Evaluation of Effectiveness of CO2 Sequestration Using Portland Cement in Geological Reservoir Based on Unified Pipe-network Method
Abstract
1. Introduction
2. Methodology
2.1. Description of the Reactive Transport Code
2.2. UPM Model for Solute Transport
2.3. Calculation of Chemical Reaction
3. Validation
3.1. Homogeneous Reaction in Porous Media
3.2. Precipitation Reaction in Fractured Porous Media
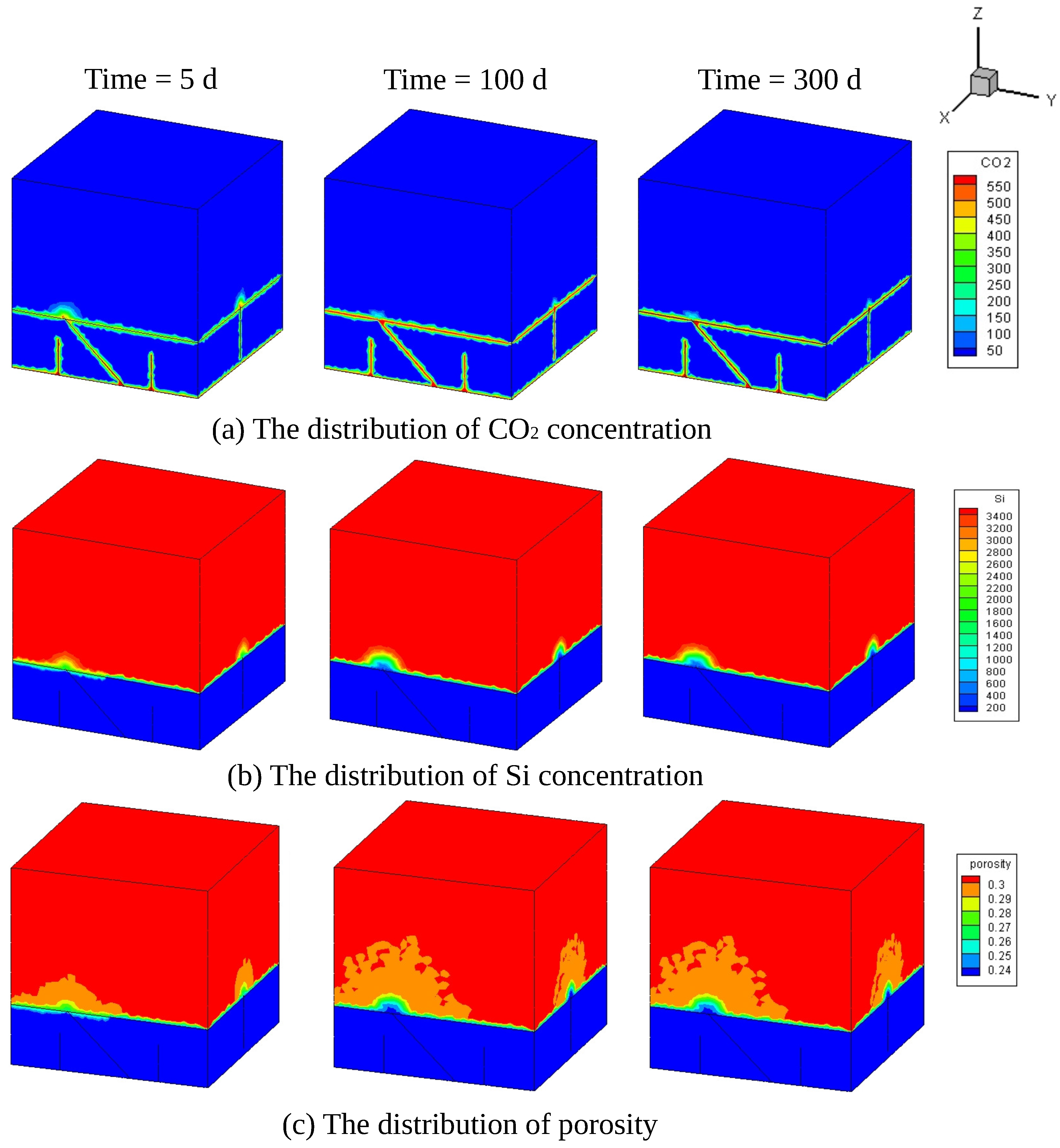
4. Simulation of CO Sequestration in Rock Masses with Fracture Networks
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UPM | Unified Pipe network Method |
Appendix A. The Derivation of the Equivalent Coefficient of 3D Matrix Pipe
Appendix B. The Derivation of the Equivalent Coefficient of 3D Fracture Pipe
References
- Wasch, L.J.; Wollenweber, J.; Neele, F.; Fleury, M. Mitigating CO2 leakage by immobilizing CO2 into solid reaction products. Energy Procedia 2017, 114, 4214–4226. [Google Scholar] [CrossRef]
- Dávila, G.; Cama, J.; Gali, S.; Luquot, L.; Soler, J.M. Efficiency of magnesium hydroxide as engineering seal in the geological sequestration of CO2. Int. J. Greenh. Gas Control 2016, 48, 171–185. [Google Scholar] [CrossRef]
- Dalkhaa, C.; Shevalier, M.; Nightingale, M.; Mayer, B. 2-d reactive transport modeling of the fate of CO2 injected into a saline aquifer in the wabamun lake area, alberta, canada. Appl. Geochem. 2013, 38, 10–23. [Google Scholar] [CrossRef]
- Sahimi, M. Flow and Transport in Porous Media and Fractured Rock: From Classical Methods to Modern Approaches; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Seifert, D.; Engesgaard, P. Sand box experiments with bioclogging of porous media: Hydraulic conductivity reductions. J. Contam. Hydrol. 2012, 136, 1–9. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Dzombak, D.A.; Lowry, G.V.; Thaulow, N. Degradation of well cement by CO2 under geologic sequestration conditions. Environ. Sci. Technol. 2007, 41, 4787–4792. [Google Scholar] [CrossRef]
- Dewaele, P.; Reardon, E.; Dayal, R. Permeability and porosity changes associated with cement grout carbonation. Cement Concr. Res. 1991, 21, 441–454. [Google Scholar] [CrossRef]
- Scherer, G.W.; Celia, M.A.; Prevost, J.-H.; Bachu, S.; Bruant, R.; Duguid, A.; Fuller, R.; Gasda, S.E.; Radonjic, M.; Vichit-Vadakan, W. Leakage of CO2 through abandoned wells: Role of corrosion of cement. In Carbon Dioxide Capture for Storage in Deep Geologic Formations; Elsevier: Amsterdam, The Netherlands, 2015; pp. 827–848. [Google Scholar]
- Bachu, S.; Bennion, D.B. Experimental assessment of brine and/or CO2 leakage through well cements at reservoir conditions. Int. J. Greenh. Gas Control 2009, 3, 494–501. [Google Scholar] [CrossRef]
- Duguid, A. An estimate of the time to degrade the cement sheath in a well exposed to carbonated brine. Energy Procedia 2009, 1, 3181–3188. [Google Scholar] [CrossRef]
- Luquot, L.; Abdoulghafour, H.; Gouze, P. Hydro-dynamically controlled alteration of fractured portland cements flowed by CO2-rich brine. Int. J. Greenh. Gas Control 2013, 16, 167–179. [Google Scholar] [CrossRef]
- Babaei, M.; Sedighi, M. Impact of phase saturation on wormhole formation in rock matrix acidizing. Chem. Eng. Sci. 2018, 177, 39–52. [Google Scholar] [CrossRef]
- Ellery, A.J.; Simpson, M.J. An analytical method to solve a general class of nonlinear reactive transport models. Chem. Eng. J. 2011, 169, 313–318. [Google Scholar] [CrossRef]
- Kim, Y.; Wan, J.; Kneafsey, T.J.; Tokunaga, T.K. Dewetting of silica surfaces upon reactions with supercritical CO2 and brine: Pore-scale studies in micromodels. Environ. Sci. Technol. 2012, 46, 4228–4235. [Google Scholar] [CrossRef]
