The Influence of Residence Time during Hydrothermal Carbonisation of Miscanthus on Bio-Coal Combustion Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Carbonisation
2.3. Analysis
2.3.1. Inorganic Analysis
2.3.2. Organic Analysis, Combustion Properties and Ash Measurement
2.3.3. Prediction of Slagging and Fouling Propensity
2.3.4. Volatile Component Analysis
3. Results
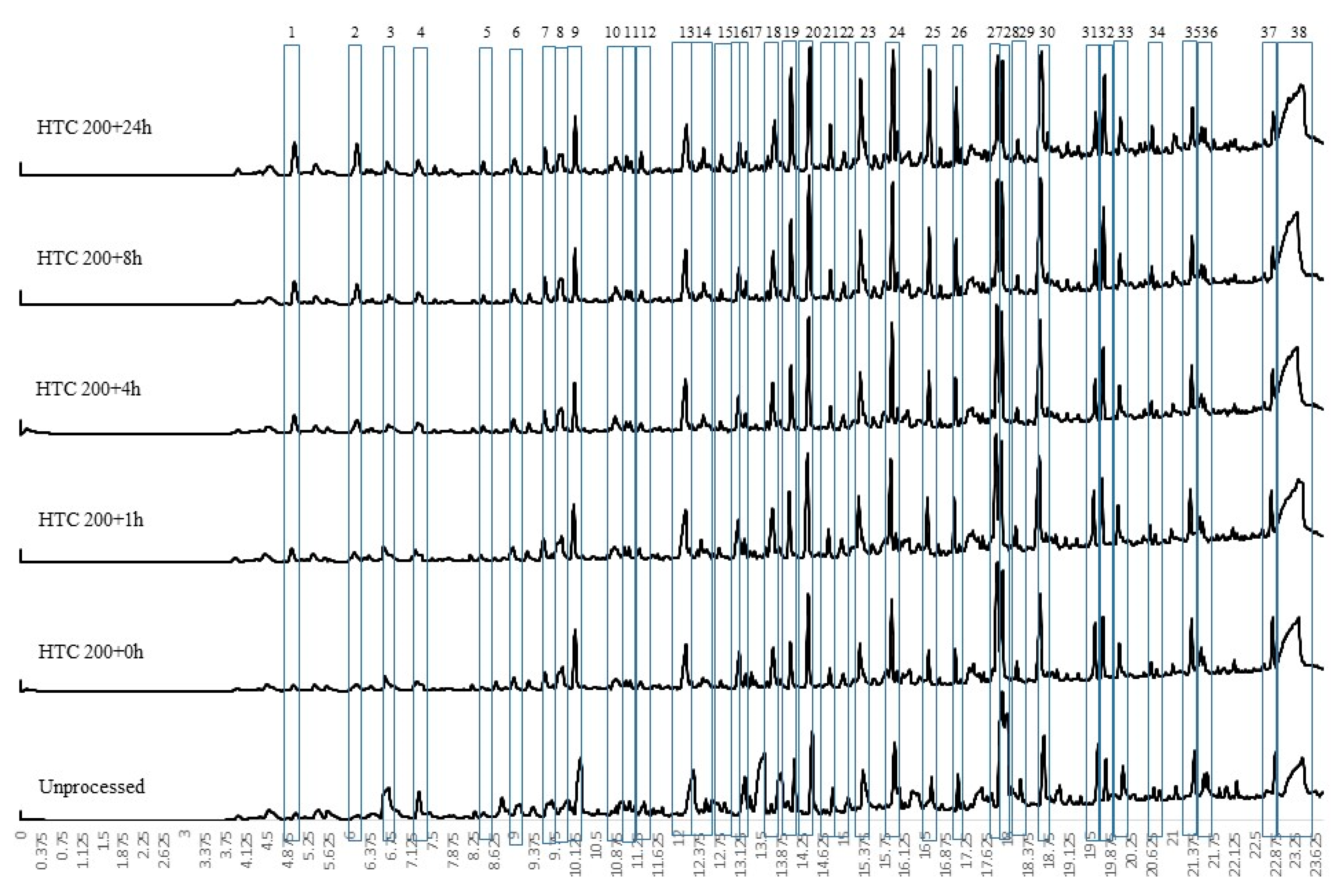
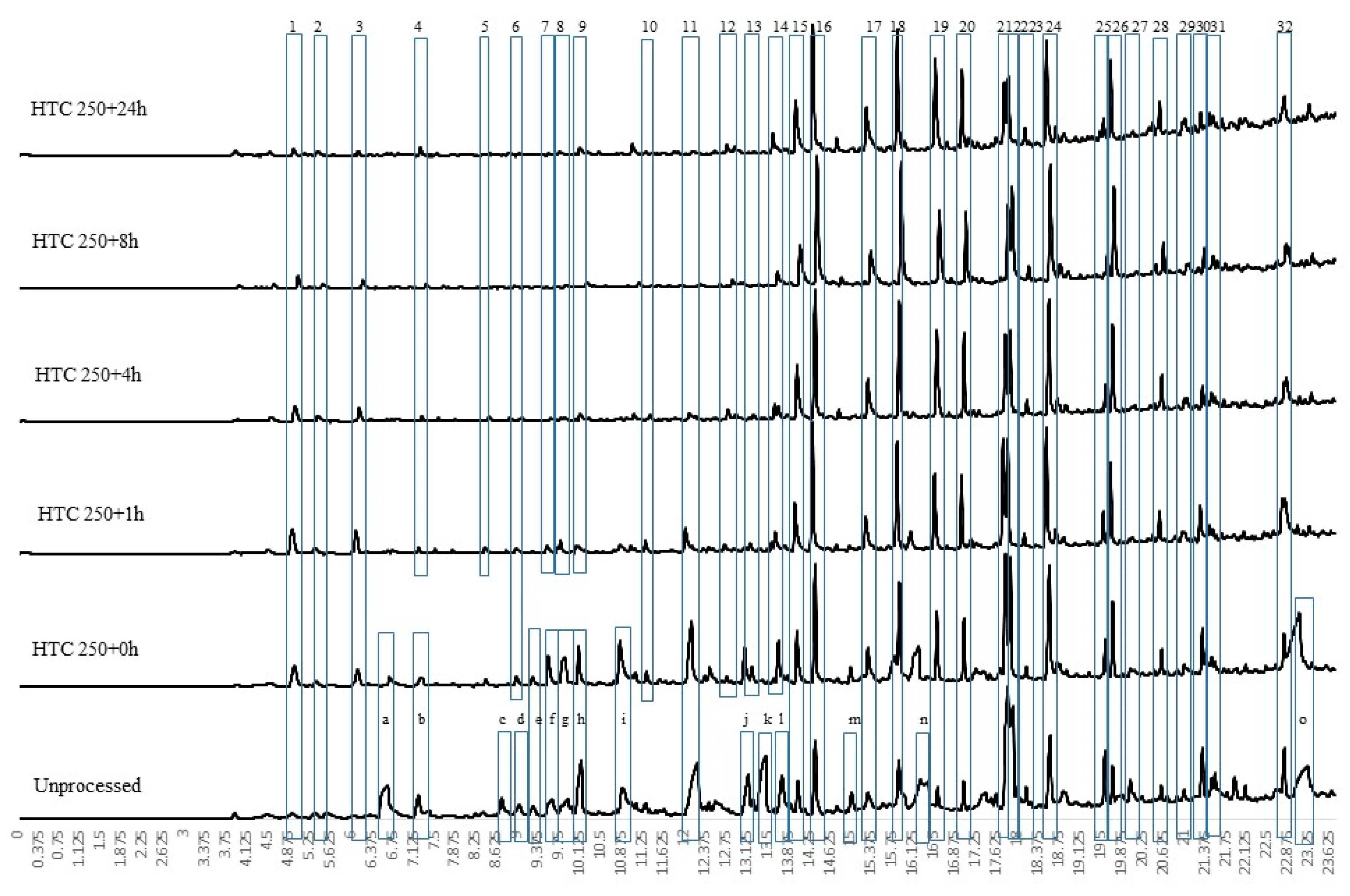
3.1. Influence of Residence Time on the Bio-Coal Organic Chemistry
3.2. Influence of Residence Time on the Bio-Coal Inorganic Chemistry
3.3. Influence of Residence Time on the Bio-Coal Combustion Behaviour
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Smith, A.M.; Whittaker, C.; Shield, I.; Ross, A.B. The potential for production of high quality bio-coal from early harvested Miscanthus by hydrothermal carbonisation. Fuel 2018, 220, 546–557. [Google Scholar] [CrossRef]
- Joo, J.B.; Kim, Y.J.; Kim, W.; Kim, P.; Yi, J. Simple synthesis of graphitic porous carbon by hydrothermal method for use as a catalyst support in methanol electro-oxidation. Catal. Commun. 2008, 10, 267–271. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Xu, J.; Flora, J.R.V.; Hoque, S.; Berge, N.D. Quantifying the sensitivity of feedstock properties and process conditions on hydrochar yield, carbon content, and energy content. Bioresour. Technol. 2018, 262, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar]
- Puccini, M.; Ceccarini, L.; Antichi, D.; Seggiani, M.; Tavarini, S.; Hernandez Latorre, M.; Vitolo, S. Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture. Sustainability 2018, 10, 846. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuelsbioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Hashaikeh, R.; Fang, Z.; Butler, I.S.; Hawari, J.; Kozinski, J.A. Hydrothermal dissolution of willow in hot compressed water as a model for biomass conversion. Fuel 2007, 86, 1614–1622. [Google Scholar] [CrossRef]
- Pastor-Villegas, J.; Pastor-Valle, J.F.; Rodríguez, J.M.M.; García, M.G. Study of commercial wood charcoals for the preparation of carbon adsorbents. J. Anal. Appl. Pyrolysis 2006, 76, 103–108. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Smith, A.M.; Ross, A.B. Production of bio-coal, bio-methane and fertilizer from seaweed via hydrothermal carbonisation. Algal Res. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Volpe, M.; Goldfarb, J.L.; Fiori, L. Hydrothermal carbonization of Opuntia ficus-indica cladodes: Role of process parameters on hydrochar properties. Bioresour. Technol. 2018, 247, 310–318. [Google Scholar] [CrossRef]
