investments for flexibilization [176], but requires a resilient microbiome (Section 3.2.2.) as well as reliable process monitoring and control (Section 3.2.3). Rapid changes in feedstocks and/or temperature bear the risk of process disturbances [30], limits of flexible feeding need to be well known and considered. Model-based predictive process control [180] (Section 3.2.4) and specific digester configurations [182] can increase process stability and help to enhance flexibility, while maintaining stable conditions and thus need to be further developed.
- Another option for flexible gas formation is the separation of the hydrolysis/acidogenesis from the acetogenesis/methanogenesis stage in the two-stage processes, which enables the production of an effluent enriched with organic acids that can be stored and rapidly converted into methane on demand. Different configurations of two-stage reactor systems have been suggested for demand-driven biogas production, combining a continuous stirred tank reactor or leach-bed reactor as the first stage with an high-performance reactor such as an upflow anaerobic sludge blanket or fixed-bed reactor as the second stage [165,176,183]. Disadvantage of two-stage systems is their higher complexity which also leads to higher investment costs. Technological and financial effort is required to apply this option for demand-driven biogas production to existing biogas plants.
- Power-to-gas technologies can be used to store surplus renewable electricity by converting it into hydrogen via electrolysis of water. On demand, the hydrogen can be combined with CO2 and fed to the anaerobic digestion process to biologically convert these gases into methane [149]. This conversion may take place within an existing biogas reactor (in-situ) or in an external reactor (ex-situ) with the latter being more flexible regarding the adjustment of optimal process conditions and adapted reactor configurations [150]. Power-to-gas technologies are still in a developmental stage, a major challenge lies in the up-scaling of the developed reactor systems and technologies to commercial scale.
3.5. Integrated Biogas Production in a Circular Bioeconomy
3.5.1. Requirements
3.5.2. Coupling Anaerobic Digestion with Other Production Systems of the Bioeconomy
3.5.3. Learning from Environmental Impact Assessments of Biogas Production
- Future biogas needs to be based increasingly on residues, and to a lesser degree on energy crops exclusively grown for biogas production, as these are often responsible for a main fraction of greenhouse gas emissions [71,226,227]. Plants mostly based on animal manures have higher environmental benefits than those including energy crops [228].
- Transport distances of feedstocks need to be low, especially for liquid or bulky feedstocks with relatively low energy content [224].
- Digestate management is important. To avoid greenhouse gas and ammonia emissions, eutrophication and acidification, digestate stores have to be covered [71,226,232], transport distances to the field need to be low [224] and digestate should be injected into the soil or incorporated immediately after at field application [233]. Appropriate digestate treatment can further improve the environmental performance [233].
- Heat utilization is of great importance. Increased heat usage from cogeneration can reduce environmental impacts [188,228,234], while low heat usage in combination with longer feedstock transports, can even result in biogas plants with negative impacts [235]. The importance of heat usage also explains why biomethane feeding into the gas grid with subsequent high heat usage can be advantageous over local combustion with low heat usage [236].
- Biorefinery concepts (Section 3.5.2) can increase the environmental efficiency of the processes involved [3,237], but feedstock selection is equally important since even similar feedstocks such as alfalfa or clovergrass can have substantially different impacts [195].
- Compared to other options that generate electricity from residues biogas is usually among the options with the lowest environmental impacts. However, depending on specific conditions, and especially for relatively dry substrates with lower nitrogen contents, combustion to generate heat and electric energy, or material use can also be a viable option [238,239].
3.5.4. Business Environments and Business Models
3.5.5. Modelling Biogas Systems
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kemausour, F.; Adaramola, M.S.; Morken, J. A review of commercial biogas systems and lessons for Africa. Energies 2018, 11, 2984. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Sravan, J.S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hagman, L.; Blumenthal, A.; Eklund, M.; Svensson, N. The role of biogas solutions in sustainable biorefineries. J. Clean. Prod. 2018, 172, 3982–3989. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Siegmeier, T.; Blumenstein, B.; Möller, D. Farm biogas production in organic agriculture: System implications. Agric. Syst 2015, 139, 196–209. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Development process and probable future transformations of rural biogas in China. Renew. Sustain. Energy Rev. 2016, 55, 703–712. [Google Scholar] [CrossRef]
- Adams, P.W.R.; Mezzullo, W.G.; McManus, M.C. Biomass sustainability criteria: Greenhouse gas accounting issues for biogas and biomethane facilities. Energy Policy 2015, 87, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Agostini, A.; Battini, F.; Padella, M.; Giuntoli, J.; Baxter, D.; Marelli, L.; Amaducci, S. Economics of GHG emissions mitigation via biogas production from sorghum, maize and dairy farm manure digestion in the Po valley. Biomass Bioenergy 2016, 89, 58–66. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Sweeney, S.; Feng, Y. Household biogas use in rural China: A study of opportunities and constraints. Renew. Sustain. Energy Rev. 2010, 14, 545–549. [Google Scholar] [CrossRef]
- Daniel-Gromke, J.; Rensberg, N.; Denysenko, V.; Stinner, W.; Schmalfuß, T.; Scheftelowitz, M.; Nelles, M.; Liebetrau, J. Current developments in production and utilization of biogas and biomethane in Germany. Chem. Ing. Tech. 2018, 90, 17–35. [Google Scholar] [CrossRef]
- Purkus, A.; Gawel, E.; Szarka, N.; Lauer, M.; Lenz, V.; Ortwein, A.; Tafarte, P.; Eichhorn, M.; Thrän, D. Contributions of flexible power generation from biomass to a secure and cost-effective electricity supply—A review of potentials, incentives and obstacles in Germany. Energy Sustain. Soc. 2018, 8, 18. [Google Scholar] [CrossRef]
- Britz, W.; Delzeit, R. The impact of German biogas production on European and global agricultural markets, land use and the environment. Energy Policy 2013, 62, 1268–1275. [Google Scholar] [CrossRef]
- German Biogas Association. Biogas market data in Germany 2017/2018. Available online: https://www.biogas.org/edcom/webfvb.nsf/id/DE_Branchenzahlen/$file/18-07-05_Biogasindustryfigures-2017-2018_english.pdf (accessed on 16 November 2018).
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Statistical Yearbook 2001. Available online: http://www.digizeitschriften.de/dms/toc/?PID=PPN635628112_2003 (accessed on 17 November 2018).
- Statistisches Bundesamt. Statistical Yearbook 2013. Available online: https://www.destatis.de/DE/Publikationen/StatistischesJahrbuch/StatistischesJahrbuch2014.pdf?__blob=publicationFile (accessed on 17 November 2018).
- Bundesverband der Energie- und Wasserwirtschaft. Strompreisanalyse 2018. Available online: https://www.bdew.de/media/documents/1805018_BDEW-Strompreisanalyse-Mai-2018.pdf (accessed on 16 November 2018).
- Herbes, C.; Jirka, E.; Braun, J.P.; Pukall, K. The Social Discourse on the “Maize Cap” before and after the 2012 Amendment of the German Renewable Energies Act (EEG). GAIA 2014, 23, 100–108. [Google Scholar] [CrossRef]
- Gevers, J.; Høye, T.T.; Topping, C.J.; Glemnitz, M.; Schröder, B. Biodiversity and the mitigation of climate change through bioenergy: Impacts of increased maize cultivation on farmland wildlife. GCB Bioenergy 2011, 3, 472–482. [Google Scholar] [CrossRef]
- Lüker-Jans, N.; Simmering, D.; Otte, A. The impact of biogas plants on regional dynamics of permanent grassland and maize area—The example of Hesse, Germany (2005–2010). Agric. Ecosyst. Environ. 2017, 241, 24–38. [Google Scholar] [CrossRef]
- Stein, S.; Krug, A. The boom in biomass production—A challenge for grassland biodiversity? Grassl. Sci. Eur. 2008, 13, 730–732. [Google Scholar]
- Appel, F.; Ostermeyer-Wiethaup, A.; Balmann, A. Effects of the German Renewable Energy Act on structural change in agriculture—The case of biogas. Util. Policy 2016, 41, 172–182. [Google Scholar] [CrossRef]
- Bundesministerium für Wirtschaft und Energie: Bruttostromerzeugung in Deutschland 2017. Available online: https://www.bmwi.de/Redaktion/DE/Infografiken/Energie/Energiedaten/Energietraeger/energiedaten-energietraeger-28.html (accessed on 30 December 2018).
