The Influence of Cerium on the Hydrogen Storage Properties of La1-xCexNi5 Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
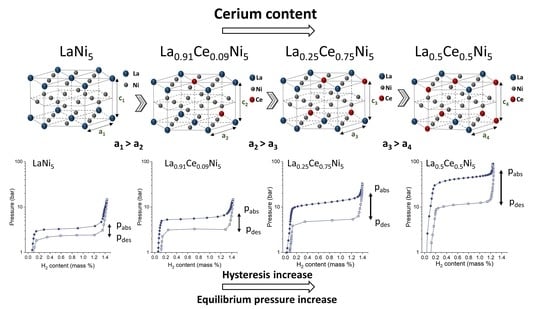
3.1. Structural and Phase Analysis by X-ray Diffraction
3.2. Hydrogen Absorption and Desorption PCT Isotherms
3.3. Microstructure and Chemical Compositions
3.4. Microhardness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hirscher, M. Handbook of Hydrogen Storage: New Materials for Future Energy Storage; WILEY-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Introduction. In Nanomaterials for Solid State Hydrogen Storage; Springer: Boston, MA, USA, 2009; pp. 1–81. [Google Scholar]
- Ellinger, F.H.; Holley, C.E.J.; McInteer, B.B.; Pavone, D.; Potter, R.M.; Staritzky, E.; Zachariasen, W.H. The Preparation and Some Properties of Magnesium Hydride. J. Am. Chem. Soc. 1955, 77, 2647–2648. [Google Scholar] [CrossRef]
- Kennelley, J.A.; Varwig, J.W.; Myers, H.W. Magnesium-hydrogen relationships. J. Phys. Chem. 1960, 64, 703–704. [Google Scholar] [CrossRef]
- Stampfer, J.F.J.; Holley, C.E.J.; Suttle, J.F. The magnesium-hydrogen system. J. Am. Chem. Soc. 1960, 82, 3504–3508. [Google Scholar] [CrossRef] [Green Version]
- Reilly, J.J.; Wiswall, R.H.J. Iron Titanium Hydride: Its Formation, Properties and Application; Laboratory, B.N., Ed.; Brookheaven National Laboratory: New York, NY, USA, 1973. [Google Scholar]
- Reilly, J.J.; Wiswall, R.H. Formation and properties of iron titanium hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Van Vucht, J.H.N.; Kuijpers, F.A.; Bruning, H.C.A.M. Reversible room-temperature absorption of large quantities of hydrogen by intermetallic compounds. Phillips Res. Rep. 1970, 25, 133–140. [Google Scholar]
- Zjilstra, H.; Westendorp, F.F. Influence of hydrogen on the magnetic properties of SmCo5. Solid State Commun. 1969, 7, 857–859. [Google Scholar] [CrossRef]
- Boser, O. Hydrogen sorption in LaNi5. J. Less Common Met. 1976, 46, 91–99. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Van Mal, H.H. Phase relations and hydrogen absorption in the lanthanum-nickel system. J. Less Common Met. 1972, 29, 203–210. [Google Scholar] [CrossRef]
- Van Mal, H.H.; Buschow, K.H.J.; Miedema, A.R. Hydrogen absorption in in LaNi5 and related compounds: Experimental observations and their explanation. J. Less Common Met. 1974, 35, 65–76. [Google Scholar] [CrossRef]
- Tanaka, S.; Flanagan, T.B. Thermodynamics of the solution of hydrogen in LaNi5 at small hydrogen LaNi5 at small hydrogen contents. J. Less Common Met. 1977, 51, 79–91. [Google Scholar] [CrossRef]
- Dayan, D.; Mintz, M.H.; Dariel, M.P. Hysteresis effects in cerium-containing LaNi5-type compounds. J. Less Common Met. 1980, 73, 15–24. [Google Scholar] [CrossRef]
- Meyer-Liautaud, F.; Pasturel, A.; Allibert, C.H.; Colinet, C. Thermodynamic study of the valence state of cerium and hydrogen storage in Ce(Ni1−xCux)5 compounds. J. Less Common Met. 1985, 110, 119–126. [Google Scholar] [CrossRef]
- Clay, K.R.; Goudy, A.J.; Schweibenz, R.G.; Zarynow, A. The effect of the partial replacement of lanthanum in LaNi5-H with cerium, praseodymium, and neodymium on absorption and desorption kinetics. J. Less Common Met. 1990, 166, 153–162. [Google Scholar] [CrossRef]