- Noiriel, C.; Gouze, P.; Bernard, D. Investigation of porosity and permeability effects from microstructure changes during limestone dissolution. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Noiriel, C.; Bernard, D.; Gouze, P.; Thibault, X. Hydraulic properties and microgeometry evolution accompanying limestone dissolution by acidic water. Oil Gas Sci. Technol. 2005, 60, 177–192. [Google Scholar] [CrossRef]
- Li, L.; Peters, C.A.; Celia, M.A. Upscaling geochemical reaction rates using pore-scale network modeling. Adv. Water Resour. 2006, 29, 1351–1370. [Google Scholar] [CrossRef]
- De Anna, P.; Dentz, M.; Tartakovsky, A.; Le Borgne, T. The filamentary structure of mixing fronts and its control on reaction kinetics in porous media flows. Geophys. Res. Lett. 2014, 41, 4586–4593. [Google Scholar] [CrossRef]
- Algive, L.; Bekri, S.; Robin, M.; Vizika, O. Reactive transport: Experiments and pore network modelling. In Proceedings of the International Symposium of the Society of Core Analysts, Calgary, AB, Canada, 10–12 September 2007; pp. 10–12. [Google Scholar]
- Gramling, C.M.; Harvey, C.F.; Meigs, L.C. Reactive transport in porous media: A comparison of model prediction with laboratory visualization. Environ. Sci. Technol. 2002, 36, 2508–2514. [Google Scholar] [CrossRef]
- Raje, D.S.; Kapoor, V. Experimental study of bimolecular reaction kinetics in porous media. Environ. Sci. Technol. 2000, 34, 1234–1239. [Google Scholar] [CrossRef]
- Lagneau, V. Influence des Processus géochimiques sur le Transport en Milieu Poreux–Application au Colmatage de Barrières de Confinement Potentielles Dans un Stockage en Formation Géologique. Ph.D. Thesis, École des Mines de Paris, Paris, France, 2000. [Google Scholar]
- Emmanuel, S.; Berkowitz, B. Mixing-induced precipitation and porosity evolution in porous media. Adv. Water Resour. 2005, 28, 337–344. [Google Scholar] [CrossRef]
- ATartakovsky, M.; Redden, G.; Lichtner, P.C.; Scheibe, T.D.; Meakin, P. Mixing-induced precipitation: Experimental study and multiscale numerical analysis. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Chasset, C.; Jarsjö, J.; Erlström, M.; Cvetkovic, V.; Destouni, G. Scenario simulations of CO2 injection feasibility, plume migration and storage in a saline aquifer, Scania, Sweden. Int. J. Greenh. Gas Control 2011, 5, 1303–1318. [Google Scholar] [CrossRef]
- De Windt, L.; Pellegrini, D.; van Der Lee, J. Coupled modeling of cement/claystone interactions and radionuclide migration. J. Contam. Hydrol. 2004, 68, 165–182. [Google Scholar] [CrossRef]
- Gaucher, E.C.; Blanc, P. Cement/clay interactions—A review: Experiments, natural analogues, and modeling. Waste Manag. 2006, 26, 776–788. [Google Scholar] [CrossRef]
- Kosakowski, G.; Berner, U. The evolution of clay rock/cement interfaces in a cementitious repository for low-and intermediate level radioactive waste. Phys. Chem. Earth Parts A/B/C 2013, 64, 65–86. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K.; Yamamoto, H. Numerical modeling of injection and mineral trapping of CO2 with H2S and SO2 in a sandstone formation. Chem. Geol. 2007, 242, 319–346. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Gaus, I.; Audigane, P.; André, L.; Lions, J.; Jacquemet, N.; Durst, P.; Czernichowski-Lauriol, I.; Azaroual, M. Geochemical and solute transport modelling for CO2 storage, what to expect from it? Int. J. Greenh. Gas Control 2008, 2, 605–625. [Google Scholar] [CrossRef]
- Steefel, C.I.; Lasaga, A.C. A coupled model for transport of multiple chemical species and kinetic precipitation/dissolution reactions with application to reactive flow in single phase hydrothermal systems. Am. J. Sci. 1994, 294, 529–592. [Google Scholar] [CrossRef]