- Mäkelä, M.; Kwong, C.W.; Broström, M.; Yoshikawa, K. Hydrothermal treatment of grape marc for solid fuel applications. Energy Convers. Manag. 2017, 145, 371–377. [Google Scholar] [CrossRef]
- Lane, D.J.; Truong, E.; Larizza, F.; Chiew, P.; de Nys, R.; van Eyk, P.J. Effect of Hydrothermal Carbonization on the Combustion and Gasification Behavior of Agricultural Residues and Macroalgae: Devolatilization Characteristics and Char Reactivity. Energy Fuels 2017. [Google Scholar] [CrossRef]
- PetroviĿ, J.; PerišiĿ, N.; MaksimoviĿ, J.D.; MaksimoviĿ, V.; KragoviĿ, M.; StojanoviĿ, M.; LauševiĿ, M.; MihajloviĿ, M. Hydrothermal conversion of grape pomace: Detailed characterization of obtained hydrochar and liquid phase. J. Anal. Appl. Pyrolysis 2016, 118, 267–277. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Tran, K.-Q.; Skreiberg, Ø. Accelerating wet torrefaction rate and ash removal by carbon dioxide addition. Fuel Process. Technol. 2015, 140, 297–303. [Google Scholar] [CrossRef]
- Broch, A.; Jena, U.; Hoekman, S.; Langford, J. Analysis of Solid and Aqueous Phase Products from Hydrothermal Carbonization of Whole and Lipid-Extracted Algae. Energies 2014, 7, 62–79. [Google Scholar] [CrossRef]
- Benavente, V.; Calabuig, E.; Fullana, A. Upgrading of moist agro-industrial wastes by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 89–98. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Mosteiro-Romero, M.; Vogel, F.; Wokaun, A. Liquefaction of wood in hot compressed water: Part 1—Experimental results. Chem. Eng. Sci. 2014, 109, 111–122. [Google Scholar] [CrossRef]
- Kruse, A.; Koch, F.; Stelzl, K.; Wüst, D.; Zeller, M. Fate of Nitrogen during Hydrothermal Carbonization. Energy Fuels 2016, 30, 8037–8042. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Hydrothermal processing of lignocellulosic materials. Holz Als Roh- Und Werkst. 1999, 57, 191–202. [Google Scholar]