- Kalt, G.; Kranzl, L. Assessing the economic efficiency of bioenergy technologies in climate mitigation and fossil fuel replacement in Austria using a techno-economic approach. Appl. Energy 2011, 88, 3665–3684. [Google Scholar] [CrossRef]
- Balussou, D.; McKenna, R.; Möst, D.; Fichtner, W. A model-based analysis of the future capacity expansion for German biogas plants under different legal frameworks. Renew. Sustain. Energy Rev. 2018, 96, 119–131. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Statistical Yearbook 2018. Available online: https://www.destatis.de/DE/Publikationen/StatistischesJahrbuch/LandForstwirtschaft.pdf;jsessionid=DDFC81B0FCBE8840938B64EB83899F32.InternetLive1?__blob=publicationFile (accessed on 17 November 2018). (In German).
- Arthurson, V. Closing the global energy and nutrient cycles through application of biogas residue to agricultural land—Potential benefits and drawbacks. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef]
- Lauer, M.; Thrän, D. Flexible biogas in future energy systems—Sleeping beauty for a cheaper power generation. Energies 2018, 11, 761. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process disturbances in agricultural biogas production—Causes, mechanisms and effects on the biogas microbiome: A review. Energies 2019, 12, 365. [Google Scholar] [CrossRef]
- Carballa, M.; Regueiro, L.; Lema, J.M. Microbial management of anaerobic digestion: Exploiting the microbiome-functionality nexus. Curr. Opin. Biotechnol. 2015, 33, 103–111. [Google Scholar] [CrossRef]
- Calusinska, M.; Goux, X.; Fossépré, M.; Muller, E.E.L.; Wilmes, P.; Delfosse, P. A year of monitoring 20 mesophilic full-scale bioreactors reveals the existence of stable but different core microbiomes in bio-waste and wastewater anaerobic digestion systems. Biotechnol. Biofuels 2018, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Hassa, J.; Maus, I.; Off, S.; Pühler, A.; Scherer, P.; Klocke, M.; Schlüter, A. Metagenome, metatranscriptome, and metaproteome approaches unraveled compositions and functional relationships of microbial communities residing in biogas plants. Appl. Microbiol. Biotechnol. 2018, 102, 5045–5063. [Google Scholar] [CrossRef] [Green Version]
- Kundu, K.; Sharma, S.; Sreekrishnan, T.R. Influence of process parameters on anaerobic digestion microbiome in bioenergy production: Towards an improved understanding. Bioenergy Res. 2017, 10, 288–303. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Heiermann, M.; De Vrieze, J. Marker microbiome clusters are determined by operational parameters and specific key taxa combinations in anaerobic digestion. Bioresour. Technol. 2018, 263, 128–135. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.G.; Campanaro, S.; Bassani, I.; Angelidaki, I. Deeper insight into the structure of the anaerobic digestion microbial community; the biogas microbiome database is expanded with 157 new genomes. Bioresour. Technol. 2016, 216, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Loh, K.-C.; Lim, J.W.; Zhang, J. Bioinformatics analysis of metagenomics data of biogas-producing microbial communities in anaerobic digesters: A review. Renew. Sustain. Energy Rev. 2019, 100, 110–126. [Google Scholar] [CrossRef]
- Bouchez, T.; Blieux, A.L.; Dequiedt, S.; Domaizon, I.; Dufresne, A.; Ferreira, S.; Godon, J.J.; Joulian, H.; Quaiser, A.; Martin-Laurent, F.; et al. Molecular microbiology methods for environmental diagnosis. Environ. Chem. Lett. 2016, 14, 423–441. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Amato, C.; Pozo, C.; González-Martínez, A.; González-López, J. New concepts in anaerobic digestion processes: Recent advances and biological aspects. Appl. Microbiol. Biotechnol. 2018, 102, 5065–5076. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Christiaens, M.E.R.; Verstraete, W. The microbiome as engineering tool: Manufacturing and trading between microorganisms. New Biotechnol. 2017, 39, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, A. Biogas Production: Microbiology and Technology. In Anaerobes in Biotechnology. Advances in Biochemical Engineering/Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer: Cham, Switzerland, 2016; Volume 156, pp. 195–234. ISBN 978-3-319-45651-5. [Google Scholar]
- Lloyd, K.G.; Steen, A.D.; Ladau, J.; Yin, J.; Crosby, L. Phylogenetically novel uncultured microbial cells dominate earth microbiomes. mSystems 2018, 3, e00055-18. [Google Scholar] [CrossRef] [PubMed]
- Shendure, J.; Balasubramanian, S.; Church, G.M.; Gilbert, W.; Rogers, J.; Schloss, J.A.; Waterston, R.H. DNA sequencing at 40: Past, present and future. Nature 2017, 550, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Calus, S.T.; Ijaz, U.Z.; Pinto, A.J. NanoAmpli-Seq: A workflow for amplicon sequencing for mixed microbial communities on the nanopore sequencing platform. GigaScience 2018, 7. [Google Scholar] [CrossRef]
- Cuscó, A.; Catozzi, C.; Viñes, J.; Sánchez, A.; Francino, O. Microbiota profiling with long amplicons using Nanopore sequencing: Full-length 16S rRNA gene and whole rrn operon. bioRxiv 2018. [Google Scholar] [CrossRef]
- Kerkhof, L.J.; Dillon, K.P.; Häggblom, M.M.; McGuinness, L.R. Profiling bacterial communities by MinION sequencing of ribosomal operons. Microbiome 2017, 5, 116. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef]
- Karimi, B.; Maron, P.A.; Chemidlin-Prevost Boure, N.; Bernard, N.; Gilbert, D.; Ranjard, L. Microbial diversity and ecological networks as indicators of environmental quality. Environ. Chem. Lett. 2017, 15, 265. [Google Scholar] [CrossRef]
- Bonk, F.; Popp, D.; Weinrich, S.; Sträuber, H.; Kleinsteuber, S.; Harms, H.; Centler, F. Intermittent fasting for microbes: How discontinuous feeding increases functional stability in anaerobic digestion. Biotechnol. Biofuels 2018, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Isaksson, S.; Karlsson Lindsjö, O.; Schnürer, A. Microbial community adaptability to altered temperature conditions determines the potential for process optimisation in biogas production. Appl. Energy 2018, 226, 838–848. [Google Scholar] [CrossRef]
- Ferguson, R.M.W.; Coulon, F.; Villa, R. Understanding microbial ecology can help improve biogas production in AD. Sci. Total Environ. 2018, 642, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Drosg, B. Process monitoring in biogas plants. In IEA Bioenergy Task 37—Energy from Biogas; Frost, P., Baxter, D., Eds.; IEA Bioenergy: Paris, France, 2013; ISBN 978-1-910154-03-8. [Google Scholar]
- Jimenez, J.; Latrille, E.; Harmand, J.; Robles, A.; Ferrer, J.; Gaida, D.; Wolf, C.; Mairet, F.; Bernard, O.; Alcaraz-Gonzalez, V.; et al. Instrumentation and control of anaerobic digestion processes: A review and some research challenges. Rev. Environ. Sci. Biotechnol. 2015, 14, 615–648. [Google Scholar] [CrossRef]
- Eccleston, R.; Wolf, C.; Balsam, M.; Schulte, F.; Bongards, M.; Rehorek, A. Mid-infrared spectroscopy for monitoring of anaerobic digestion processes- prospects and challenges. Chem. Eng. Technol. 2016, 39, 627–636. [Google Scholar] [CrossRef]
