Experimental Study of a Passive Thermal Management System Using Expanded Graphite/Polyethylene Glycol Composite for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. Preparation of Composite PCMs
- (1)
- First, PEG1500 was heated in a water bath at 70 °C until complete melting of all samples occurred, and then it was maintained at an elevated temperature to ensure the PEG1500 remained in a molten state.
- (2)
- Subsequently, EG was measured using an electronic balance, transferred into a beaker, and the weight in grams accurately recorded.
- (3)
- The molten PEG1500 and EG were combined using a magnetic stirrer and agitated at 800 RPM for an additional half hour, followed by a resting period of one hour.
- (4)
- Following a period of rest, the specimen was subsequently placed in a drying oven and subjected to desiccation at 70 °C for a duration of 6 h.
- (5)
- After the completion of the drying process, the sample was removed.
- (1)
- The batteries used in this study were cylindrical lithium-ion batteries (Sony VTC6, dimensions: diameter = 18 mm, height = 65 mm), and their real capacity was nearly 2600 mAh, which was used to calculate the C-rate;
- (2)
- Battery test equipment (AODAN CD1810U5, China);
- (3)
- A data logger (Keysight, 34970A, USA);
- (4)
- A temperature chamber (GZP 360BE, China).
2.2.2. Battery Pack Production
2.3. PCM-1 Performance Test
2.4. Battery Performance Test
2.5. Uncertainty Analysis
3. Results and Discussion
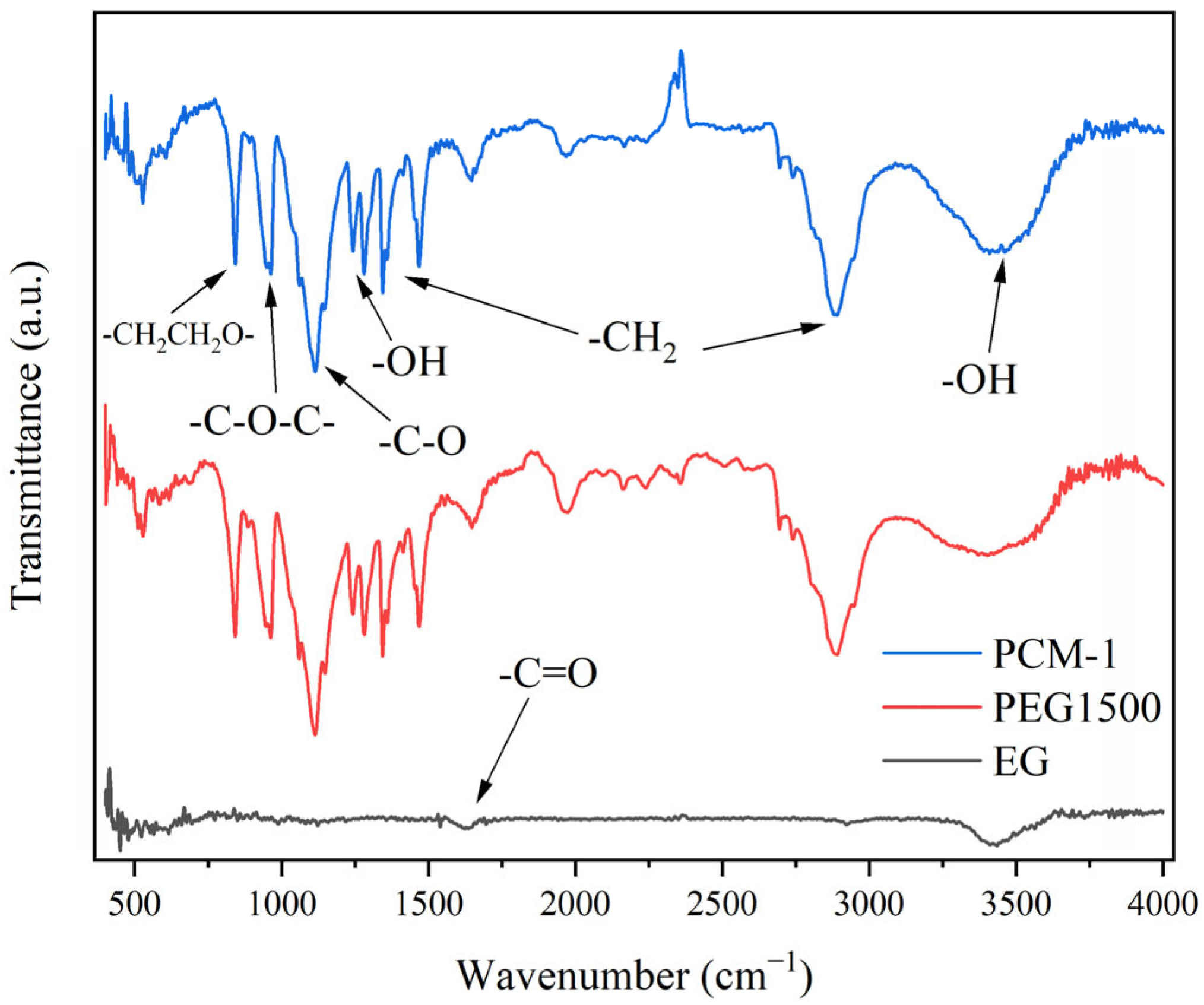