- Pollock, D.W. Semianalytical computation of path lines for finite-difference models. Groundwater 1988, 26, 743–750. [Google Scholar] [CrossRef]
- Voss, C.I. A finite-Element Simulation Model for Saturated-Unsaturated, Fluid-Density-Dependent Ground-Water Flow with Energy Transport or Chemically-Reactive Single-Species Solute Transport; US Geological Survey: Reston, VA, USA, 1984; Volume 84.
- Löhner, R.; Morgan, K.; Peraire, J.; Vahdati, M. Finite element flux-corrected transport (fem–fct) for the euler and navier–stokes equations. Int. J. Numer. Methods Fluids 1987, 7, 1093–1109. [Google Scholar] [CrossRef]
- Trefry, M.G.; Muffels, C. Feflow: A finite-element ground water flow and transport modeling tool. Groundwater 2007, 45, 525–528. [Google Scholar] [CrossRef]
- Diersch, H.-J.G. FEFLOW: Finite Element Modeling of Flow, Mass and Heat Transport in Porous and Fractured Media; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Geiger, S.; Roberts, S.; Matthäi, S.; Zoppou, C.; Burri, A. Combining finite element and finite volume methods for efficient multiphase flow simulations in highly heterogeneous and structurally complex geologic media. Geofluids 2004, 4, 284–299. [Google Scholar] [CrossRef]
- Saripalli, K.P.; Meyer, P.D.; Bacon, D.H.; Freedman, V.L. Changes in hydrologic properties of aquifer media due to chemical reactions: A review. Crit. Rev. Environ. Sci. Technol. 2001, 31, 311–349. [Google Scholar] [CrossRef]
- Luo, H.; Quintard, M.; Debenest, G.; Laouafa, F. Properties of a diffuse interface model based on a porous medium theory for solid–liquid dissolution problems. Comput. Geosci. 2012, 16, 913–932. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, L.; Valocchi, A.J.; Viswanathan, H.S. Pore-scale study of dissolution-induced changes in permeability and porosity of porous media. J. Hydrol. 2014, 517, 1049–1055. [Google Scholar] [CrossRef]
- Kang, Q.; Lichtner, P.C.; Zhang, D. Lattice boltzmann pore-scale model for multicomponent reactive transport in porous media. J. Geophys. Res. Solid Earth 2006, 111. [Google Scholar] [CrossRef]
- Parmigiani, A.; Huber, C.; Bachmann, O.; Chopard, B. Pore-scale mass and reactant transport in multiphase porous media flows. J. Fluid Mech. 2011, 686, 40–76. [Google Scholar] [CrossRef]
- Maloszewski, P.; Zuber, A. Tracer experiments in fractured rocks: Matrix diffusion and the validity of models. Water Resour. Res. 1993, 29, 2723–2735. [Google Scholar] [CrossRef]
- Sudicky, E.; Frind, E. Contaminant transport in fractured porous media: Analytical solutions for a system of parallel fractures. Water Resour. Res. 1982, 18, 1634–1642. [Google Scholar] [CrossRef]
- Bigi, S.; Battaglia, M.; Alemanni, A.; Lombardi, S.; Campana, A.; Borisova, E.; Loizzo, M. CO2 flow through a fractured rock volume: Insights from field data, 3d fractures representation and fluid flow modeling. Int. J. Greenh. Gas Control 2013, 18, 183–199. [Google Scholar] [CrossRef]
- Lee, I.-H.; Ni, C.-F. Fracture-based modeling of complex flow and CO2 migration in three-dimensional fractured rocks. Comput. Geosci. 2015, 81, 64–77. [Google Scholar] [CrossRef]
- Pan, P.-Z.; Rutqvist, J.; Feng, X.-T.; Yan, F. Modeling of caprock discontinuous fracturing during CO2 injection into a deep brine aquifer. Int. J. Greenh. Gas Control 2013, 19, 559–575. [Google Scholar] [CrossRef]
- Ren, F.; Ma, G.; Wang, Y.; Fan, L. Pipe network model for unconfined seepage analysis in fractured rock masses. Int. J. Rock Mech. Min. Sci. 2016, 88, 183–196. [Google Scholar] [CrossRef]