- Ward, A.J. Near-Infrared Spectroscopy for Determination of the Biochemical Methane Potential: State of the Art. Chem. Eng. Technol. 2016, 39, 611–619. [Google Scholar] [CrossRef]
- Kretzschmar, J.; Böhme, P.; Liebetrau, J.; Mertig, M.; Harnisch, F. Microbial electrochemical sensors for anaerobic digestion process control—Performance of electroactive biofilms under real conditions. Chem. Eng. Technol. 2018, 41, 687–695. [Google Scholar] [CrossRef]
- Jin, X.; Angelidaki, I.; Zhang, Y. Microbial electrochemical monitoring of volatile fatty acids during anaerobic digestion. Environ. Sci. Technol. 2016, 50, 4422–4429. [Google Scholar] [CrossRef] [Green Version]
- Röhlen, D.L.; Pilas, J.; Dahmen, M.; Keusgen, M.; Selmer, T.; Schöning, M.J. Toward a hybrid biosensor system for analysis of organic and volatile fatty acids in fermentation processes. Front. Chem. 2018, 6, 284. [Google Scholar] [CrossRef] [PubMed]
- Enitan, A.M.; Adeyemo, J.; Swalaha, F.M.; Kumari, S.; Bux, F. Optimization of biogas generation using anaerobic digestion models and computational intelligence approaches. Rev. Chem. Eng. 2017, 33, 309–335. [Google Scholar] [CrossRef]
- Lauwers, J.; Appels, L.; Thompson, I.P.; Degrève, J.; Van Impe, J.F.; Dewil, R. Mathematical modelling of anaerobic digestion of biomass and waste: Power and limitations. Prog. Energy Combust. Sci. 2013, 39, 383–402. [Google Scholar] [CrossRef] [Green Version]
- Dach, J.; Koszela, K.; Boniecki, P.; Zaborowicz, M.; Lewicki, A.; Czekała, W.; Skwarcz, J.; Qiao, W.; Piekarska-Boniecka, H.; Białobrzewski, I. The use of neural modelling to estimate the methane production from slurry fermentation processes. Renew. Sustain. Energy Rev. 2016, 56, 603–610. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA anaerobic digestion model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Batstone, D.J.; Puyol, D.; Flores-Alsina, X.; Rodríguez, J. Mathematical modelling of anaerobic digestion processes: Applications and future needs. Rev. Environ. Sci. Biotechnol. 2015, 14, 595. [Google Scholar] [CrossRef]
- Luo, G.; De Francisci, D.; Kougias, P.G.; Treu, L.; Zhu, X.; Angelidaki, I. New steady-state microbial community compositions and process performances in biogas reactors induced by temperature disturbances. Biotechnol. Biofuels 2015, 8, 3. [Google Scholar] [CrossRef]
- Biggs, M.B.; Medlock, G.L.; Kolling, G.L.; Papin, J.A. Metabolic network modeling of microbial communities. Wiley Interdiscip. Rev. Syst. Biol. Med. 2015, 7, 317–334. [Google Scholar] [CrossRef] [Green Version]
- Schölkopf, B.; Platt, J.C.; Shawe-Taylor, J.; Smola, A.J.; Williamson, R.C. Estimating the support of a high-dimensional distribution. Neural Comput. 2001, 13, 1443–1471. [Google Scholar] [CrossRef]
- Ruff, L.; Görnitz, N.; Deecke, L.; Siddiqui, S.A.; Vandermeulen, R.; Binder, A.; Müller, E.; Kloft, M. Deep one-class classification. In Proceedings of the Thirty-Fifth Intetnational Conference on Machine Learning, Stockholm, Sweden, 10–15 July 2018. [Google Scholar]
- Ji, S.; Carin, L. Cost-sensitive feature acquisition and classification. Pattern Recognit. 2007, 40, 1474–1485. [Google Scholar] [CrossRef]
- Maliah, S.; Shani, G. MDP-based cost sensitive classification using decision trees. In Proceedings of the Thirty-Second AAAI Conference on Artificial Intelligence, New Orleans, LA, USA, 2–7 February 2018. [Google Scholar]
- Meyer-Aurich, A.; Schattauer, A.; Hellebrand, H.-J.; Klauss, H.; Plöchl, M.; Berg, W. Impacts of uncertainties on greenhouse gas mitigation potential of biogas production from agricultural resources. Renew. Energy 2012, 37, 277–284. [Google Scholar] [CrossRef]
- Einarsson, R.; Persson, U.M. Analyzing key constraints to biogas production from crop residues and manure in the EU—A spatially explicit model. PLoS ONE 2017, 121, e0171001. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Fahl, F.; Dallemand, J.-F.; Monforti, F.; Motola, V. A spatial analysis of biogas potential from manure in Europe. Renew. Sustain. Energy Rev. 2018, 94, 915–930. [Google Scholar] [CrossRef]
- Scheftelowitz, M.; Thrän, D. Unlocking the energy potential of manure—An assessment of the biogas production potential at the farm level in Germany. Agriculture 2016, 6, 20. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Liu, Y.; Dach, J.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
- Bacenetti, J.; Bava, L.; Zucali, M.; Lovarelli, D.; Sandrucci, A.; Tamburini, A.; Fiala, M. Anaerobic digestion and milking frequency as mitigation strategies of the environmental burden in the milk production system. Sci. Total Environ. 2016, 539, 450–459. [Google Scholar] [CrossRef]
- de Vries, J.W.; Groenestein, C.M.; Schröder, J.J.; Hoogmoedd, W.B.; Sukkel, W.; Groot Koerkamp, P.W.G.; de Boer, I.J.M. Integrated manure management to reduce environmental impact: II. Environmental impact assessment of strategies. Agric. Syst. 2015, 138, 88–99. [Google Scholar] [CrossRef]
- Kafle, G.P.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef]
- de Mendonça Costa, M.S.S.; de Lucas, J., Jr.; de Mendonça Costa, L.A.; Orrico, A.C.A. A highly concentrated diet increases biogas production and the agronomic value of young bull’s manure. Waste Manag. 2016, 48, 521–527. [Google Scholar] [CrossRef]
- Miranda, N.D.; Granell, R.; Tuomisto, H.L.; McCulloch, M.D. Meta-analysis of methane yields from anaerobic digestion of dairy cattle manure. Biomass Bioenergy 2016, 86, 65–75. [Google Scholar] [CrossRef]
- Møller, H.B.; Moset, V.; Brask, M.; Weisbjerg, M.R.; Lund, P. Feces composition and manure derived methane yield from dairy cows: Influence of diet with focus on fat supplement and roughage type. Atmos. Environ. 2014, 94, 36–43. [Google Scholar] [CrossRef]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Mayerle, S.F.; de Figueiredo, J.N. Designing optimal supply chains for anaerobic bio-digestion/energy generation complexes with distributed small farm feedstock sourcing. Renew. Energy 2016, 90, 46–54. [Google Scholar] [CrossRef]
- Herrmann, C.; Prochnow, A.; Heiermann, M.; Idler, C. Biomass from landscape management used for biogas production: Effects of harvest date and silage additives on feedstock quality and methane yields. Grass Forage Sci. 2014, 69, 549–566. [Google Scholar] [CrossRef]
- Melts, I.; Normak, A.; Nurk, L.; Heinsoo, K. Chemical characteristics of biomass from nature conservation management for methane production. Bioresour. Technol. 2014, 167, 226–231. [Google Scholar] [CrossRef] [PubMed]
- van Meerbeek, K.; Appels, L.; Dewil, R.; van Beek, J.; Bellings, L.; Liebert, K.; Muys, B.; Hermy, M. Energy potential for combustion and anaerobic digestion of biomass from low-input high-diversity systems in conservation areas. GCB Bioenergy 2015, 7, 888–898. [Google Scholar] [CrossRef]
- Blokhina, Y.; Prochnow, A.; Plöchl, M.; Luckhaus, C.; Heiermann, M. Concepts and profitability of biogas production from landscape management grass. Bioresour. Technol. 2011, 102, 2086–2092. [Google Scholar] [CrossRef]