3.1. FT-IR Analysis of CPCMs
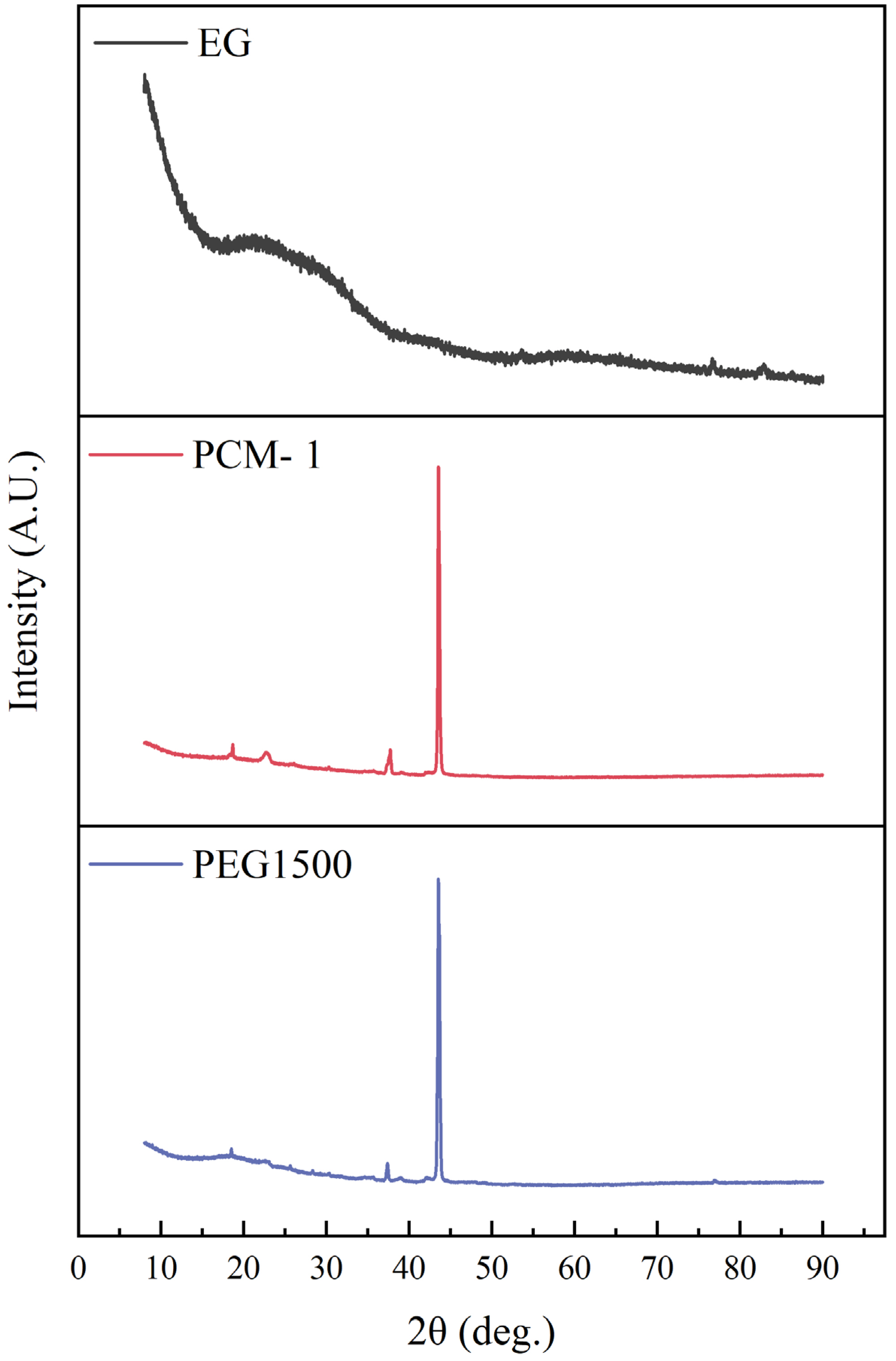
3.2. XRD Analysis of CPCMs
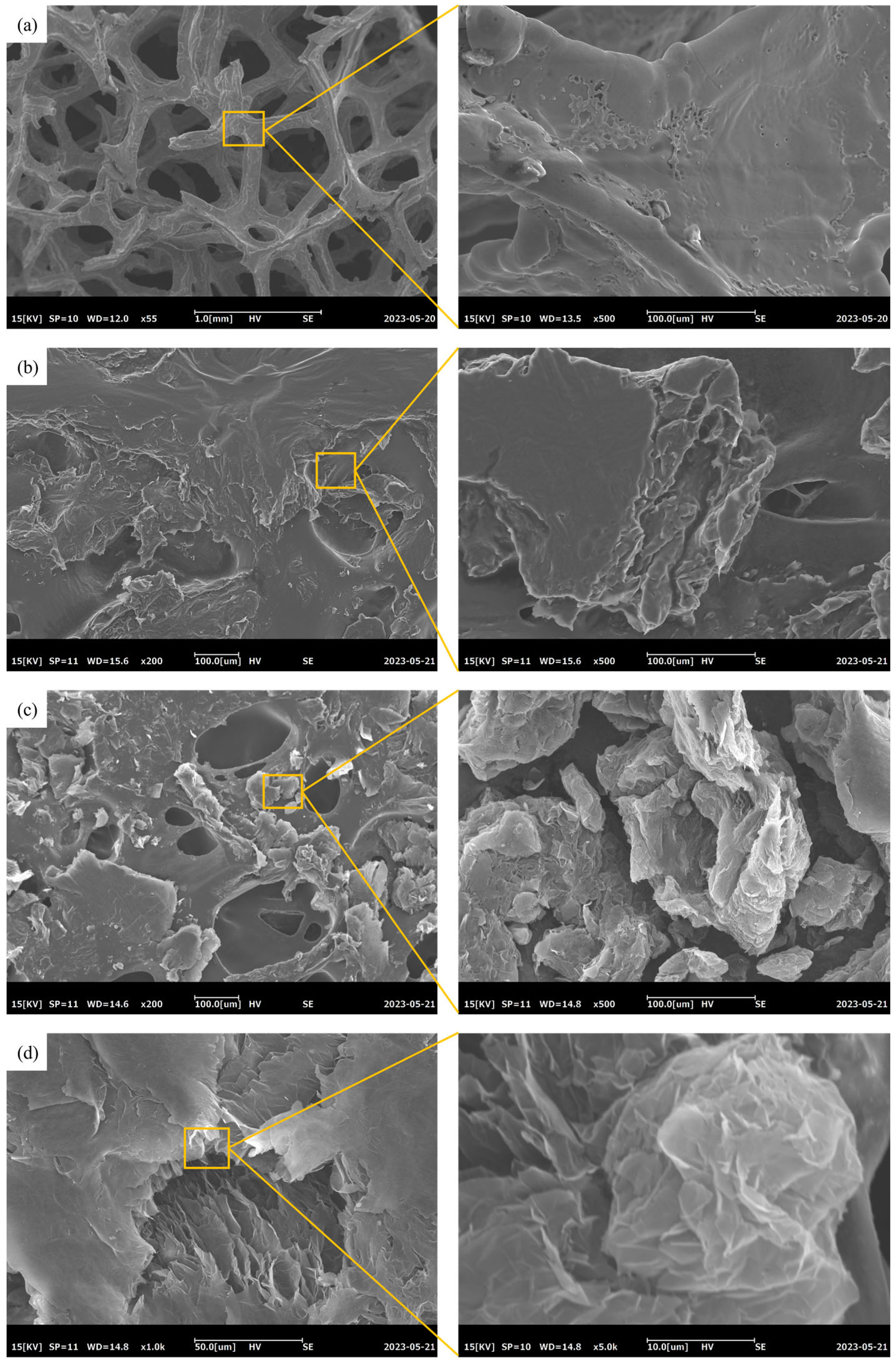
3.3. SEM Analysis of CPCMs
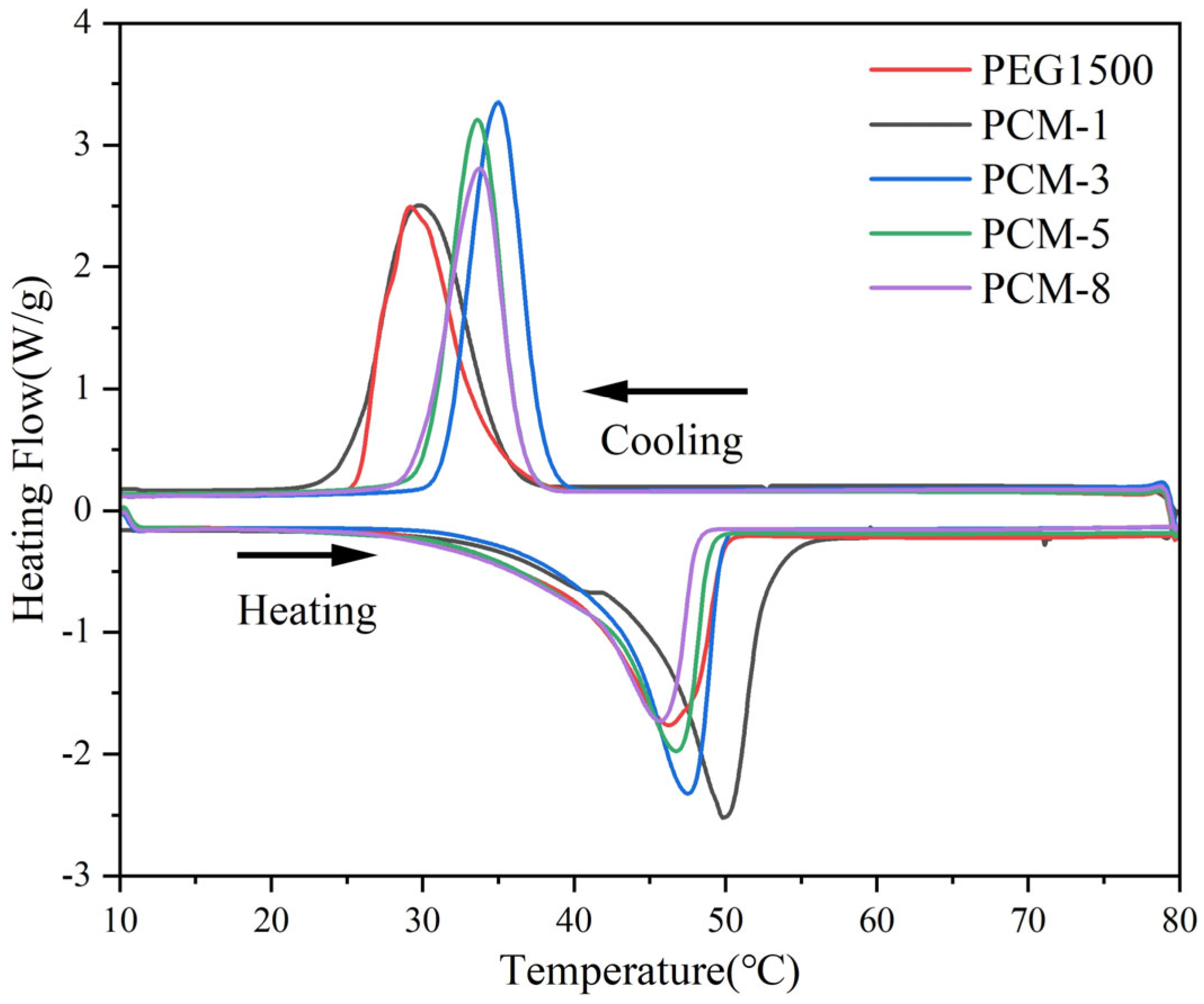
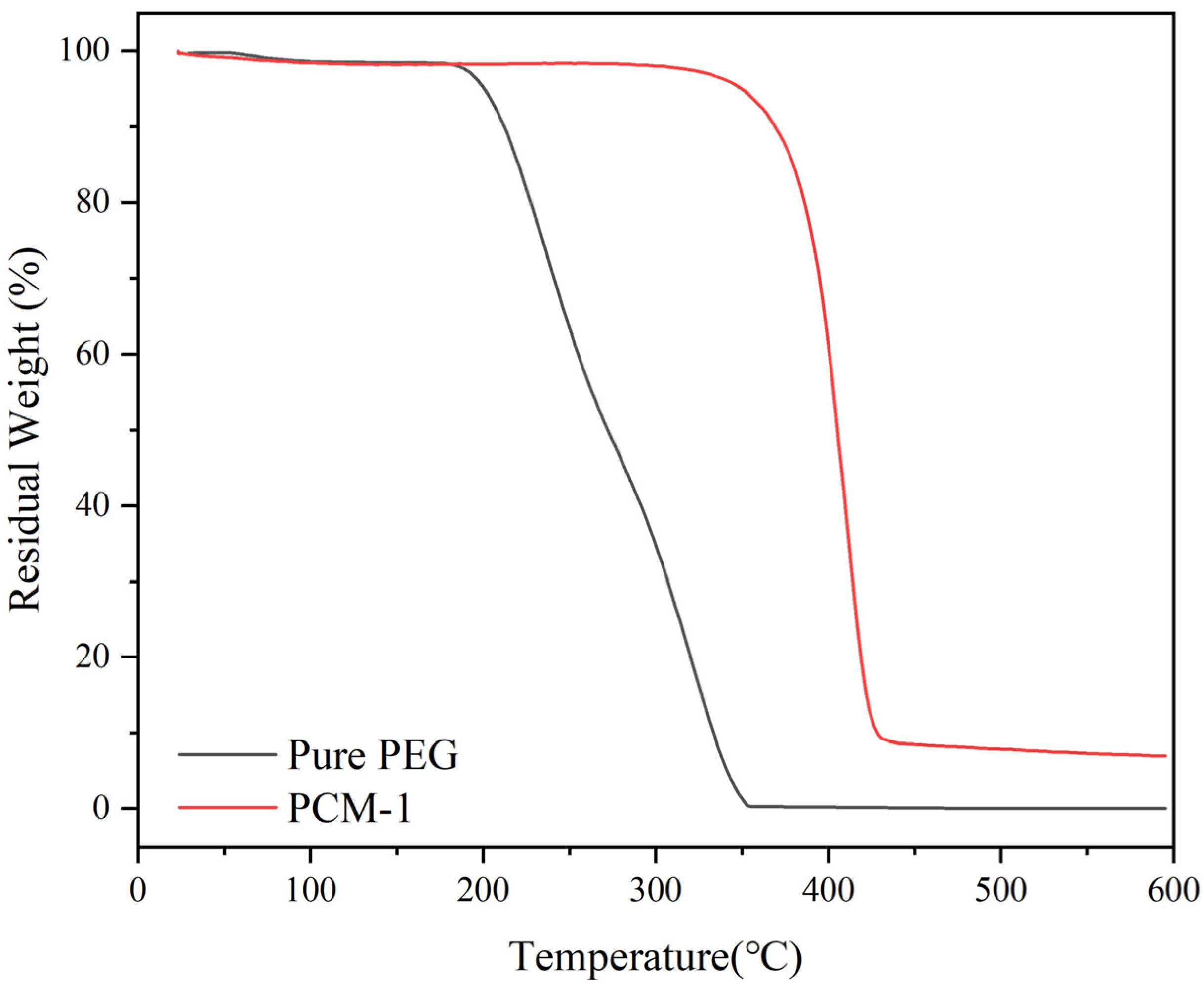