- Ren, F.; Ma, G.; Wang, Y.; Fan, L.; Zhu, H. Two-phase flow pipe network method for simulation of CO2 sequestration in fractured saline aquifers. Int. J. Rock Mech. Min. Sci. 2017, 98, 39–53. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y.; Jin, Y.; Wang, H. Modelling temperature-influenced acidizing process in fractured carbonate rocks. Int. J. Rock Mech. Min. Sci. 2018, 105, 73–84. [Google Scholar] [CrossRef]
- Ma, G.; Li, T.; Wang, Y.; Chen, Y. A semi-continuum model for numerical simulations of mass transport in 3-d fractured rock masses. Rock Mech. Rock Eng. 2019, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Pichler, C.; Yuan, Y.; Zeiml, M.; Lackner, R. Micromechanics-based multifield framework for early-age concrete. Eng. Struct. 2013, 47, 16–24. [Google Scholar] [CrossRef]
- Pesavento, F.; Gawin, D.; Wyrzykowski, M.; Schrefler, B.; Simoni, L. Modeling alkali-silica reaction in non-isothermal, partially saturated cement based materials. Comput. Methods Appl. Mech. Eng. 2012, 225–228, 95–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeiml, M.; Pichler, C.; Lackner, R. Model-based risk assessment of concrete spalling in tunnel linings under fire loading. Eng. Struct. 2014, 77, 207–215. [Google Scholar] [CrossRef]
- Gawin, D.; Pesavento, F.; Schrefler, B. Hygro-thermo-chemo-mechanical modelling of concrete at early ages and beyond. Part I: Hydration and hygro-thermal phenomena. Int. J. Numer. Methods Eng. 2006, 67, 299–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeiml, M.; Maier, M.; Yuan, Y.; Lackner, R. Fast assessing spalling risk of tunnel linings under rabt fire: From a coupled thermo-hydro-chemo-mechanical model towards an estimation method. Eng. Struct. 2017, 142, 1–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Lackner, R.; Zeiml, M.; Mang, H. Strong discontinuity embedded approach with standard SOS formulation: Element formulation, energy-based crack-tracking strategy, and validations. Comput. Methods Appl. Mech. Eng. 2015, 287, 335–366. [Google Scholar] [CrossRef]
- Wu, J.-Y. A unified phase-field theory for the mechanics of damage and quasi-brittle failure. J. Mech. Phys. Solids 2017, 103, 72–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, X. Cracking elements: A self-propagating strong discontinuity embedded approach for quasi-brittle fracture. Finite Elem. Anal. Des. 2018, 144, 84–100. [Google Scholar] [CrossRef]
- Wu, J.-Y. A geometrically regularized gradient-damage model with energetic equivalence. Comput. Methods Appl. Mech. Eng. 2018, 328, 612–637. [Google Scholar] [CrossRef]
- Ren, H.; Zhuang, X.; Cai, Y.; Rabczuk, T. Dual-horizon peridynamics. Int. J. Numer. Methods Eng. 2016, 108, 1451–1476. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, X. Cracking elements method for dynamic brittle fracture. Theor. Appl. Fract. Mech. 2019, 102, 1–9. [Google Scholar] [CrossRef]
- Rabczuk, T.; Ren, H. A peridynamics formulation for quasi-static fracture and contact in rock. Eng. Geol. 2017, 225, 42–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Li, Y.; Zhuang, X. On the crack opening and energy dissipation in a continuum based disconnected crack model. Finite Elem. Anal. Des. 2019. [Google Scholar] [CrossRef]
- Nikolić, M.; Do, X.N.; Ibrahimbegovic, A.; Nikolić, Ž. Crack propagation in dynamics by embedded strong discontinuity approach: Enhanced solid versus discrete lattice model. Comput. Methods Appl. Mech. Eng. 2018, 340, 480–499. [Google Scholar] [CrossRef]
- Haeberlein, F. Time Space Domain Decomposition Methods for Reactive Transport-Application to CO2 Geological Storage. 2011. Available online: https://tel.archives-ouvertes.fr/tel-00634507/file/transparents.pdf (accessed on 20 October 2019).