- Roos, A.A.; Persson, T.; Krawczyk, H.; Zacchi, G.; Stålbrand, H. Extraction of water-soluble hemicelluloses from barley husks. Bioresour. Technol. 2009, 100, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Myint, A.A.; Lee, H.W.; Yoon, J.; Lee, Y.-W. Evaluation of hot compressed water pretreatment and enzymatic saccharification of tulip tree sawdust using severity factors. Bioresour. Technol. 2013, 144, 460–466. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.; Miles, T. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Bapat, D.; Kulkarni, S.; Bhandarkar, V. Design and Operating Experience on Fluidized Bed Boiler Burning Biomass Fuels with High Alkali Ash; American Society of Mechanical Engineers: New York, NY, USA, 1997. [Google Scholar]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Demirbaş, A. Relationships between lignin contents and fixed carbon contents of biomass samples. Energy Convers. Manag. 2003, 44, 1481–1486. [Google Scholar] [CrossRef]
- Haenel, M.W. Recent progress in coal structure research. Fuel 1992, 71, 1211–1223. [Google Scholar] [CrossRef]
- Kei-ichi, S.; Yoshihisa, I.; Hitoshi, I. Catalytic Activity of Lanthanide(III) Ions for the Dehydration of Hexose to 5-Hydroxymethyl-2-furaldehyde in Water. Bull. Chem. Soc. Jpn. 2001, 74, 1145–1150. [Google Scholar]
- Patil, S.K.R.; Lund, C.R.F. Formation and Growth of Humins via Aldol Addition and Condensation during Acid-Catalyzed Conversion of 5-Hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Lakshmanan, C.M.; Hoelscher, H.E. Production of Levoglucosan by Pyrolysis of Carbohydrates. Pyrolysis in Hot Inert Gas Stream. Prod. Rd 1970, 9, 57–59. [Google Scholar]
- Saddawi, A.; Jones, J.M.; Williams, A. Influence of alkali metals on the kinetics of the thermal decomposition of biomass. Fuel Process. Technol. 2012, 104, 189–197. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T. Catalyzed Carbon–NO Reaction Studied by Scanning Tunneling Microscopy and ab Initio Molecular Orbital Calculations. J. Catal. 1999, 185, 286–296. [Google Scholar] [CrossRef]
- Backreedy, R.I.; Jones, J.M.; Pourkashanian, M.; Williams, A. Modeling the reaction of oxygen with coal and biomass chars. Proc. Combust. Inst. 2002, 29, 415–421. [Google Scholar] [CrossRef]
- Jones, J.M.; Darvell, L.I.; Pourkashanian, M.; Williams, A. The role of metals in biomass char combustion. In Proceedings of the European Combustion Meeting, Louvain-la-Neuve, Belgium, 3–6 April 2005. [Google Scholar]
