Short-Term Nitrogen Uptake of Barley from Differently Processed Biogas Digestate in Pot Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Analysis of Digestate Variants
2.2. Chemical Composition of Digestate Variants
2.3. Plant Growth Experiments
2.4. Statistical Analysis
3. Results and Discussion
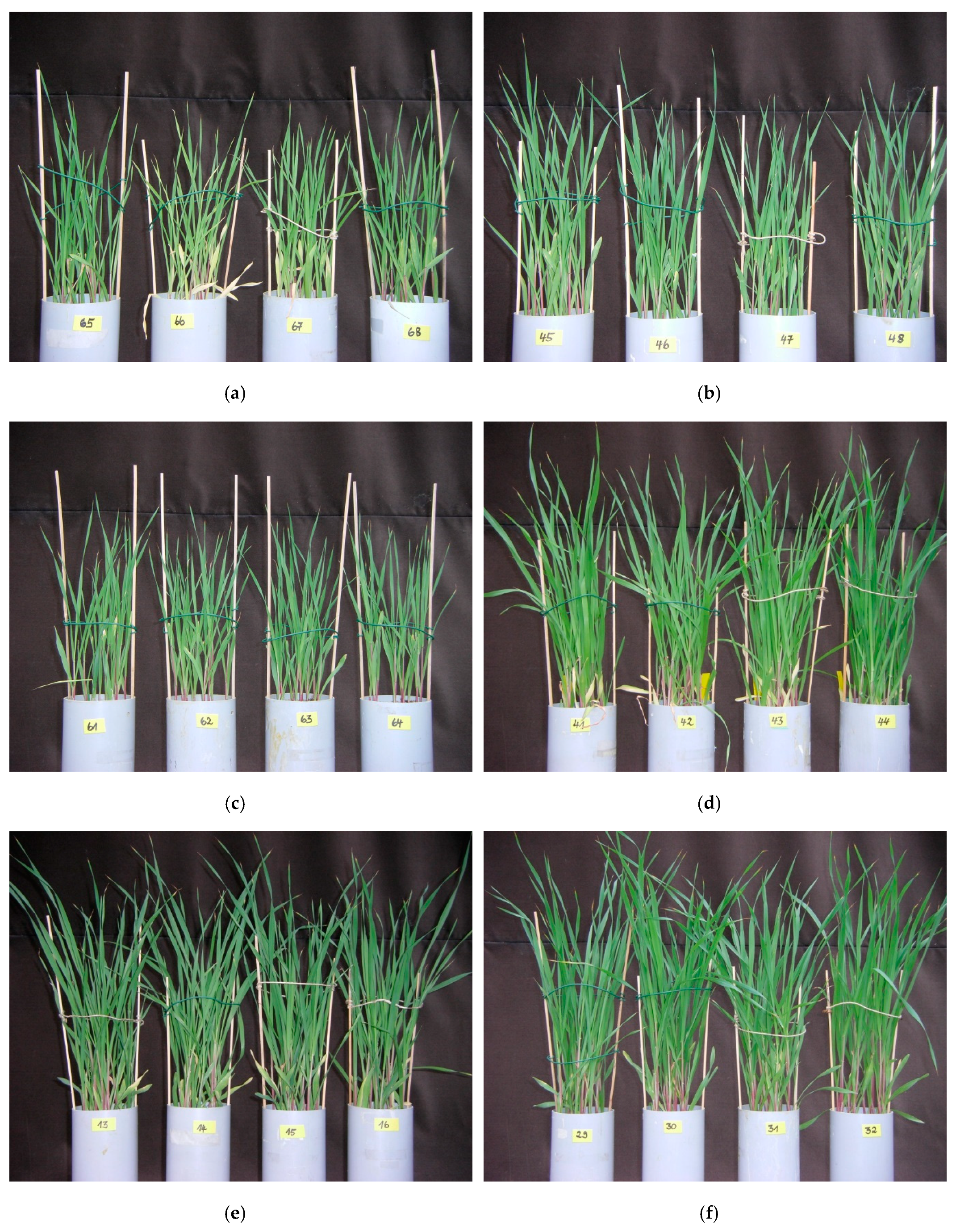
3.1. Visual Observation of Plant Development
3.2. Above-Ground Biomass Yield
3.3. Nitrogen Content of Above-Ground Biomass
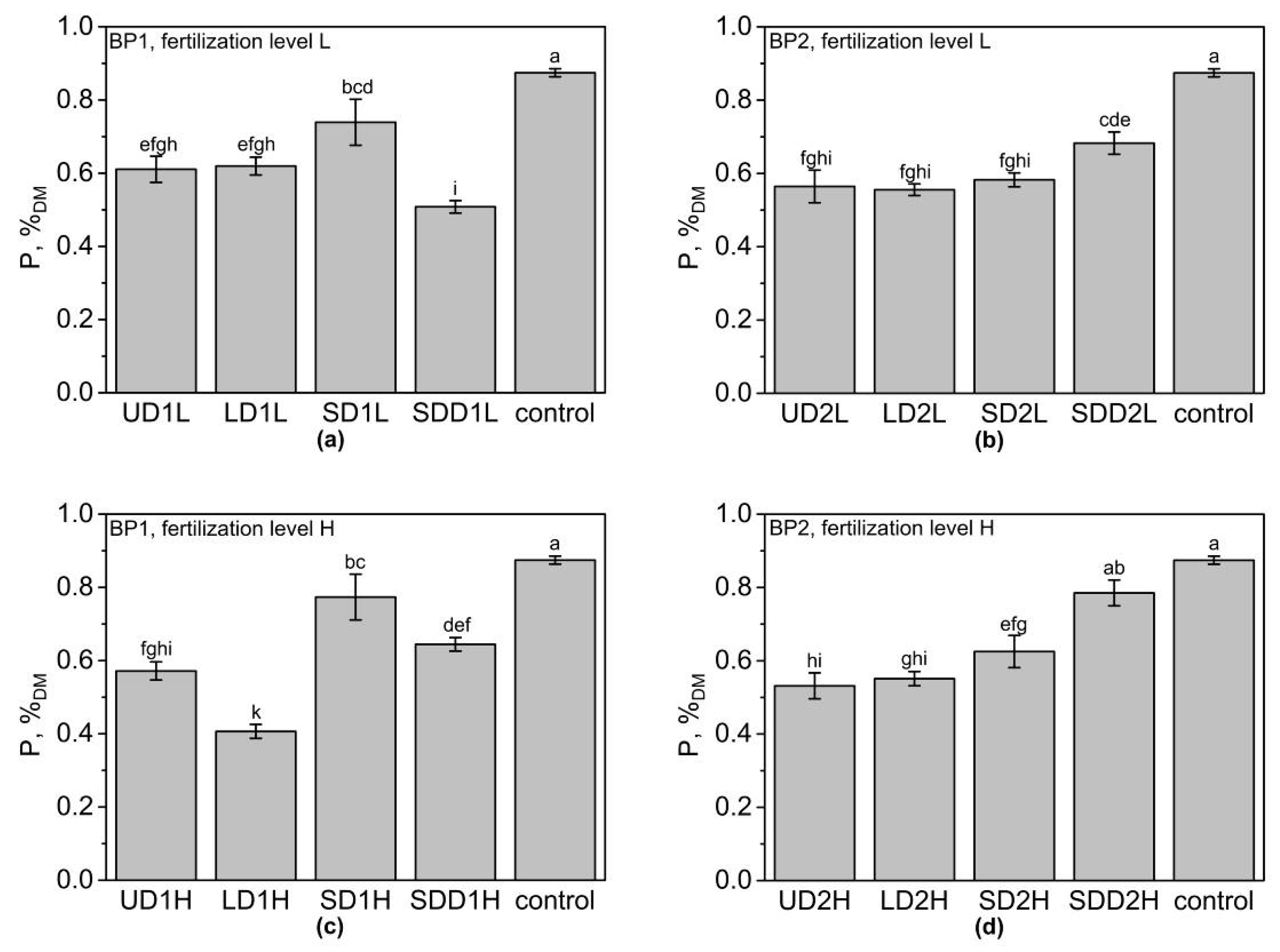
3.4. Phosphorus Content of Above-Ground Biomass
3.5. Nitrogen Removal from Soil Substrate and Plant Uptake Efficiency of Nitrogen
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Möller, K.; Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosyst. 2008, 82, 209–232. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef] [PubMed]
- European Council. Council Directive 91/676/EEC of 12 December 1991 Concering the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31991L0676 (accessed on 27 January 2019).