- Zimmerman, R.W.; Bodvarsson, G.S. Hydraulic conductivity of rock fractures. Transp. Porous Media 1996, 23, 1–30. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Q.; Carey, B.; Tao, W.-Q. Pore-scale study of diffusion–reaction processes involving dissolution and precipitation using the lattice boltzmann method. Int. J. Heat Mass Transf. 2014, 75, 483–496. [Google Scholar] [CrossRef]
- Lasaga, A.C. Chemical kinetics of water-rock interactions. J. Geophys. Res. Solid Earth 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Mineral sequestration of carbon dioxide in a sandstone–shale system. Chem. Geol. 2005, 217, 295–318. [Google Scholar] [CrossRef]
- Liu, M.; Mostaghimi, P. Numerical simulation of fluid-fluid-solid reactions in porous media. Int. J. Heat Mass Transf. 2018, 120, 194–201. [Google Scholar] [CrossRef]
- Ito, T.; Xu, T.; Tanaka, H.; Taniuchi, Y.; Okamoto, A. Possibility to remedy CO2 leakage from geological reservoir using CO2 reactive grout. Int. J. Greenh. Gas Control 2014, 20, 310–323. [Google Scholar] [CrossRef]
- Verma, A.; Pruess, K. Thermohydrological conditions and silica redistribution near high-level nuclear wastes emplaced in saturated geological formations. J. Geophys. Res. Solid Earth 1988, 93, 1159–1173. [Google Scholar] [CrossRef]