- Boscaro, D.; Pezzuolo, A.; Grigolato, S.; Cavalli, R.; Marinello, F.; Sartori, L. Preliminary analysis on mowing and harvesting grass along riverbanks for the supply of anaerobic digestion plants in north-eastern Italy. J. Agric. Eng. 2015, 46, 100–104. [Google Scholar] [CrossRef]
- van Meerbeek, K.; Ottoy, S.; de Meyer, A.; van Schaeybroeck, T.; van Orshoven, J.; Muys, B.; Hermy, M. The bioenergy potential of conservation areas and roadsides for biogas in an urbanized region. Appl. Energy 2015, 154, 742–751. [Google Scholar] [CrossRef]
- Auburger, S.; Petig, E.; Bahrs, E. Assessment of grassland as biogas feedstock in terms of production costs and greenhouse gas emissions in exemplary federal states of Germany. Biomass Bioenergy 2017, 101, 44–52. [Google Scholar] [CrossRef]
- Blumenstein, B.; Buhle, L.; Wachendorf, M.; Möller, D. Economic assessment of the integrated generation of solid fuel and biogas from biomass (IFBB) in comparison to different energy recovery, animal-based and non-refining management systems. Bioresour. Technol. 2012, 119, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, D.; Pezzuolo, A.; Sartori, L.; Marinello, F.; Mattioli, A.; Bolzonella, D.; Grigolato, S. Evaluation of the energy and greenhouse gases impacts of grass harvested on riverbanks for feeding anaerobic digestion plants. J. Clean. Prod. 2018, 172, 4099–4109. [Google Scholar] [CrossRef]
- Bühle, L.; Hensgen, F.; Donnison, I.; Heinsoo, K.; Wachendorf, M. Life cycle assessment of the integrated generation of solid fuel and biogas from biomass (IFBB) in comparison to different energy recovery, animal-based and non-refining management systems. Bioresour. Technol. 2012, 111, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.K.P.; Ehimen, E.A.; Holm-Nielsen, J.B. Bioenergy production from roadside grass: A case study of the feasibility of using roadside grass for biogas production in Denmark. Resour. Conserv. Recycl. 2014, 93, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Piepenschneider, M.; Bühle, L.; Hensgen, F.; Wachendorf, M. Energy recovery from grass of urban roadside verges by anaerobic digestion and combustion after pre-processing. Biomass Bioenergy 2016, 85, 278–287. [Google Scholar] [CrossRef]
- Campuzano, R.; González-Martínez, S. Characteristics of the organic fraction of municipal solid waste and methane production: A review. Waste Manag. 2016, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Sen, B.; Aravind, J.; Kanmani, P.; Lay, C.H. State of the art and future concept of food waste fermentation to bioenergy. Renew. Sustain. Energy Rev. 2016, 53, 547–557. [Google Scholar] [CrossRef]
- Smurzyńska, A.; Dach, J.; Kozłowski, K.; Mazurkiewicz, J.; Woźniak, E.; Boniecki, P.; Kupryaniuk, K.; Janczak, D.; Brzoski, M. Relevant biogas substrate—Maize silage vs slaughterhouse waste. In Proceedings of the International Conference on Information and Communication Technologies in Agriculture, Food and Environment, Chania, Crete Island, Greece, 21–24 September 2017. [Google Scholar]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Romero Garcia, L.I.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste - Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Moratilla Soria, B.Y. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1084. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Anonymus: Verordnung über die Verwertung von Bioabfällen auf Landwirtschaftlich, Forstwirtschaftlich und Gärtnerisch Genutzten Böden (Bioabfallverordnung—BioAbfV). Available online: https://www.gesetze-im-internet.de/bioabfv/BioAbfV.pdf (accessed on 30 December 2018).
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Bahadar, A.; Bilal Khan, M. Progress in energy from microalgae: A review. Renew. Sustain. Energy Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Grala, A.; Dudek, M. Algae biomass as an alternative substrate in biogas production technologies—Review. Renew. Sustain. Energy Rev. 2013, 27, 596–604. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Moeller, L.; Bauer, A.; Wedwitschka, H.; Stinner, W.; Zehmsdorf, A. Crop characteristics of aquatic macrophytes for use as a substrate in anaerobic digestion plants—A study from Germany. Energies 2018, 11, 3016. [Google Scholar] [CrossRef]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Xia, A.; Herrmann, C.; Murphy, J.D. How do we optimize third-generation algal biofuels? Biofuels Bioprod. Biorefin. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Allen, E.; Browne, J.; Hynes, S.; Murphy, J.D. The potential of algae blooms to produce renewable gaseous fuel. Waste Manag. 2013, 33, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Herbes, C.; Brummer, V.; Roth, S.; Röhl, M. Using aquatic plant biomass from de-weeding in biogas processes—An economically viable option? Energy Sustain. Soc. 2018, 8, 21. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-D.; Li, S.; Ho, S.-H.; Wang, C.; Lin, Y.-C.; Nagarajan, D.; Chang, J.-S.; Ren, N.-Q. Integration of sludge digestion and microalgae cultivation for enhancing bioenergy and biorefinery. Renew. Sustain. Energy Rev. 2018, 96, 76–90. [Google Scholar] [CrossRef]
- Koutra, E.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Bio-based products from microalgae cultivated in digestates. Trends Biotechnol. 2018, 36, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Kalita, N.; Wall, D.; Xia, A.; Murphy, J.D. Optimised biogas production from microalgae through co-digestion with carbon-rich co-substrates. Bioresour. Technol. 2016, 214, 328–337. [Google Scholar] [CrossRef]
- Herrmann, C.; FitzGerald, J.; O’Shea, R.; Xia, A.; O’Kiely, P.; Murphy, J.D. Ensiling of seaweed for a seaweed biofuel industry. Bioresour. Technol. 2015, 196, 301–313. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current state and perspectives—Mini Review. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Monti, A. Energy crops in rotation: A review. Biomass Bioenergy 2011, 35, 12–25. [Google Scholar] [CrossRef]
- Mayer, F.; Gerin, P.A.; Noo, A.; Foucart, G.; Flammang, J.; Lemaigre, S.; Sinnaeve, G.; Dardenne, P.; Delfosse, P. Assessment of factors influencing the biomethane yield of maize silages. Bioresour. Technol. 2014, 153, 260–268. [Google Scholar] [CrossRef]
- Meyer-Aurich, A.; Lochmann, Y.; Klauss, H.; Prochnow, A. Comparative advantage of maize- and grass-silage based feedstock for biogas production with respect to greenhouse gas mitigation. Sustainability 2016, 8, 617. [Google Scholar] [CrossRef]
- Peter, C.; Glemnitz, M.; Winter, K.; Kornatz, P.; Müller, J.; Heiermann, M.; Aurbacher, J. Impact of energy crop rotation design on multiple aspects of resource efficiency. Chem. Eng. Technol. 2017, 40, 323–332. [Google Scholar] [CrossRef]
- Nilsson, D.; Rosenqvist, H.; Bernesson, S. Profitability of the production of energy grasses on marginal agricultural land in Sweden. Biomass Bioenergy 2015, 83, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Wünsch, K.; Gruber, S.; Claupein, W. Profitability analysis of cropping systems for biogas production on marginal sites in southwestern Germany. Renew Energy 2012, 45, 213–220. [Google Scholar] [CrossRef]