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; Baxter, D., Ed.; IEA Bioenergy: Berlin, Germany, 2015; ISBN 978-1-910154-16-8. [Google Scholar]
- Huang, J.; Xu, C.; Ridoutt, B.G.; Wang, X.; Ren, P. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Döhler, H.; Schliebner, P. Verfahren und Wirtschaftlichkeit der Gärrestaufbereitung; KTBL HRSG: Darmstadt, Germany, 2006; pp. 199–212. ISBN 978-3-939371-05-2. [Google Scholar]
- Delzeit, R.; Kellner, U. The impact of plant size and location on profitability of biogas plants in Germany under consideration of processing digestates. Biomass Bioenergy 2013, 52, 43–53. [Google Scholar] [CrossRef]
- Möller, K.; Schulz, R.; Müller, T. Effects of setup of centralized biogas plants on crop acreage and balances of nutrients and soil humus. Nutr. Cycl. Agroecosyst. 2011, 89, 303–312. [Google Scholar] [CrossRef]
- Dahlin, J.; Nelles, M.; Herbes, C. Biogas digestate management: Evaluating the attitudes and perceptions of German gardeners towards digestate-based soil amendments. Resour. Conserv. Recycl. 2017, 118, 27–38. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life cycle assessment of biogas digestate processing technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Maurer, C.; Müller, J. Ammonia (NH3) emissions during drying of untreated and dewatered biogas digestate in a hybrid waste-heat/solar dryer. Eng. Life Sci. 2012, 12, 321–326. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef] [Green Version]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative characterization of digestate versus pig slurry and cow manure—Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef]
- Tambone, F.; Adani, F.; Gigliotti, G.; Volpe, D.; Fabbri, C.; Provenzano, M.R. Organic matter characterization during the anaerobic digestion of different biomasses by means of CPMAS 13C NMR spectroscopy. Biomass Bioenergy 2013, 48, 111–120. [Google Scholar] [CrossRef]
- Zirkler, D.; Peters, A.; Kaupenjohann, M. Elemental composition of biogas residues: Variability and alteration during anaerobic digestion. Biomass Bioenergy 2014, 67, 89–98. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Papalia, T.; Attinà, E.; Basile, C.; Panuccio, M.R. Anaerobic co-digestion of recalcitrant agricultural wastes: Characterizing of biochemical parameters of digestate and its impacts on soil ecosystem. Sci. Total Environ. 2017, 586, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Möller, K.; Schulz, R.; Müller, T. Mit Gärresten richtig Düngen. Aktuelle Informationen für Berater. Available online: https://plantnutrition.uni-hohenheim.de/ (accessed on 27 January 2019).
- Alburquerque, J.A.; de la Fuente, C.; Bernal, M.P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- De Boer, H.C. Co-digestion of animal slurry can increase short-term nitrogen recovery by crops. J. Environ. Qual. 2008, 37, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Grigatti, M.; Di Girolamo, G.; Chincarini, R.; Ciavatta, C.; Barbanti, L. Potential nitrogen mineralization, plant utilization efficiency and soil CO2 emissions following the addition of anaerobic digested slurries. Biomass Bioenergy 2011, 35, 4619–4629. [Google Scholar] [CrossRef]
- Gunnarsson, A.; Bengtsson, F.; Caspersen, S. Use efficiency of nitrogen from biodigested plant material by ryegrass. J. Plant Nutr. Soil Sci. 2010, 173, 113–119. [Google Scholar] [CrossRef]
- Koszel, M.; Lorencowicz, E. Agricultural Use of Biogas Digestate as a Replacement Fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Lošák, T.; Zatloukalová, A.; Szostková, M.; Hlušek, J.; Fryč, J.; Vítěz, T. Comparison of the effectiveness of digestate and mineral fertilisers on yield and quality of kohlrabi (Brassica oleracea, L.). Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 2011, 59, 117–122. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Magid, J.; Jensen, L.S. Net and gross nitrogen turnover in soil amended with acidified and differently dried solids from biogas digestate. Soil Sci. Soc. Am. J. 2016, 80, 943–953. [Google Scholar] [CrossRef]