- Backreedy, R.I.; Jones, J.M.; Pourkashanian, M.; Williams, A. Burn-out of pulverised coal and biomass chars. Fuel 2003, 82, 2097–2105. [Google Scholar] [CrossRef]
- Kannan, M.P.; Richards, G.N. Gasification of biomass chars in carbon dioxide: Dependence of gasification rate on the indigenous metal content. Fuel 1990, 69, 747–753. [Google Scholar] [CrossRef]
- Stojanowska, G.; Jones, J. Influence of added calcium on thermal decomposition of biomass, lignite and their blends. Arch. Combust. 2006, 26, 91. [Google Scholar]
- Titirici, M.-M. Sustainable Carbon Materials from Hydrothermal Processes; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wohlgemuth, S.-A.; Vilela, F.; Titirici, M.-M.; Antonietti, M. A one-pot hydrothermal synthesis of tunable dual heteroatom-doped carbon microspheres. Green Chem. 2012, 14, 741–749. [Google Scholar] [CrossRef]
- Marschner, H.; Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Korbee, R.; Kiel, J.; Zevenhoven, M.; Skrifvars, B.; Jensen, P.; Frandsen, F. Investigation of biomass inorganic matter by advanced fuel analysis and conversion experiments. In Power Production in the 21st Century: Impacts of Fuel Quality and Operations; United Engineering Foundation Advanced Combustion Engineering Research Center: Snowbird, UT, USA, 2001. [Google Scholar]
- Antal, M.J.; Mok, W.S.L.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Z.; Moriya, T.; Kishida, H.; Higashijima, H.; Enomoto, H. Controlling Hydrothermal Reaction Pathways To Improve Acetic Acid Production from Carbohydrate Biomass. Environ. Sci. Technol. 2005, 39, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Domazetis, G.; James, B.D. Molecular models of brown coal containing inorganic species. Org. Geochem. 2006, 37, 244–259. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, T.; Che, D. Effect of water washing on fuel properties, pyrolysis and combustion characteristics, and ash fusibility of biomass. Fuel Process. Technol. 2013, 106, 712–720. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A.; Le Coeur, C. Commodity fuels from biomass through pretreatment and torrefaction: Effects of mineral content on torrefied fuel characteristics and quality. Energy Fuels 2012, 26, 6466–6474. [Google Scholar] [CrossRef]