- Lichtner, P.C. The quasi-stationary state approximation to coupled mass transport and fluid-rock interaction in a porous medium. Geochim. Cosmochim. Acta 1988, 52, 143–165. [Google Scholar] [CrossRef]
- Ren, F.; Ma, G.; Fu, G.; Zhang, K. Investigation of the permeability anisotropy of 2d fractured rock masses. Eng. Geol. 2015, 196, 171–182. [Google Scholar] [CrossRef]
- Ren, F.; Ma, G.; Wang, Y.; Li, T.; Zhu, H. Unified pipe network method for simulation of water flow in fractured porous rock. J. Hydrol. 2017, 547, 80–96. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Han, W.; Ma, G.; Zhang, Y. Simulating the filtration effects of cement-grout in fractured porous media with the 3d unified pipe-network method. Processes 2019, 7, 46. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Liu, R.; Xu, Z.; Li, S.; Zhang, Y. Transient analysis of grout penetration with time-dependent viscosity inside 3d fractured rock mass by unified pipe-network method. Water 2018, 10, 1122. [Google Scholar] [CrossRef]
- Poskozim, P.S. General chemistry, principles and modern applications, (petrucci, ralph h.; harwood, william s.) and general chemistry, (whitten, kenneth w.; davis, raymond e.; peck, m. larry). J. Chem. Educ. 1997. [Google Scholar] [CrossRef]