- Rigby, H.; Smith, S.R. Nitrogen availability and indirect measurements of greenhouse gas emissions from aerobic and anaerobic biowaste digestates applied to agricultural soils. Waste Manag. 2013, 33, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Adani, F. Nitrogen mineralization from digestate in comparison to sewage sludge, compost and urea in a laboratory incubated soil experiment. J. Plant Nutr. Soil Sci. 2017, 180, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Vaneeckhaute, C.; Meers, E.; Michels, E.; Ghekiere, G.; Accoe, F.; Tack, F.M.G. Closing the nutrient cycle by using bio-digestion waste derivatives as synthetic fertilizer substitutes: A field experiment. Biomass Bioenergy 2013, 55, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, D.; Cabassi, G.; Borrelli, L.; Fuccella, R.; Degano, L.; Bechini, L.; Marino, P. Nitrogen fertiliser value of digested dairy cow slurry, its liquid and solid fractions, and of dairy cow slurry. Ital. J. Agron. 2014, 9, 71–78. [Google Scholar] [CrossRef]
- Cavalli, D.; Cabassi, G.; Borrelli, L.; Geromel, G.; Bechini, L.; Degano, L.; Marino Gallina, P. Nitrogen fertilizer replacement value of undigested liquid cattle manure and digestates. Eur. J. Agron. 2016, 73, 34–41. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci. Total Environ. 2017, 599, 1885–1894. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Angers, D.A.; Bélanger, G.; Rochette, P.; Eriksen-Hamel, N.; Bittman, S.; Buckley, K.; Massé, D.; Gasser, M.-O. Yield and Nutrient Export of Grain Corn Fertilized with Raw and Treated Liquid Swine Manure. Agron. J. 2008, 100, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
- Duan, N.; Ran, X.; Li, R.; Kougias, P.; Zhang, Y.; Lin, C.; Liu, H. Performance Evaluation of Mesophilic Anaerobic Digestion of Chicken Manure with Algal Digestate. Energies 2018, 11, 1829. [Google Scholar] [CrossRef]
- Da Borso, F.; Di Marzo, C.; Zuliani, F.; Danuso, F.; Baldini, M. Harvest time and ensilage suitability of giant reed and miscanthus for bio-methane production and characterization of digestate for agronomic use. Agron. Res. 2018, 16, 22–40. [Google Scholar] [CrossRef]
- Baryga, A.; Połeć, B.; Małczak, E. Technological value of raw materials from sugar beet growing area fertilized with digestate from sugar beet pulp biogas plant. Plant Soil Environ. 2017, 63, 207–212. [Google Scholar] [Green Version]
- Ronga, D.; Setti, L.; Salvarani, C.; De Leo, R.; Bedin, E.; Pulvirenti, A.; Milc, J.; Pecchioni, N.; Francia, E. Effects of solid and liquid digestate for hydroponic baby leaf lettuce (Lactuca sativa L.) cultivation. Sci. Hortic. 2019, 244, 172–181. [Google Scholar] [CrossRef]
- Kouřimská, L.; Poustková, I.; Babička, L. The use of digestate as a replacement of mineral fertilizers for vegetables growing. Scientia agriculturae bohemica 2012, 43, 121–126. [Google Scholar] [CrossRef]
- Ehmann, A.; Bach, I.-M.; Bilbao, J.; Lewandowski, I.; Müller, T. Phosphates recycled from semi-liquid manure and digestate are suitable alternative fertilizers for ornamentals. Sci. Hortic. 2019, 243, 440–450. [Google Scholar] [CrossRef]
- German Institute for Standardization (DIN). Characterization of Sludges—Determination of Dry Residue and Water Content; German Institute for Standardization: Berlin, Germany, 2001. [Google Scholar]
- German Institute for Standardization (DIN). Sludge, treated biowaste and soil - Determination of pH. Available online: https://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwir7sT34MvgAhVNxosBHe8bCyYQFjAAegQIARAC&url=https%3A%2F%2Flakeohridniva.files.wordpress.com%2F2015%2F07%2Fbs-en-15933-2012sludge-treated-biowaste-and.pdf&usg=AOvVaw0JykqvQZsvUIPlRY9AEEpd (accessed on 27 January 2019).