- Miles, T.R.; Miles, T., Jr.; Baxter, L.; Bryers, R.; Jenkins, B.; Oden, L. Alkali Deposits Found in Biomass Power Plants: A Preliminary Investigation of Their Extent and Nature. Volume 1; National Renewable Energy Lab.: Golden, CO, USA; Miles (Thomas R.): Portland, OR, USA; Sandia National Labs.: Livermore, CA, USA; Foster Wheeler Development Corp.: Livingston, NJ, USA; California Univ.: Davis, CA, USA; Bureau of Mines: Albany, OR, USA; Albany Research Center: Albany, OR, USA, 1995. [Google Scholar]
- Koppejan, J.; Van Loo, S. The Handbook of Biomass Combustion and Co-Firing; Routledge: London, UK, 2012. [Google Scholar]
- Riedl, R.; Dahl, J.; Obernberger, I.; Narodoslawsky, M. Corrosion in fire tube boilers of biomass combustion plants. In Proceedings of the China International Corrosion Control Conference, Beijing, China, 1999. [Google Scholar]
- Grimm, A.; Skoglund, N.; Boström, D.; Ohman, M. Bed agglomeration characteristics in fluidized quartz bed combustion of phosphorus-rich biomass fuels. Energy Fuels 2011, 25, 937–947. [Google Scholar] [CrossRef]
- Uberoi, M.; Punjak, W.A.; Shadman, F. The kinetics and mechanism of alkali removal from flue gases by solid sorbents. Prog. Energy Combust. Sci. 1990, 16, 205–211. [Google Scholar] [CrossRef]
- Kyi, S.; Chadwick, B.L. Screening of potential mineral additives for use as fouling preventatives in Victorian brown coal combustion. Fuel 1999, 78, 845–855. [Google Scholar] [CrossRef]
- Llorente, M.J.F.; Arocas, P.D.; Nebot, L.G.; García, J.E.C. The effect of the addition of chemical materials on the sintering of biomass ash. Fuel 2008, 87, 2651–2658. [Google Scholar] [CrossRef]
- Steenari, B.-M.; Lundberg, A.; Pettersson, H.; Wilewska-Bien, M.; Andersson, D. Investigation of Ash Sintering during Combustion of Agricultural Residues and the Effect of Additives. Energy Fuels 2009, 23, 5655–5662. [Google Scholar] [CrossRef]
- Gilbe, C.; Öhman, M.; Lindström, E.; Boström, D.; Backman, R.; Samuelsson, R.; Burvall, J. Slagging Characteristics during Residential Combustion of Biomass Pellets. Energy Fuels 2008, 22, 3536–3543. [Google Scholar] [CrossRef]
- Lindström, E.; Sandström, M.; Boström, D.; Öhman, M. Slagging Characteristics during Combustion of Cereal Grains Rich in Phosphorus. Energy Fuels 2007, 21, 710–717. [Google Scholar] [CrossRef]