- Mason, P.M.; Glover, K.; Smith, J.A.C.; Willis, K.J.; Woods, J.; Thompson, I.P. The potential of CAM crops as a globally significant bioenergy resource: Moving from ‘fuel or food’ to ‘fuel and more food’. Energy Environ. Sci. 2015, 8, 2320–2329. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Abhilash, P.C. Exploring marginal and degraded lands for biomass and bioenergy production: An Indian scenario. Renew. Sustain. Energy Rev. 2016, 54, 1537–1551. [Google Scholar] [CrossRef]
- Mandl, M.G. Status of green biorefining in Europe. Biofuels Bioprod. Biorefin. 2010, 4, 268–274. [Google Scholar] [CrossRef]
- Laasasenaho, K.; Lensu, A.; Rintala, J. Planning land use for biogas energy crop production: The potential of cutaway peat production lands. Biomass Bioenergy 2016, 85, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Graß, R.; Heuser, F.; Stülpnagel, R.; Piepho, H.-P.; Wachendorf, M. Energy crop production in double-cropping systems: Results from an experiment at seven sites. Eur. J. Agron. 2013, 51, 120–129. [Google Scholar] [CrossRef]
- Negri, M.; Bacenetti, J.; Brambilla, M.; Manfredini, A.; Cantore, A.; Bocchi, S. Biomethane production from different crop systems of cereals in Northern Italy. Biomass Bioenergy 2014, 63, 321–329. [Google Scholar] [CrossRef]
- Strauß, C.; Vetter, A.; Dickeduisberg, M.; Von Felde, A. Biogas Production and Energy Cropping. In Energy from Organic Materials (Biomass), 2nd ed.; Kaltschmitt, M., Ed.; Springer: New York, NY, USA, 2017; Volume 2, pp. 113–164. ISBN 978-1-4939-7812-0. [Google Scholar]
- Molinuevo-Salces, B.; Fernández-Varela, R.; Uellendahl, H. Key factors influencing the potential of catch crops for methane production. Environ. Technol. 2014, 35, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Gansberger, M.; Montgomery, F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- De Mol, F.; Tamms, L.; Gerowitt, B. Biodiversität einer mehrjährigen Wildpflanzenmischung für die Biogasproduktion (Biodiversity of a perennial wild flower mixture for biogas production). In Proceedings of the 28th German Conference on Weed Biology and Weed Control, Braunschweig, Germany, 27 February–1 March 2018; Nordmeyer, H., Ulber, L., Eds.; p. 458. [Google Scholar] [CrossRef]
- Hahn, J.; Westerman, P.R.; Heiermann, M.; Gerowitt, B. Wildflower mixtures as biogas feedstock - Can seeds survive the process? In Proceedings of the Biogas Science 2018, International Conference on Anaerobic Digestion, Lingotto Conference center, Torino, Italy, 17–19 September 2018; p. 95. [Google Scholar]
- Holst, G.S.; Musshoff, O.; Doerschner, T. Policy impact analysis of penalty and reward scenarios to promote flowering cover crops using a business simulation game. Biomass Bioenergy 2014, 70, 196–206. [Google Scholar] [CrossRef]
- Meyer, A.K.P.; Ehimen, E.A.; Holm-Nielsen, J.B. Future European biogas: Animal manure, straw and grass potentials for a sustainable European biogas production. Biomass Bioenergy 2018, 111, 154–164. [Google Scholar] [CrossRef]
- Murphy, J.D.; Power, N.M. An argument for using biomethane generated from grass as a biofuel in Ireland. Biomass Bioenergy 2009, 33, 504–512. [Google Scholar] [CrossRef]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Linke, B.; Idler, C.; Amon, T.; Hobbs, P. Bioenergy from permanent grassland—A review: I. Biogas. Bioresour. Technol. 2009, 100, 4931–4944. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.; Holland, R.A.; Taylor, G.; Richter, G.M. Grassland futures in Great Britain—Productivity assessment and scenarios for land use change opportunities. Sci. Total Environ. 2018, 634, 1108–1118. [Google Scholar] [CrossRef]
- de Meyer, A.; Cattrysse, D.; van Orshoven, J. Considering biomass growth and regeneration in the optimisation of biomass supply chains. Renew. Energy 2016, 87, 990–1002. [Google Scholar] [CrossRef] [Green Version]
- Tilvikiene, V.; Kadziuliene, Z.; Dabkevicius, Z.; Venslauskas, K.; Navickas, K. Feasibility of tall fescue, cocksfoot and reed canary grass for anaerobic digestion: Analysis of productivity and energy potential. Ind. Crops Prod. 2016, 84, 87–96. [Google Scholar] [CrossRef]
- Wahid, R.; Nielsen, S.F.; Hernandez, V.M.; Ward, A.J.; Gislum, R.; Jørgensen, U.; Møller, H.B. Methane production potential from Miscanthus sp. Effect of harvesting time, genotypes and plant fractions. Biosyst. Eng. 2015, 133, 71–80. [Google Scholar] [CrossRef]
- McEniry, J.; Allen, E.; Murphy, J.D.; O’Kiely, P. Grass for biogas production: The impact of silage fermentation characteristics on methane yield in two contrasting biomethane potential test systems. Renew. Energy 2014, 63, 524–530. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Pretreatment techniques used in biogas production from grass. Renew. Sustain. Energy Rev. 2017, 68, 1193–1204. [Google Scholar] [CrossRef]
- Persson, T.; Murphy, J.; Jannasch, A.-K.; Ahern, E.; Liebetrau, J.; Trommler, M.; Toyama, J. A Perspective on the Potential Role of Biogas in Smart Energy Grids. In IEA Bioenergy 2014, Technical Brochure. Available online: https://www.dbfz.de/fileadmin/user_upload/Referenzen/Studien/Smart_Grids_Final_web.pdf (accessed on 17 December 2018).
- Strübing, D.; Moeller, A.B.; Mößnang, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Anaerobic thermophilic trickle bed reactor as a promising technology for flexible and demand-oriented H2/CO2 biomethanation. Appl. Energy 2018, 232, 543–554. [Google Scholar] [CrossRef]
- Herrmann, C.; Ramm, P.; Murphy, J.D. The relationship between bioreactor design and feedstock for optimal biogas production. In Bioreactors for Microbial Biomass and Energy Conversion; Liao, Q., Chang, J.-S., Herrmann, C., Xia, A., Eds.; Springer: Singapore, 2018; pp. 163–197. ISBN 978-981-10-7677-0. [Google Scholar]
- Burkhardt, M.; Koschack, T.; Busch, G. Biocatalytic methanation of hydrogen and carbon dioxide in an anaerobic three-phase system. Bioresour. Technol. 2015, 178, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Biological methanation of CO2 in a novel biofilm plug-flow reactor: A high rate and low parasitic energy process. Appl. Energy 2017, 202, 238–247. [Google Scholar] [CrossRef]
- Budzianowski, W.M. A review of potential innovations for production, conditioning and utilization of biogas with multiple-criteria assessment. Renew. Sustain. Energy Rev. 2016, 54, 1148–1171. [Google Scholar] [CrossRef]
- André, L.; Pauss, A.; Ribeiro, T. Solid anaerobic digestion: State-of-art, scientific and technological hurdles. Bioresour. Technol. 2018, 247, 1027–1037. [Google Scholar] [CrossRef]
- Carrère, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Silvestri, D.; Padil, V.V.T.; Wacławek, M.; Černík, M.; Varma, R.S. Disintegration of wastewater activated sludge (WAS) for improved biogas production. Energies 2019, 12, 21. [Google Scholar] [CrossRef]
- Budde, J.; Prochnow, A.; Plöchl, M.; Suárez-Quinones, T.; Heiermann, M. Energy balance, greenhouse gas emissions, and profitability of thermobarical pretreatment of cattle waste in anaerobic digestion. Waste Manag. 2015, 49, 390–410. [Google Scholar] [CrossRef]