- Deutsches Institut fur Normung. German Standard Methods for the Examination of Water, Waste Water and Sludge; Beuth Verlag GmbH: Berlin, Germany, 1983. [Google Scholar]
- German Institute for Standardization (DIN). Soil Quality: Determination of Total Nitrogen: Modified Kjeldahl Method. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11261:ed-1:v1:en (accessed on 27 January 2019).
- VDLUFA. Methodenbuch Band VII Umweltanalytik, 3rd ed.; VDLUFA: Darmstadt, Germany 2008. [Google Scholar]
- Schilling, G.; Kerschberger, M. Pflanzenernährung und Düngung; Ulmer: Stuttgart, Germany 2000. [Google Scholar]
- Finck, A. Pflanzenernährung und Düngung in Stichworten; Borntraeger: Stuttgart, Germany, 2007; ISBN 978-3-443-03116-9. [Google Scholar]
- Döhler, H. Landbauliche Verwertung stickstoffreicher Abfallstoff; Wasser&Boden: Berlin, Germany, 1996; pp. 7–16. ISSN 0043-0951. [Google Scholar]
- Gericke, S.; Kurmies, B. Colorimetrische Bestimmung der Phosphorsäure mit Vanadat-Molybdat. In Colorimetrische Bestimmung der Phosphorsäure mit Vanadat-Molybdat; Springer: Berlin, Germany, 1952; pp. 15–22. [Google Scholar]
- Benke, A.P.; Rieps, A.-M.; Wollmann, I.; Petrova, I.; Zikeli, S.; Möller, K. Fertilizer value and nitrogen transfer efficiencies with clover-grass ley biomass based fertilizers. Nutr. Cycl. Agroecosyst. 2017, 107, 395–411. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Pereira, A.; Cabanas, J.E.; Dias, L.; Pires, J.; Arrobas, M. Crops use-efficiency of nitrogen from manures permitted in organic farming. Eur. J. Agron. 2006, 25, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Zorn, W.; Marks, G.; Hubert, H.; Werner, B. Handbuch zur visuellen Diagnose von Ernährungsstörungen bei Kulturpflanzen; Springer Spektrum: Berlin, Germany, 2016. [Google Scholar]
- Neubert, P.; Bergmann, W.; Cerling, V.V.; Gollmick, F.; Hundt, I.; Schurich, W.; Vanselow, G.; Vielemeyer, H.-P. Pflanzenanalyse. In Pflanzendiagnose und Pflanzenanalyse zur Ermittlung von Ernährungsstörungen und des Ernährungszustandes der Kulturpflanzen; Fischer VEB: Jena, Germany, 1976. [Google Scholar]



| Name | Description | Code | |
|---|---|---|---|
| Feedstock Manure (75%, BP1) | Feedstock Energy Crops (BP2) | ||
| Untreated digestate | Untreated digestate from post-fermentation tank | UD1 | UD2 |
| Liquid digestate fraction | Liquid fraction from digestate separated by screw extruder | LD1 | LD2 |
| Solid digestate fraction | Solid fraction from digestate separated by screw extruder | SD1 | SD2 |
| Solid digestate fraction, dried | Solid fraction from digestate separated by screw extruder and dried at 60 °C | SDD1 | SDD2 |
| Code | DM | pH | Corg | Nt | NH4-N | P | K | Ca | Mg | S | Cu | Zn | Cd | Cr | Ni | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | - | g kg−1 | mg kg−1 | |||||||||||||
| UD1 | 7.5 | 8.1 | 392 | 56.7 | 34.0 | 14.7 | 55.1 | 27.6 | 6.8 | 5.3 | 79.9 | 312.3 | 0.3 | 6.4 | 6.5 | 2.2 |