- Pandey, S.K.; Srivastava, A.; Srivastava, O.N. Improvement in hydrogen storage capacity in LaNi5 through substitution of Ni by Fe. Int. J. Hydrog. Energy 2007, 32, 2461–2465. [Google Scholar] [CrossRef]
- Rożdżyńska-Kiełbik, B.; Iwasieczko, W.; Drulis, H.; Pavlyuk, V.V.; Bala, H. Hydrogenation equilibria characteristics of LaNi5−xZnx intermetallics. J. Alloys Compd. 2000, 298, 237–243. [Google Scholar] [CrossRef]
- Drašner, A.; Blažina, Ž.Ž. Hydrogen sorption properties of the LaNi5−xGax alloys. J. Alloys Compd. 2003, 359, 180–185. [Google Scholar] [CrossRef]
- Gondek, Ł.; Selvaraj, N.B.; Czub, J.; Figiel, H.; Chapelle, D.; Kardjilov, N.; Hilger, A.; Manke, I. Imaging of an operating LaNi4.8Al0.2–based hydrogen storage container. Int. J. Hydrog. Energy 2011, 36, 9751–9757. [Google Scholar] [CrossRef]
- Guzik, M.N.; Lang, J.; Huot, J.; Sartori, S. Effect of Al presence and synthesis method on phase composition of the hydrogen absorbing La–Mg–Ni-based compounds. Int. J. Hydrog. Energy 2017, 42, 30135–30144. [Google Scholar] [CrossRef] [Green Version]
- Ngameni, R.; Mbemba, N.; Grigoriev, S.A.; Millet, P. Comparative analysis of the hydriding kinetics of LaNi5, La0.8Nd0.2Ni5 and La0.7Ce0.3Ni5 compounds. Int. J. Hydrog. Energy 2011, 36, 4178–4184. [Google Scholar] [CrossRef]
- Van Mal, H.H.; Buschow, K.H.J.; Kuijpers, F.A. Hydrogen absorption and magnetic properties of LaCo5xNi5−5x compounds. J. Less Common Met. 1973, 32, 289–296. [Google Scholar] [CrossRef]
- Borzone, E.M.; Blanco, M.V.; Baruj, A.; Meyer, G.O. Stability of LaNi5−xSnx cycled in hydrogen. Int. J. Hydrog. Energy 2014, 39, 8791–8796. [Google Scholar] [CrossRef]
- Han, J.I.; Lee, J.-Y. Influence of oxygen impurity on the hydrogenation properties of LaNi5, LaNi4.7Al0.3 and MmNi4.5Al0.5 during long-term pressure-induced hydriding-dehydriding cycling. J. Less Common Met. 1989, 152, 329–338. [Google Scholar] [CrossRef]
- Tousignant, M.; Huot, J. Hydrogen sorption enhancement in cold rolled LaNi5. J. Alloys Compd. 2014, 595, 22–27. [Google Scholar] [CrossRef]
- Simičić, M.V.; Zdujić, M.; Jelovac, D.M.; Rakin, P.M. Hydrogen storage material based on LaNi5 alloy produced by mechanical alloying. J. Power Sources 2001, 92, 250–254. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, H.; Xiong, J.; Pan, S.; Luo, J. Microstructure, electromagnetic and microwave absorbing properties of plate-like LaCeNi powder. J. Mater. Sci. Mater. Electron. 2018, 29, 18030–18035. [Google Scholar] [CrossRef]
- Odysseos, M.; De Rango, P.; Christodoulou, C.N.; Hlil, E.K.; Steriotis, T.; Karagiorgis, G.; Charalambopoulou, G.; Papapanagiotou, T.; Ampoumogli, A.; Psycharis, V.; et al. The effect of compositional changes on the structural and hydrogen storage properties of (La–Ce)Ni5 type intermetallics towards compounds suitable for metal hydride hydrogen compression. J. Alloys Compd. 2013, 580, S268–S270. [Google Scholar] [CrossRef]
- Uchida, H.; Masayoshi, T.; Huang, Y.C. The influence of cerium, praseodymium, neodymium and samarium on hydrogen absorption in LaNi5 alloys. J. Less Common Met. 1982, 88, 81–87. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, Y.; Li, Y.; Shao, H. The performances of La1−xCexNi5 (0≤x≤1) hydrogen storage alloys studied by powder microelectrode. J. Alloys Compd. 2008, 453, 79–86. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.-S.; Ma, Z.-F.; Xu, N. Characteristics of LaNi5-based hydrogen storage alloys modified by partial substituting La for Ce. J. Alloys Compd. 2003, 359, 300–306. [Google Scholar] [CrossRef]