- Alhashmi, Z.; Blunt, M.; Bijeljic, B. Predictions of dynamic changes in reaction rates as a consequence of incomplete mixing using pore scale reactive transport modeling on images of porous media. J. Contam. Hydrol. 2015, 179, 171–181. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous nacl solutions from 273 to 533 k and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, G.; Ren, F.; Li, T. A constrained delaunay discretization method for adaptively meshing highly discontinuous geological media. Comput. Geosci. 2017, 109, 134–148. [Google Scholar] [CrossRef]


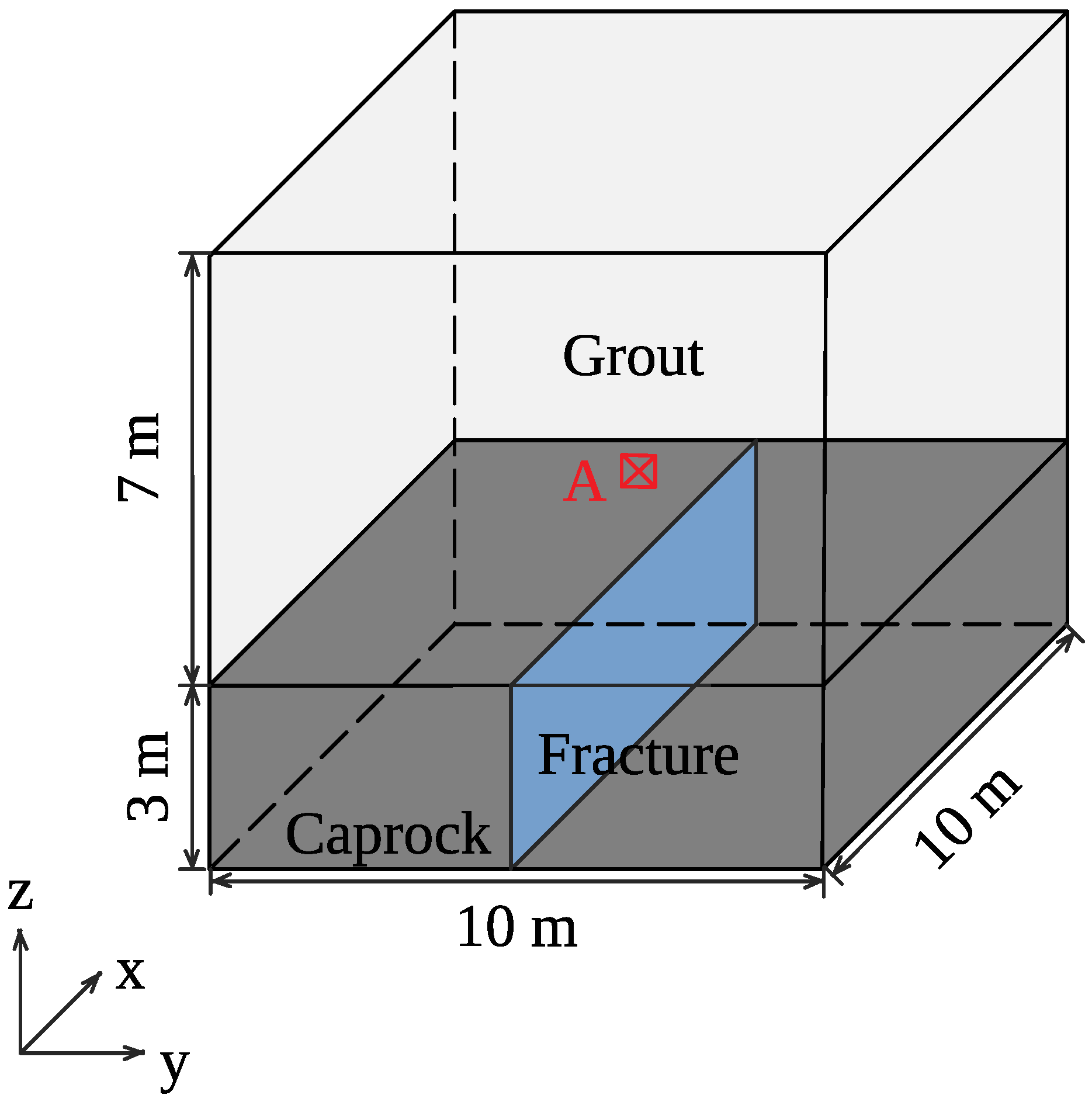

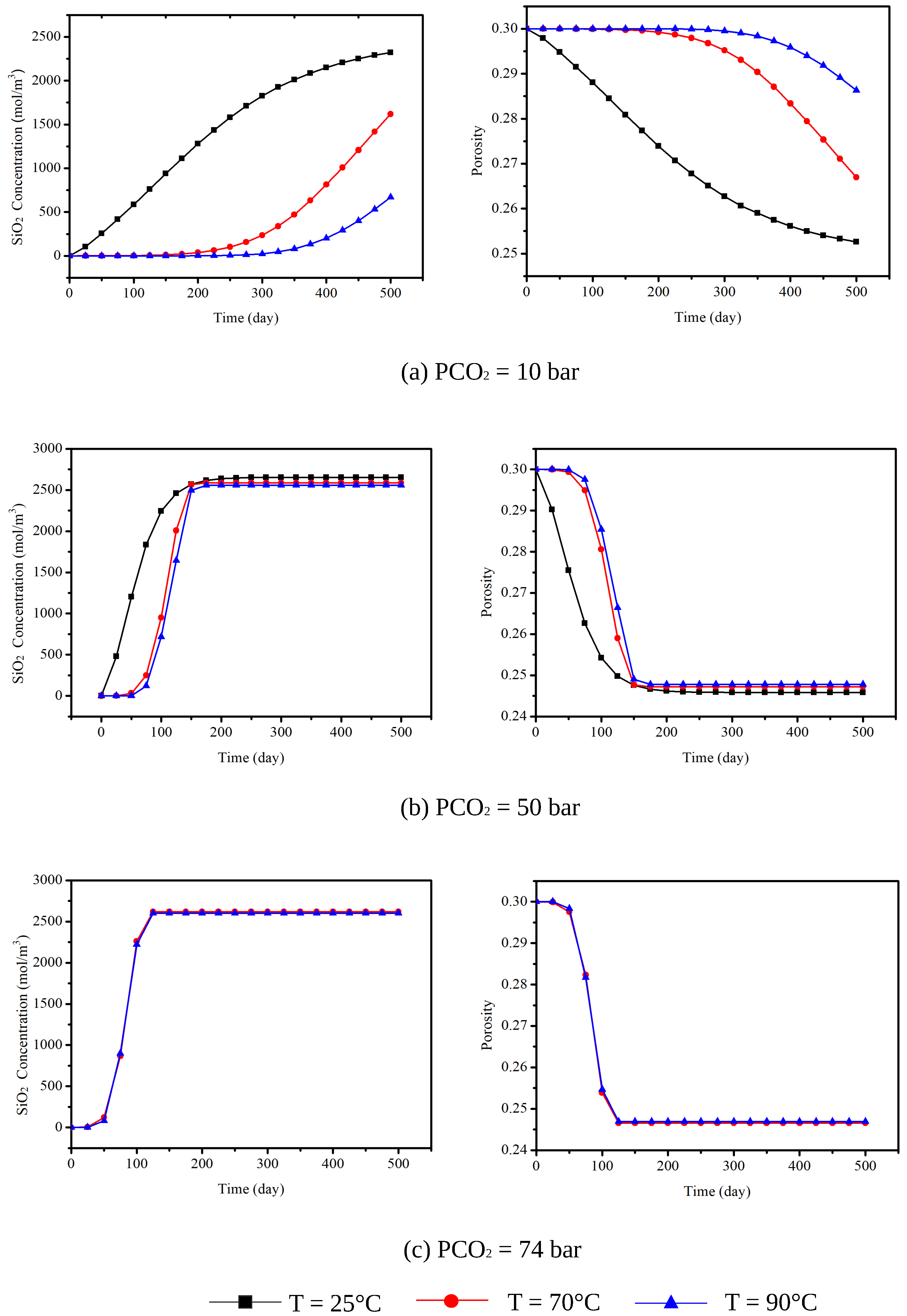
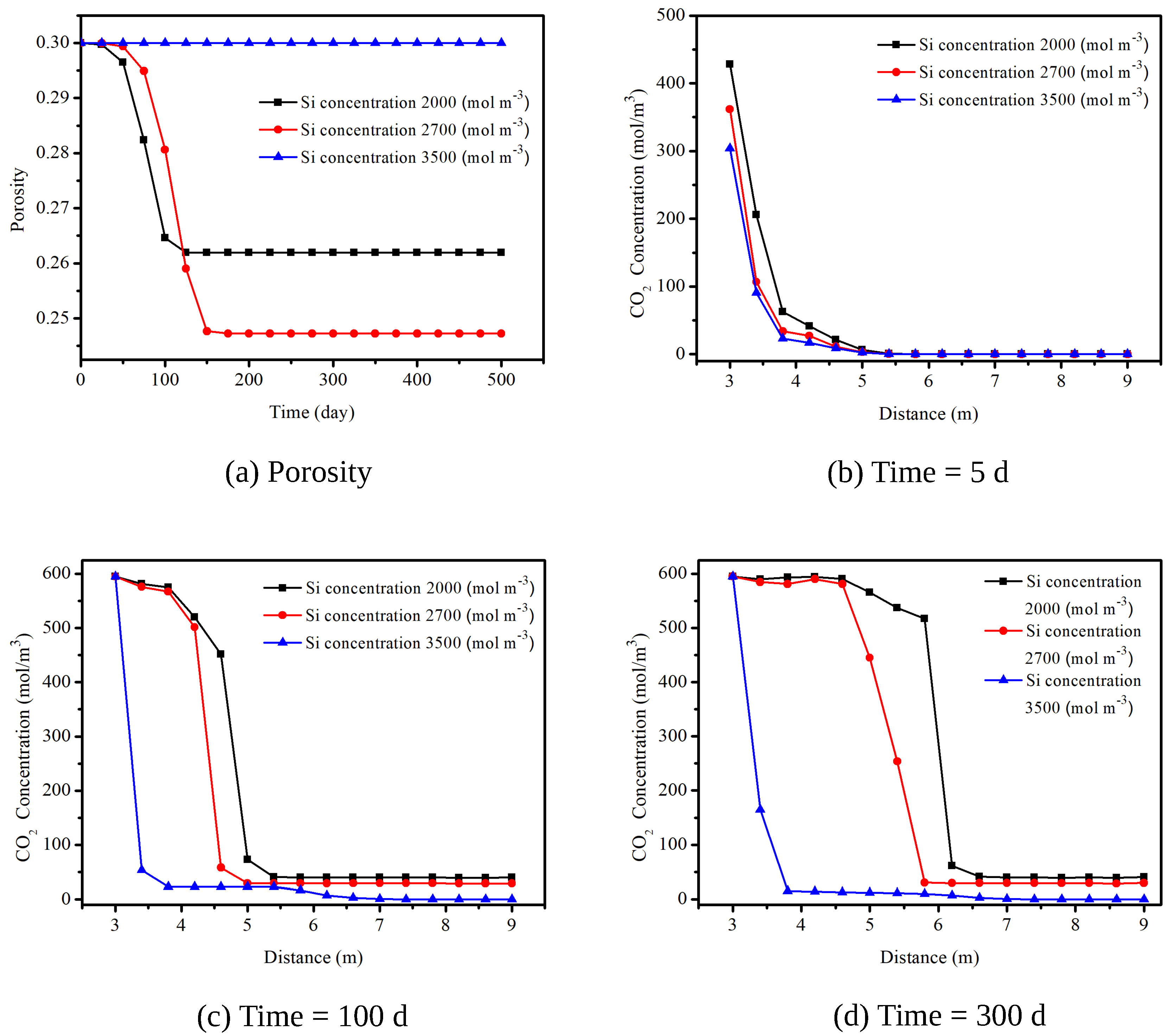



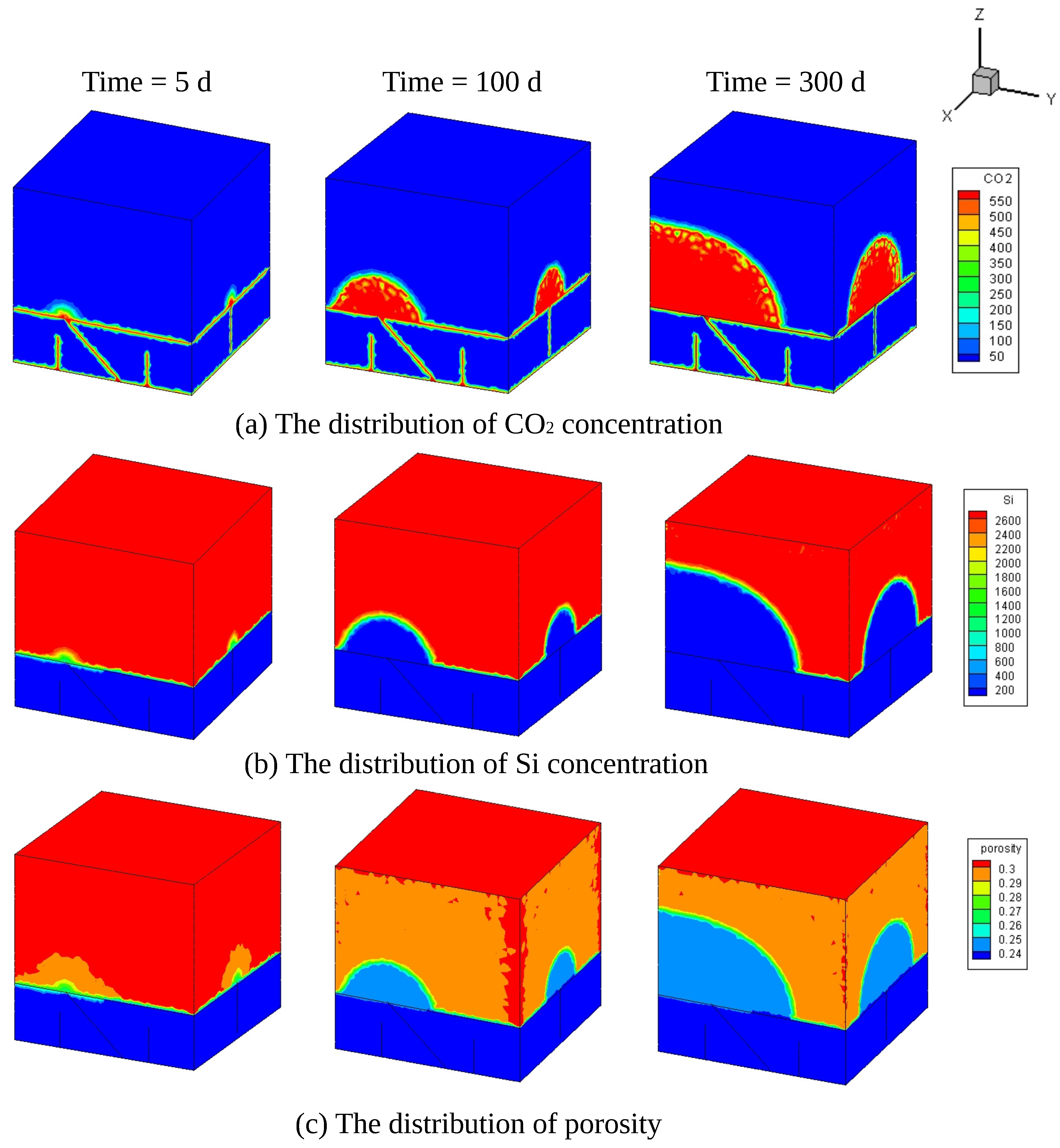


| Parameters | Symbol | Unit | Value | Reference |
|---|---|---|---|---|
| Acid fluid viscosity | Ito et al. [73] | |||
| Acid fluid density | 1000 | Ito et al. [73] | ||
| Initial porosity of aquifer | Ito et al. [73] | |||
| Intrinsic permeability of aquifer | 4 × | Ito et al. [73] | ||
| Initial porosity of caprock | Ito et al. [73] | |||
| Intrinsic permeability of caprock | 0 | Ito et al. [73] | ||
| Molecular diffusion-dispersion coefficient | Dvila et al. [2] | |||
| Reaction rate constant at 25 C | Ito et al. [73] | |||
| Activation energy | E | Ito et al. [73] | ||
| Gas constant | R | Ito et al. [73] | ||
| Reaction equilibrium constant | Chen et al. [69] | |||
| Precipitation growth threshold | Chen et al. [69] | |||
| Initial specific reactive surface area | A | Ito et al. [73] | ||