- Herrmann, C.; Prochnow, A.; Heiermann, M.; Idler, C. Particle size reduction during harvest of crop feedstock for biogas production: 2. Energy balance, greenhouse gas balance and profitability. Bioenergy Res. 2012, 5, 937–948. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trénel, P.; Angelidaki, I. Improving the energy balance of grass-based anaerobic digestion through combined harvesting and pretreatment. Anaerobe 2017, 46, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Biotechnol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Dodd, I.C.; Herbert, B.M.J.; Li, H.; Ricketts, L.; Semple, K.T. High solid anaerobic digestion: Operational challenges and possibilities. Environ. Technol. Innov. 2015, 4, 268–284. [Google Scholar] [CrossRef]
- Linke, B.; Rodríguez-Abalde, Á.; Jost, C.; Krieg, A. Performance of a novel two-phase continuously fed leach bed reactor for demand-based biogas production from maize silage. Bioresour. Technol. 2015, 177, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mumme, J.; Linke, B.; Tölle, R. Novel upflow anaerobic solid-state (UASS) reactor. Bioresour. Technol. 2010, 101, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.M.; Schmidt, T.; Lv, Z.; Liebetrau, J.; Richnow, H.H.; Kleinsteuber, S.; Nikolausz, M. Reduction of the hydraulic retention time at constant high organic loading rate to reach the microbial limits of anaerobic digestion in various reactor systems. Bioresour. Technol. 2016, 217, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.; Schropp, D.; Bengelsdorf, F.R.; Othman, M.; Kazda, M. Dynamics of biofilm formation during anaerobic digestion of organic waste. Anaerobe 2014, 29, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.; Jewiarz, M.; Mudryk, K.; Frączek, J.; Dziedzic, K. Conceptual design of the mobile granulation line for production fertilizers from digestates and ash mixtures. MATEC Web Conf. 2018, 168, 04003. [Google Scholar] [CrossRef]
- Ramm, P.; Abendroth, C.; Latorre Pérez, A.; Herrmann, C.; Sebök, S.; Geißler, A.; Vilanova, C.; Porcar, M.; Dornack, C.; Bürger, C.; et al. Ammonia removal during leach-bed acidification leads to optimized organic acid production from chicken manure. Renew. Energy 2019. under review. [Google Scholar]
- Kuo, W.-C.; Lai, W.-L. Treatment of kitchen waste using a mobile thermophilic anaerobic digestion system. Renew. Energy 2010, 35, 2335–2339. [Google Scholar] [CrossRef]
- Moreira, C.; Pazmiño-Hernandez, M.A.; Pazmiño-Barreno, M.A.; Griffin, K.; Pullammanappallil, P. Design and construction of a solar mobile anaerobic digester for rural communities. In Proceedings of the 15th LACCEI International Multi-Conference for Engineering, Education and Technology, Boca Raton, FL, USA, 19–21 July 2017. [Google Scholar]
- Umweltbundesamt. Renewable Energies—The Figures. 1 October 2018. Available online: https://www.umweltbundesamt.de/themen/klima-energie/erneuerbare-energien/erneuerbare-energien-in-zahlen#emissionsbilanz (accessed on 16 December 2018).
- Hahn, H.; Krautkremer, B.; Hartmann, K.; Wachendorf, M. Review of concepts for a demand-driven biogas supply for flexible power generation. Renew. Sustain. Energy Rev. 2014, 29, 383–393. [Google Scholar] [CrossRef]
- Bekkering, J.; Broekhuis, A.A.; van Gemert, W.J.T.; Hengeveld, E.J. Balancing gas supply and demand with a sustainable gas supply chain—A study based on field data. Appl. Energy 2013, 111, 842–852. [Google Scholar] [CrossRef]
- Hahn, H.; Ganagin, W.; Hartmann, K.; Wachendorf, M. Cost analysis of concepts for a demand oriented biogas supply for flexible power generation. Bioresour. Technol. 2014, 170, 211–220. [Google Scholar] [CrossRef]
- Schaaf, T.; Grünig, J.; Schuster, M.R.; Rothenfluh, T.; Orth, A. Methanation of CO2—Storage of renewable energy in a gas distribution system. Energy Sustain. Soc. 2014, 4, 4–29. [Google Scholar] [CrossRef]
- De Vrieze, J.; Verstraete, W.; Boon, N. Repeated pulse feeding induces functional stability in anaerobic digestion. Microb. Biotechnol. 2013, 6, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Mulat, D.G.; Jacobi, H.F.; Feilberg, A.; Adamsen, A.P.S.; Richnow, H.-H.; Nikolausz, M. Changing Feeding Regimes to Demonstrate Flexible Biogas Production: Effects on Process Performance, Microbial Community Structure, and Methanogenesis Pathways. Appl. Environ. Microbiol. 2016, 82, 438–449. [Google Scholar] [CrossRef]
- Mauky, E.; Weinrich, S.; Jacobi, H.-F.; Nägele, H.-J.; Liebetrau, J.; Nelles, M. Demand-driven biogas production by flexible feeding in full-scale—Process stability and flexibility potentials. Anaerobe 2017, 46, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Z.-H.; Zheng, Y.; Liu, J.-B.; Xiong, W.-P.; Zhang, Y.-R.; Lu, Y.; Xue, W.-J.; Fan, C.-Z. Organic loading rate and hydraulic retention time shape distinct ecological networks of anaerobic digestion related microbiome. Bioresour. Technol. 2018, 262, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Terboven, C.; Ramm, P.; Herrmann, C. Demand-driven biogas production from sugar beet silage in a novel fixed bed disc reactor under mesophilic and thermophilic conditions. Bioresour. Technol. 2017, 241, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.M.; Allen, E.; O’Shea, R.; O’Kiely, P.; Murphy, J.D. Investigating two-phase digestion of grass silage for demand-driven biogas applications: Effect of particle size and rumen fluid addition. Renew. Energy 2016, 86, 1215–1223. [Google Scholar] [CrossRef]
- Rostkowski, K.H.; Criddle, C.S.; Lepech, M.D. Cradle-to-gate life cycle assessment for a cradle-to-cradle cycle: Biogas-to-bioplastic (and back). Environ. Sci. Technol. 2012, 46, 9822–9829. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, W.; Bringezu, S.; Wächter, N. Economic assessment of CO2-based methane, methanol and polyoxymethylene production. J. CO2 Util. 2018, 27, 170–178. [Google Scholar] [CrossRef]
- Zain, M.M.; Mohamed, A.R. An overview on conversion technologies to produce value added products from CH4 and CO2 as major biogas constituents. Renew. Sustain. Energy Rev. 2018, 98, 56–63. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.C.; Lorenzo, P.; Medrano, E.; Castilla, N.; Soriano, T.; Baille, A. Effect of variable CO2 enrichment on greenhouse production in mild winter climates. Agric. Meteorol. 2005, 132, 244–252. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, X.T.; Clift, R. Life cycle analysis of a biogas-centred integrated dairy farm-greenhouse system in British Columbia. Process Saf. Environ. Prot. 2015, 93, 18–30. [Google Scholar] [CrossRef]
- Rogelj, J.; Shindell, D.; Jiang, K.; Fifita, S.; Forster, P.; Ginzburg, V.; Handa, C.; Kheshgi, H.; Kobayashi, S.; Kriegler, E.; et al. Mitigation Pathways Compatible with 1.5 °C in the Context of Sustainable Development, Global warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. 2018. Available online: https://www.ipcc.ch/site/assets/uploads/sites/2/2018/11/SR15_Chapter2_Low_Res.pdf (accessed on 25 December 2018).