- Cao, X.X.; Yang, F.S.; Wu, Z.; Wang, Y.Q.; Zhang, Z.X. Experimental Study on the Hydrogen Storage Properties of LaNi5 Alloy in Repeated Hydriding/Dehydriding Cycles. Adv. Mater. Res. 2013, 815, 25–30. [Google Scholar] [CrossRef]
- An, X.H.; Gu, Q.F.; Zhang, J.Y.; Chen, S.L.; Yu, X.B.; Li, Q. Experimental investigation and thermodynamic reassessment of La–Ni and LaNi5–H systems. Calphad 2013, 40, 48–55. [Google Scholar] [CrossRef]
- Quian, S.; Northwood, D.O. Hysteresis in metal-hydrogen systems: A critical review of the experimental observations and theoretical models. Int. J. Hydrog. Energy 1988, 13, 25–35. [Google Scholar] [CrossRef]
- Quian, S.; Northwood, D.O. Elastic and plastic accommodation effects on hysteresis during hydride formation and decomposition. Int. J. Hydrog. Energy 1990, 15, 649–654. [Google Scholar] [CrossRef]
- Panas, A.J.; Fikus, B.; Płatek, P.; Kunce, I.; Witek, K.; Kuziora, P.; Olejarczyk, A.; Dyjak, S.; Michalska-Domańska, M.; Jaroszewicz, L.; et al. Pressurised-cell test stand with oscillating heating for investigation heat transfer phenomena in metal hydride beds. Int. J. Hydrog. Energy 2016, 41, 16974–16983. [Google Scholar] [CrossRef]









| Sample La1-xCexNi5 | Parameter a (Å) | Parameter c (Å) | Volume (Å3) | Relative Phase Content (%) | Other Phases Present |
|---|---|---|---|---|---|
| LaNi5 | 5.017 | 3.977 | 86.724 | 100 | none |
| La0.91Ce0.09Ni5 | 5.005 | 3.982 | 86.424 | 100 | none |
| La0.75Ce0.25Ni5 | 4.988 | 3.988 | 85.944 | 100 | none |
| La0.5Ce0.5Ni5 | 4.954 | 3.992 | 84.869 | 90.3 ± 1.5 | La2NiO4, X-unidentified phase |
| Sample La1-xCexNi5 | Peq_abs 35 °C (bar) | Peq_abs 40 °C (bar) | Peq_abs 45 °C (bar) | Peq_des 35 °C (bar) | Peq_des 40 °C (bar) | Peq_des 45 °C (bar) |
|---|---|---|---|---|---|---|
| LaNi5 | 3.35 | 4.09 | 4.79 | 2.36 | 2.91 | 3.59 |
| La0.91Ce0.09Ni5 | 5.56 | 6.70 | 7.92 | 3.29 | 3.83 | 4.85 |
| La0.75Ce0.25Ni5 | 12.44 | 14.66 | 17.30 | 5.01 | 5.87 | 7.22 |
| La0.5Ce0.5Ni5 | 43.21 | 48.74 | 56.88 | 11.52 | 14.26 | 17.54 |
| Sample La1-xCexNi5 | ΔHabs (kJ·mol−1·H2) | ΔHdes (kJ·mol−1·H2) | ΔSabs (J·mol−1·K−1·H2) | ΔSdes (J·mol−1·K−1·H2) |
|---|---|---|---|---|
| LaNi5 | −29.08 | 34.09 | −104.47 | 117.78 |
| La0.91Ce0.09Ni5 | −28.79 | 31.69 | −107.73 | 112.65 |
| La0.75Ce0.25Ni5 | −26.84 | 29.74 | −108.08 | 109.84 |
| La0.5Ce0.5Ni5 | −22.37 | 34.29 | −103.87 | 131.60 |
| Sample La1-xCexNi5 | HV 0.05 (Vickers Hardness) |
|---|---|
| LaNi5 | 631 ± 23 |
| La0.91Ce0.09Ni5 | 661 ± 18 |
| La0.75Ce0.25Ni5 | 703 ± 19 |
| La0.5Ce0.5Ni5 | 681 ± 44; 582 ± 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pęska, M.; Dworecka-Wójcik, J.; Płociński, T.; Polański, M. The Influence of Cerium on the Hydrogen Storage Properties of La1-xCexNi5 Alloys. Energies 2020, 13, 1437. https://doi.org/10.3390/en13061437
Pęska M, Dworecka-Wójcik J, Płociński T, Polański M. The Influence of Cerium on the Hydrogen Storage Properties of La1-xCexNi5 Alloys. Energies. 2020; 13(6):1437. https://doi.org/10.3390/en13061437
Chicago/Turabian StylePęska, Magda, Julita Dworecka-Wójcik, Tomasz Płociński, and Marek Polański. 2020. "The Influence of Cerium on the Hydrogen Storage Properties of La1-xCexNi5 Alloys" Energies 13, no. 6: 1437. https://doi.org/10.3390/en13061437