| LD1 | 4.1 | 8.2 | 368 | 66.1 | 45.9 | 9.3 | 177.1 | 11.8 | 1.0 | 6.4 | 50.7 | 214.0 | 0.2 | 6.0 | 8.7 | 1.1 |
| SD1 | 30.6 | 8.3 | 447 | 18.7 | 5.7 | 15.0 | 13.9 | 15.8 | 9.6 | 3.9 | 35.9 | 119.0 | 0.1 | 4.4 | 5.2 | 1.3 |
| SDD1 | 92.3 | 7.3 | 449 | 12.8 | 1.0 | 13.1 | 12.5 | 14.6 | 8.4 | 3.5 | 29.8 | 108.7 | 0.1 | 4.0 | 4.8 | 1.3 |
| UD2 | 7.5 | 7.9 | 427 | 80.1 | 30.4 | 7.9 | 59.2 | 13.1 | 4.4 | 4.4 | 27.2 | 139.3 | 0.4 | 4.5 | 7.0 | 2.7 |
| LD2 | 6.8 | 7.9 | 419 | 70.3 | 33.1 | 8.7 | 67.8 | 15.3 | 4.9 | 4.8 | 25.9 | 152.0 | 0.5 | 5.5 | 9.4 | 3.0 |
| SD2 | 22.4 | 8.3 | 467 | 25.6 | 6.5 | 10.1 | 20.4 | 6.6 | 7.0 | 2.9 | 14.1 | 44.1 | 0.1 | 3.6 | 5.9 | 1.4 |
| SDD2 | 94.2 | 7.3 | 477 | 16.5 | 0.3 | 9.6 | 17.9 | 5.7 | 6.7 | 2.4 | 21.4 | 35.4 | 0.1 | 2.5 | 4.0 | 1.1 |
| Compound | Nitrogen | Phosphorus | Potassium | Magnesium |
|---|---|---|---|---|
| NH4NO3 | Ca(H2PO4)2 | K2SO4 | MgSO4·7H2O | |
| Ammonium Nitrate | Monocalcium Phosphate | Potassium Sulfate | Magnesium Sulfate Heptahydrate | |
| Added N, P, K, Mg, mg kg−1 | 25 | 150 | 200 | 100 |
| Code | Fertilization Level L: 300 mg N kg−1 | Fertilization level H: 500 mg N kg−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Digestate per pot, g | Nt, mg | NH4-N, mg | P, mg | K, mg | Mg, mg | Digestate per pot, g | Nt, mg | NH4-N, mg | P, mg | K, mg | Mg, mg | |
| UD1 | 90 | 407 | 254 | 249 | 228 | 114 | 155 | 684 | 420 | 321 | 248 | 124 |
| LD1 | 129 | 375 | 268 | 199 | 238 | 103 | 223 | 629 | 444 | 235 | 266 | 104 |
| SD1 | 94 | 562 | 189 | 440 | 322 | 239 | 155 | 950 | 307 | 893 | 411 | 340 |
| SDD1 | 66 | 804 | 87 | 946 | 847 | 859 | 113 | 1358 | 131 | 1407 | 1307 | 1400 |
| UD2 | 81 | 512 | 210 | 198 | 227 | 106 | 140 | 866 | 344 | 233 | 247 | 110 |
| LD2 | 91 | 460 | 230 | 204 | 229 | 106 | 173 | 780 | 381 | 243 | 250 | 111 |
| SD2 | 100 | 599 | 171 | 376 | 302 | 133 | 158 | 1018 | 278 | 541 | 377 | 157 |
| SDD2 | 57 | 909 | 43 | 666 | 1050 | 373 | 98 | 1544 | 55 | 1037 | 1661 | 570 |
| Fertilization Level | Code | |||||||
|---|---|---|---|---|---|---|---|---|
| UD1 | LD1 | SD1 | SDD1 | UD2 | LD2 | SD2 | SDD2 | |
| Low (L), % | 54 | 61 | 41 | 15 | 42 | 45 | 12 | 5 |
| High (H), % | 45 | 13 | 46 | 12 | 42 | 47 | 21 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, C.; Seiler-Petzold, J.; Schulz, R.; Müller, J. Short-Term Nitrogen Uptake of Barley from Differently Processed Biogas Digestate in Pot Experiments. Energies 2019, 12, 696. https://doi.org/10.3390/en12040696
Maurer C, Seiler-Petzold J, Schulz R, Müller J. Short-Term Nitrogen Uptake of Barley from Differently Processed Biogas Digestate in Pot Experiments. Energies. 2019; 12(4):696. https://doi.org/10.3390/en12040696
Chicago/Turabian StyleMaurer, Claudia, Julia Seiler-Petzold, Rudolf Schulz, and Joachim Müller. 2019. "Short-Term Nitrogen Uptake of Barley from Differently Processed Biogas Digestate in Pot Experiments" Energies 12, no. 4: 696. https://doi.org/10.3390/en12040696