- Minx, J.C.; Lamb, W.F.; Callaghan, M.W.; Fuss, S.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; De Oliveira Garcia, W.; Hartmann, J.; et al. Negative emissions—Part 1: Research landscape and synthesis. Environ. Res. Lett. 2018, 13, 063001. [Google Scholar] [CrossRef]
- Valentino, F.; Gottardo, M.; Micolucci, F.; Pavan, P.; Bolzonella, D.; Rossetti, S.; Majone, M. Organic fraction of municipal solid waste recovery by conversion into added-value polyhydroxyalkanoates and biogas. ACS Sustain. Chem. Eng. 2018, 6, 16375–16385. [Google Scholar] [CrossRef]
- Demichelis, F.; Fiore, S.; Pleissner, D.; Venus, J. Technical and economic assessment of food waste valorization through a biorefinery chain. Renew. Sustain. Energy Rev. 2018, 94, 38–48. [Google Scholar] [CrossRef]
- Albornoz, S.; Wyman, V.; Palma, C.; Carvajal, A. Understanding of the contribution of the fungal treatment conditions in a wheat straw biorefinery that produces enzymes and biogas. Biochem. Eng. J. 2018, 140, 140–147. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Corona, A.; Ambye-Jensen, M.; Vega, G.C.; Hauschild, M.Z.; Birkved, M. Techno-environmental assessment of the green biorefinery concept: Combining process simulation and life cycle assessment at an early design stage. Sci. Total Environ. 2018, 635, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Santamaría-Fernández, M.; Molinuevo-Salces, B.; Lübeck, M.; Uellendahl, H. Biogas potential of green biomass after protein extraction in an organic biorefinery concept for feed, fuel and fertilizer production. Renew Energy 2018, 129, 769–775. [Google Scholar] [CrossRef]
- Haag, N.L.; Nägele, H.-J.; Fritz, T.; Oechsner, H. Effects of ensiling treatments on lactic acid production and supplementary methane formation of maize and amaranth—An advanced green biorefining approach. Bioresour. Technol. 2015, 178, 217–225. [Google Scholar] [CrossRef]
- Andersen, L.; Lamp, A.; Dieckmann, C.; Baetge, S.; Schmidt, L.M.; Kaltschmitt, M. Biogas plants as key units of biorefinery concepts: Options and their assessment. J. Biotechnol. 2015, 283, 130–139. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy recovery from industrial crop wastes by dry anaerobic digestion: A review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Horváth, I.S. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Insam, H.; Gomez-Brandon, M.; Ascher, J. Manure-based biogas fermentation residues—Friend or foe of soil fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Jones, D.L.; Edwards-Jones, G.; Williams, A.P. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J. Plant Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Ehmann, A.; Thumm, U.; Lewandowski, I. Fertilizing potential of separated biogas digestates in annual and perennial biomass production systems. Front. Sustain. Food Syst. 2018, 2, 1–14. [Google Scholar] [CrossRef]
- Nhu, T.T.; Dewulf, J.; Serruys, P.; Huysveld, S.; Nguyen, C.V.; Sorgeloos, P.; Schaubroeck, T. Resource usage of integrated Pig-Biogas-Fish system: Partitioning and substitution within attributional life cycle assessment. Resour. Conserv. Recycl. 2015, 102, 27–38. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grünewald, C.F.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, W.; Liu, Y. A sustainable biorefinery to convert agricultural residues into value-added chemicals. Biotechnol. Biofuels 2016, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Wobiwo, F.A.; Alleluya, V.K.; Emaga, T.H.; Boda, M.; Fokoud, E.; Gillet, S.; Deleu, M.; Gerin, P.A. Recovery of fibers and biomethane from banana peduncles biomass through anaerobic digestion. Energy Sustain. Dev. 2017, 37, 60–65. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient recovery from digestate of anaerobic digestion of livestock manure: A review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Baute, K.A.; Robinson, D.E.; van Eerd, L.L.; Edson, M.; Sikkema, P.H.; Gilroyed, B.H. Survival of seeds from perennial biomass species during commercial-scale anaerobic digestion. Weed Res. 2016, 56, 258–266. [Google Scholar] [CrossRef]
- Fröschle, B.; Heiermann, M.; Lebuhn, M.; Messelhäusser, U.; Plöchl, M. Hygiene and sanitation in biogas plants. Adv. Biochem. Eng. Biotechnol. 2015, 151, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Massé, D.I.; Gilbert, Y. Potential of biological processes to eliminate antibiotics in livestock manure: An overview. Animals 2014, 4, 146–163. [Google Scholar]
- Pivato, A.; Vanin, S.; Raga, R.; Lavagnolo, M.C.; Barausse, A.; Rieple, A.; Laurent, A.; Cossu, R. Use of digestate from a decentralized on-farm biogas plant as fertilizer in soils: An ecotoxicological study for future indicators in risk and life cycle assessment. Waste Manag. 2016, 49, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauss, T.; Wings, T.K.; Brunner, J.S.; Glaeser, S.P.; Dott, W.; Kämpfer, P. Bacterial diversity and antibiotic resistances of abundant aerobic culturable bacteria in input and output samples of 15 German biogas plants. J. Appl. Microbiol. 2016, 121, 1673–1684. [Google Scholar] [CrossRef]
- Thomas, C.; Idler, C.; Ammon, C.; Herrmann, C.; Amon, T. Inactivation of ESBL-/AmpC-producing Escherichia coli during mesophilic and thermophilic anaerobic digestion of chicken manure. Waste Manag. 2019, 84, 74–82. [Google Scholar] [CrossRef]
- Bian, B.; Wu, H.S.; Lv, L.; Fan, Y.; Lu, H. Health risk assessment of metals in food crops and related soils amended with biogas slurry in Taihu Basin: Perspective from field experiment. Environ. Sci. Pollut. Res. 2015, 22, 14358–14366. [Google Scholar] [CrossRef] [PubMed]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic characteristics of five different urban waste digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Wu, F.; Tong, X.; Jiang, B. Emergy-based sustainability assessment of an integrated production system of cattle, biogas, and greenhouse vegetables: Insight into the comprehensive utilization of wastes on a large-scale farm in Northwest China. Ecol. Eng. 2013, 61, 335–344. [Google Scholar] [CrossRef]
- Muster-Slawitsch, B.; Weiss, W.; Schnitzer, H.; Brunner, C. The green brewery concept—Energy efficiency and the use of renewable energy sources in breweries. Appl. Eng. 2011, 31, 2123–2134. [Google Scholar] [CrossRef]
- Broberg Viklund, S.; Lindkvist, E. Biogas production supported by excess heat—A systems analysis within the food industry. Energy Convers. Manag. 2015, 91, 249–258. [Google Scholar] [CrossRef]
- Hijazi, O.; Munro, S.; Zerhusen, B.; Effenberger, M. Review of life cycle assessment for biogas production in Europe. Renew. Sustain. Energy Rev. 2016, 54, 1291–1300. [Google Scholar] [CrossRef]
- Poeschl, M.; Ward, S.; Owende, P. Environmental impacts of biogas deployment—Part II: Life cycle assessment of multiple production and utilization pathways. J. Clean. Prod. 2012, 24, 184–201. [Google Scholar] [CrossRef]
- Dressler, D.; Loewen, A.; Nelles, M. Life cycle assessment of the supply and use of bioenergy: Impact of regional factors on biogas production. Int. J. Life Cycle Assess. 2012, 17, 1104–1115. [Google Scholar] [CrossRef]
- Lijó, L.; González-García, S.; Bacenetti, J.; Fiala, M.; Feijoo, G.; Lema, J.M.; Moreira, M.T. Life Cycle Assessment of electricity production in Italy from anaerobic co-digestion of pig slurry and energy crops. Renew. Energy 2014, 68, 625–635. [Google Scholar] [CrossRef]