- Wang, L.; Hustad, J.E.; Skreiberg, Ø.; Skjevrak, G.; Grønli, M. A Critical Review on Additives to Reduce Ash Related Operation Problems in Biomass Combustion Applications. Energy Procedia 2012, 20, 20–29. [Google Scholar] [CrossRef]
- Su, S.; Pohl, J.H.; Holcombe, D.; Hart, J.A. Techniques to determine ignition, flame stability and burnout of blended coals in p.f. power station boilers. Prog. Energy Combust. Sci. 2001, 27, 75–98. [Google Scholar] [CrossRef]
- Williams, A.; Jones, J.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Yarin, L.P.; Hetsroni, G.; Mosyak, A. Combustion of Two-Phase Reactive Media; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Tillman, D.A.; Duong, D.N.B.; Harding, N.S. Chapter 4—Blending Coal with Biomass: Cofiring Biomass with Coal. In Solid Fuel Blending; Tillman, D.A., Duong, D.N.B., Harding, N.S., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2012; pp. 125–200. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ndibe, C.; Vonk, G.; Yuan, S.; Maier, J.; Scheffknecht, G. Characterizing the Grinding Behavior of Pre-Treated Biomass Fuels for Coal Pulverizer Application. In Proceedings of the 24th European Biomass Conference and Exhibition, Amsterdam, The Netherlands, 6–9 June 2016; pp. 457–465. [Google Scholar] [CrossRef]







| Slagging/Fouling Index | Expression | Limit | |
|---|---|---|---|
| Alkali Index | AI < 0.17 safe combustion AI > 0.17 < 0.34 probable slagging and fouling AI > 0.34 almost certain slagging and fouling [31] | EQ.3 | |
| Bed Agglomeration Index | BAI < 0.15 bed agglomeration likely [32] | EQ.4 | |
| Base to Acid Ratio | R < 0.5 low slagging risk [32] | EQ.5 |
| Temperature | Residence | % Dry Basis | HHV MJ/kg | Energy Yield (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | Nitrogen | Carbon | Hydrogen | Oxygen | Ash | Volatile Matter | Fixed Carbon | ||||
| Unprocessed | 0.7 | 52.2 | 5.2 | 38.7 | 3.1 | 84.5 | 12.3 | 18.2 | |||
| HTC 200 | 0 h | 67 | 0.7 | 51.8 | 5.6 | 39.5 | 2.4 | 80.4 | 16.0 | 18.5 | 68 |
| 1 h | 65 | 0.7 | 53.6 | 5.3 | 38.2 | 2.2 | 77.4 | 18.9 | 18.9 | 68 | |
| 4 h | 64 | 0.7 | 55.3 | 5.5 | 36.0 | 2.4 | 74.7 | 21.7 | 20.1 | 71 | |
| 8 h | 61 | 0.7 | 57.6 | 5.3 | 34.3 | 2.1 | 71.9 | 25.2 | 20.9 | 70 | |
| 24 h | 56 | 0.8 | 61.1 | 5.2 | 30.5 | 2.5 | 65.7 | 31.1 | 22.6 | 70 | |