| Power law exponent for permeability | n | 2 | Ito et al. [73] | |
| Critical porosity | Ito et al. [73] | |||
| Fracture aperture | a | m | ||
| Fracture porosity | 1 | |||
| Fracture diffusivity |
| Temperature (C) | P = 10 bar | P = 50 bar | P = 74 bar |
|---|---|---|---|
| 25 | |||
| 70 | |||
| 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Sun, Z.; Li, S.; Yang, W.; Zhang, Y. Evaluation of Effectiveness of CO2 Sequestration Using Portland Cement in Geological Reservoir Based on Unified Pipe-network Method. Energies 2020, 13, 387. https://doi.org/10.3390/en13020387
Yan X, Sun Z, Li S, Yang W, Zhang Y. Evaluation of Effectiveness of CO2 Sequestration Using Portland Cement in Geological Reservoir Based on Unified Pipe-network Method. Energies. 2020; 13(2):387. https://doi.org/10.3390/en13020387
Chicago/Turabian StyleYan, Xiao, Zizheng Sun, Shucai Li, Weimin Yang, and Yiming Zhang. 2020. "Evaluation of Effectiveness of CO2 Sequestration Using Portland Cement in Geological Reservoir Based on Unified Pipe-network Method" Energies 13, no. 2: 387. https://doi.org/10.3390/en13020387
APA StyleYan, X., Sun, Z., Li, S., Yang, W., & Zhang, Y. (2020). Evaluation of Effectiveness of CO2 Sequestration Using Portland Cement in Geological Reservoir Based on Unified Pipe-network Method. Energies, 13(2), 387. https://doi.org/10.3390/en13020387