- Venanzi, S.; Pezzolla, D.; Cecchini, L.; Pauselli, M.; Ricci, A.; Sordi, A.; Torquati, B.; Gigliotti, G. Use of agricultural by-products in the development of an agro-energy chain: A case study from the Umbria region. Sci. Total Environ. 2018, 627, 494–505. [Google Scholar] [CrossRef]
- Lansche, J.; Müller, J. Life cycle assessment of energy generation of biogas fed combined heat and power plants: Environmental impact of different agricultural substrates. Eng. Life Sci. 2012, 12, 313–320. [Google Scholar] [CrossRef]
- Michel, J.; Weiske, A.; Möller, K. The effect of biogas digestion on the environmental impact and energy balances in organic cropping systems using the life-cycle assessment methodology. Renew. Agric. Food Syst. 2010, 25, 204–218. [Google Scholar] [CrossRef]
- Oehmichen, K.; Thrän, D. Fostering renewable energy provision from manure in Germany—Where to implement GHG emission reduction incentives. Energy Policy 2017, 110, 471–477. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Styles, D.; Prade, T.; Adams, P.; Thelin, G.; Rodhe, L.; Gunnarsson, I.; D’Hertefeldt, T. Closing nutrient loops through decentralized anaerobic digestion of organic residues in agricultural regions: A multi-dimensional sustainability assessment. Resour. Conserv. Recycl. 2018, 136, 110–117. [Google Scholar] [CrossRef]
- Valli, L.; Rossi, L.; Fabbri, C.; Sibilla, F.; Gattoni, P.; Dale, B.E.; Kim, S.; Ong, R.G.; Bozzetto, S. Greenhouse gas emissions of electricity and biomethane produced using the BiogasdonerightTM system: Four case studies from Italy. Biofuels Bioprod. Biorefin. 2017, 11, 847–860. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Golkowska, K.; Lebuf, V.; Vaneeckhaute, C.; Michels, E.; Meers, E.; Benetto, E.; Koster, D. Environmental assessment of digestate treatment technologies using LCA methodology. Waste Manag. 2015, 43, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Felten, D.; Fröba, N.; Fries, J.; Emmerling, C. Energy balances and greenhouse gas-mitigation potentials of bioenergy cropping systems (Miscanthus, rapeseed, and maize) based on farming conditions in Western Germany. Renew. Energy 2013, 55, 160–174. [Google Scholar] [CrossRef]
- Muradin, M.; Joachimiak-Lechman, K.; Foltynowicz, Z. Evaluation of Eco-Efficiency of Two Alternative Agricultural Biogas Plants. Appl. Sci. 2018, 8, 2083. [Google Scholar] [CrossRef]
- Bystricky, M.; Knödlseder, T.; Weber-Blaschke, G.; Faulstich, M. Comparing environmental impacts of electricity, heat and fuel from energy crops: Evaluating biogas utilization pathways by the basket of benefit methodology. Eng. Life Sci. 2010, 10, 570–576. [Google Scholar] [CrossRef]
- Martin, M.; Svensson, N.; Fonseca, J.; Eklund, M. Quantifying the environmental performance of integrated bioethanol and biogas production. Renew. Energy 2014, 61, 109–116. [Google Scholar] [CrossRef]
- Soam, S.; Borjesson, P.; Sharma, P.K.; Gupta, R.P.; Tuli, D.K.; Kumar, R. Life cycle assessment of rice straw utilization practices in India. Bioresour. Technol. 2017, 228, 89–98. [Google Scholar] [CrossRef]
- Wagner, M.; Kiesel, A.; Hastings, A.; Iqbal, Y.; Lewandowski, I. Novel Miscanthus Germplasm-Based Value Chains: A Life Cycle Assessment. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- IPCC. Emissions from Livestock and Manure Management. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; 2006; Available online: http://www.ipcc-nggip.iges.or.jp/public/2006gl/index.html (accessed on 25 December 2018).
- Kimming, M.; Sundberg, C.; Nordberg, Å.; Baky, A.; Bernesson, S.; Norén, O.; Hansson, P.A. Biomass from agriculture in small-scale combined heat and power plants—A comparative life cycle assessment. Biomass Bioenergy 2011, 35, 1572–1581. [Google Scholar] [CrossRef]
- Liebetrau, J.; Clemens, J.; Cuhls, C.; Hafermann, C.; Friehe, J.; Weiland, P.; Daniel-Gromke, J. Methane emissions from biogas-producing facilities within the agricultural sector. Eng. Life Sci. 2010, 10, 595–599. [Google Scholar] [CrossRef]
- Fantin, V.; Giuliano, A.; Manfredi, M.; Ottaviano, G.; Stefanova, M.; Masoni, P. Environmental assessment of electricity generation from an Italian anaerobic digestion plant. Biomass Bioenergy 2015, 83, 422–435. [Google Scholar] [CrossRef]
- Styles, D.; Gibbons, J.; Williams, A.P.; Dauber, J.; Stichnothe, H.; Urban, B.; Chadwick, D.R.; Jones, D.L. Consequential life cycle assessment of biogas, biofuel and biomass energy options within an arable crop rotation. GCB Bioenergy 2015, 7, 1305–1320. [Google Scholar] [CrossRef] [Green Version]
- Pawelzik, P.; Carus, M.; Hotchkiss, J.; Narayan, R.; Selke, S.; Wellisch, M.; Weiss, M.; Wicke, B.; Patel, M.K. Critical aspects in the life cycle assessment (LCA) of bio-based materials—Reviewing methodologies and deriving recommendations. Resour. Conserv. Recycl. 2013, 73, 211–228. [Google Scholar] [CrossRef]
- Finkbeiner, M. Indirect land use change—Help beyond the hype? Biomass Bioenergy 2014, 62, 218–221. [Google Scholar] [CrossRef]
- Schmidt, J.H.; Weidema, B.P.; Brandão, M. A framework for modelling indirect land use changes in Life Cycle Assessment. J. Clean. Prod. 2015, 99, 230–238. [Google Scholar] [CrossRef]
- De Rosa, M. Land use and land-use changes in life cycle assessment: Green modelling or black boxing? Ecol. Econ. 2018, 144, 73–81. [Google Scholar] [CrossRef]
- Tonini, D.; Hamelin, L.; Astrup, T.F. Environmental implications of the use of agro-industrial residues for biorefineries: Application of a deterministic model for indirect land-use changes. GCB Bioenergy 2016, 8, 690–706. [Google Scholar] [CrossRef]
- von Bock und Polach, C.; Kunze, C.; Maaß, O.; Grundmann, P. Bioenergy as a socio-technical system: The nexus of rules, social capital and cooperation in the development of bioenergy villages in Germany. Energy Res. Soc. Sci. 2015, 6, 128–135. [Google Scholar] [CrossRef]
- Grundmann, P.; Ehlers, M.-H. Determinants of courses of action in bioenergy villages responding to changes in renewable heat utilization policy. Util. Policy 2016, 41, 183–192. [Google Scholar] [CrossRef]
- Ehlers, M.-H. Fermented Dreams—Regional Entrepreneurship and Institutional Dynamics of Germany’s Agricultural Biogas Sector; Shaker Verlag: Herzogenrath, Germany, 2018; 558p, ISBN 978-3-8440-6219-9. [Google Scholar]
- Keutmann, S.; Uckert, G.; Grundmann, P. Insights into a black box! Comparison of organizational modes and their monetary implications for the producers of short rotation coppice (SRC) in Brandenburg/Germany. Land Use Policy 2016, 57, 313–326. [Google Scholar] [CrossRef]
- Grundmann, P.; Ehlers, M.-H.; Uckert, G. Responses of agricultural bioenergy sectors in Brandenburg (Germany) to climate, economic and legal changes: An application of Holling’s adaptive cycle. Energy Policy 2012, 48, 118–129. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theuerl, S.; Herrmann, C.; Heiermann, M.; Grundmann, P.; Landwehr, N.; Kreidenweis, U.; Prochnow, A. The Future Agricultural Biogas Plant in Germany: A Vision. Energies 2019, 12, 396. https://doi.org/10.3390/en12030396
Theuerl S, Herrmann C, Heiermann M, Grundmann P, Landwehr N, Kreidenweis U, Prochnow A. The Future Agricultural Biogas Plant in Germany: A Vision. Energies. 2019; 12(3):396. https://doi.org/10.3390/en12030396
Chicago/Turabian StyleTheuerl, Susanne, Christiane Herrmann, Monika Heiermann, Philipp Grundmann, Niels Landwehr, Ulrich Kreidenweis, and Annette Prochnow. 2019. "The Future Agricultural Biogas Plant in Germany: A Vision" Energies 12, no. 3: 396. https://doi.org/10.3390/en12030396