| HTC 250 | 0 h | 52 | 0.6 | 59.2 | 4.9 | 32.9 | 2.3 | 69.1 | 27.9 | 21.1 | 60 |
| 1 h | 46 | 0.8 | 65.5 | 4.7 | 26.2 | 2.8 | 58.1 | 38.2 | 24.2 | 61 | |
| 4 h | 44 | 0.8 | 71.6 | 4.8 | 20.1 | 2.7 | 53.5 | 42.3 | 27.5 | 66 | |
| 8 h | 43 | 0.8 | 73.4 | 4.7 | 18.6 | 2.5 | 51.7 | 44.1 | 28.2 | 67 | |
| 24 h | 43 | 0.8 | 74.1 | 4.6 | 17.5 | 2.9 | 49.3 | 45.8 | 28.5 | 67 | |
| Temperature | Residence | mg/kg (Dry Basis) | AI | BAI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | Si | P | S | Cl | K | Ca | Mn | Fe | |||||
| Unprocessed | 520 | 920 | 30 | 6890 | 700 | 1130 | 3590 | 3030 | 3530 | 110 | 40 | 0.27 | 0.01 | 0.78 | |
| HTC 200 | 0 h | 100 | 540 | 80 | 6550 | 290 | 690 | 2710 | 1120 | 4060 | 50 | 90 | 0.08 | 0.08 | 0.58 |
| 1 h | 150 | 400 | 70 | 6150 | 350 | 750 | 1760 | 1130 | 2800 | 50 | 120 | 0.08 | 0.09 | 0.49 | |
| 4 h | 120 | 480 | 110 | 6620 | 610 | 970 | 1650 | 900 | 4030 | 70 | 110 | 0.06 | 0.12 | 0.55 | |
| 8 h | 140 | 470 | 60 | 6960 | 670 | 880 | 1370 | 840 | 3780 | 80 | 50 | 0.06 | 0.05 | 0.50 | |
| 24 h | 190 | 470 | 100 | 7610 | 690 | 1170 | 1530 | 740 | 3630 | 60 | 100 | 0.06 | 0.10 | 0.44 | |
| HTC 250 | 0 h | 140 | 440 | 120 | 6320 | 710 | 1290 | 3500 | 920 | 3100 | 70 | 110 | 0.06 | 0.11 | 0.48 |
| 1 h | 90 | 530 | 190 | 7840 | 1150 | 1130 | 1720 | 1040 | 5280 | 80 | 140 | 0.05 | 0.14 | 0.57 | |
| 4 h | 120 | 460 | 120 | 6770 | 1100 | 1230 | 2010 | 890 | 3570 | 80 | 120 | 0.05 | 0.12 | 0.49 | |
| 8 h | 110 | 350 | 110 | 6000 | 1290 | 1020 | 1510 | 650 | 2180 | 90 | 90 | 0.04 | 0.12 | 0.37 | |
| 24 h | 130 | 370 | 140 | 8080 | 1090 | 1070 | 1760 | 850 | 1450 | 70 | 130 | 0.04 | 0.14 | 0.24 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.M.; Ross, A.B. The Influence of Residence Time during Hydrothermal Carbonisation of Miscanthus on Bio-Coal Combustion Chemistry. Energies 2019, 12, 523. https://doi.org/10.3390/en12030523
Smith AM, Ross AB. The Influence of Residence Time during Hydrothermal Carbonisation of Miscanthus on Bio-Coal Combustion Chemistry. Energies. 2019; 12(3):523. https://doi.org/10.3390/en12030523
Chicago/Turabian StyleSmith, Aidan M., and Andrew B. Ross. 2019. "The Influence of Residence Time during Hydrothermal Carbonisation of Miscanthus on Bio-Coal Combustion Chemistry" Energies 12, no. 3: 523. https://doi.org/10.3390/en12030523