3.4. Thermal Analysis of CPCMs
4. Battery Heat Production Analysis
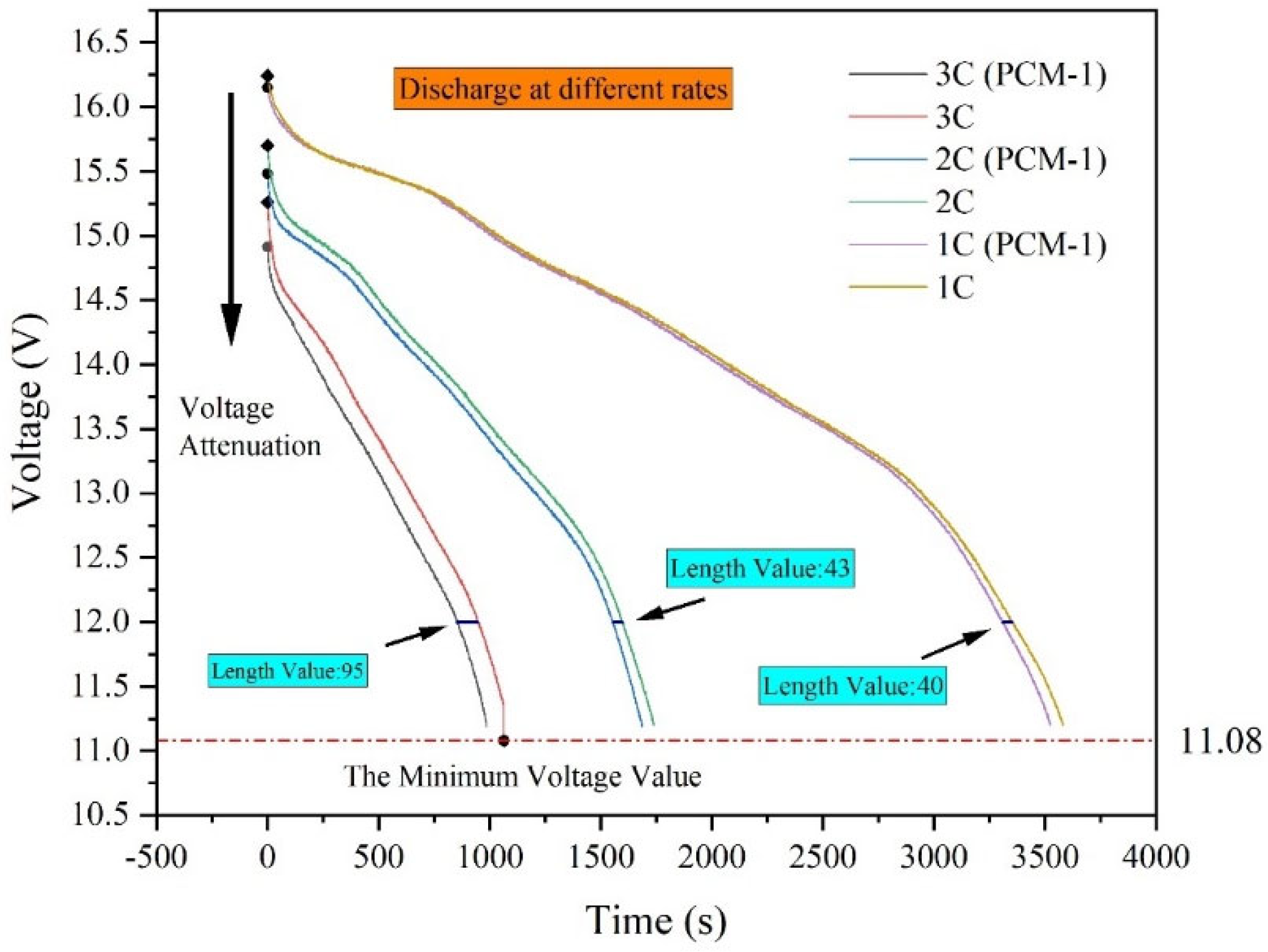
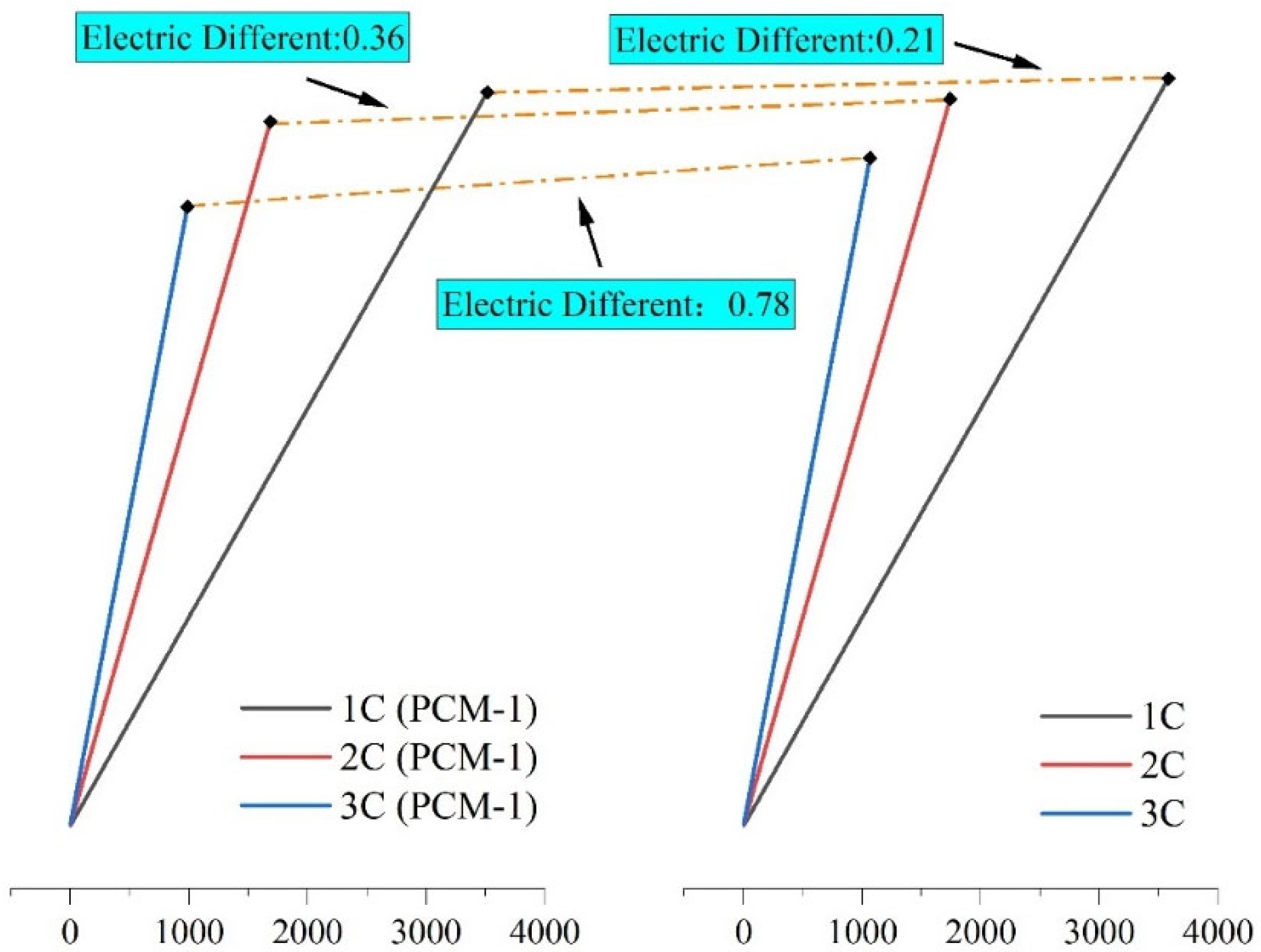
4.1. Battery Performance Changes at Different Rates
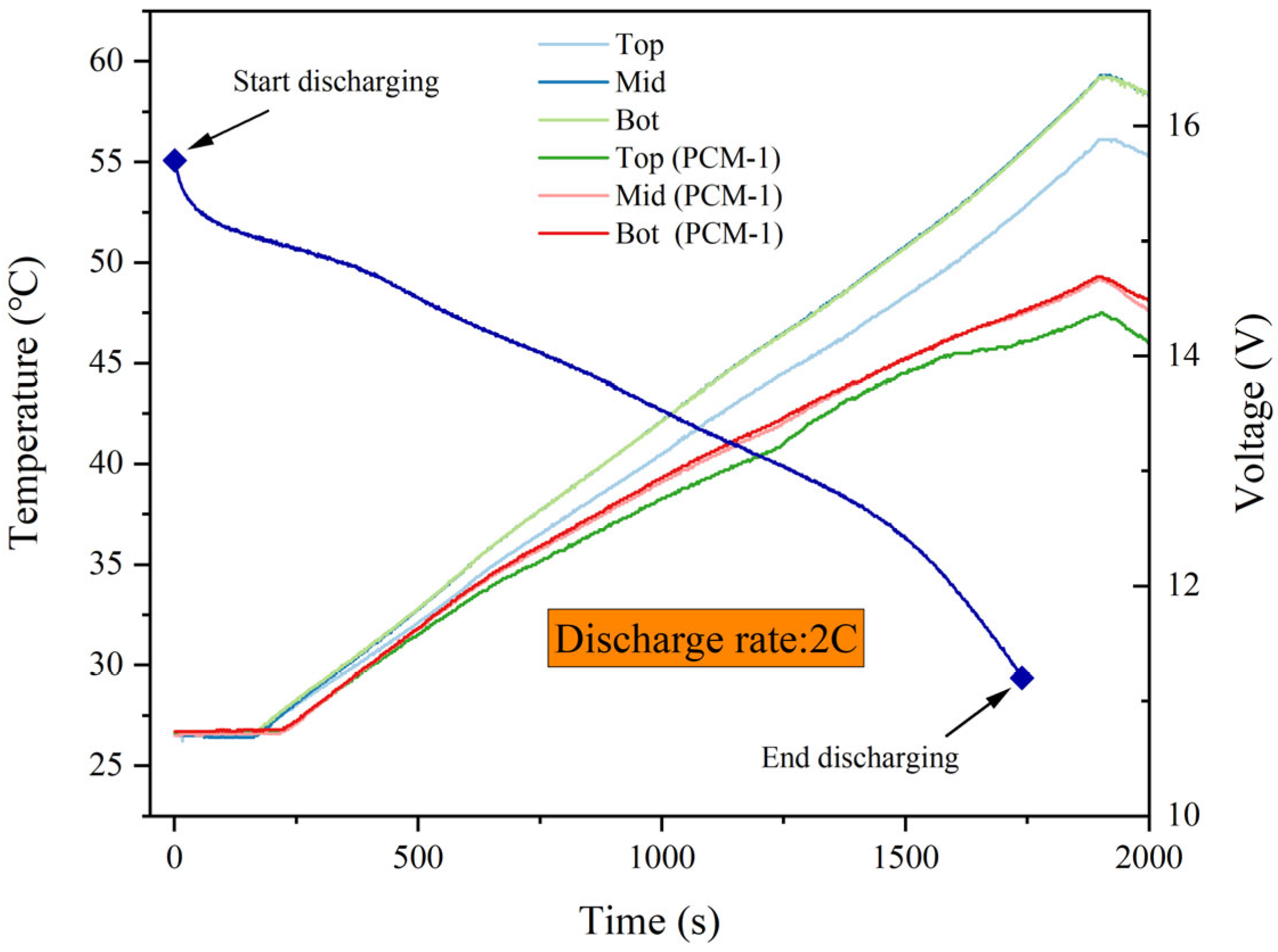
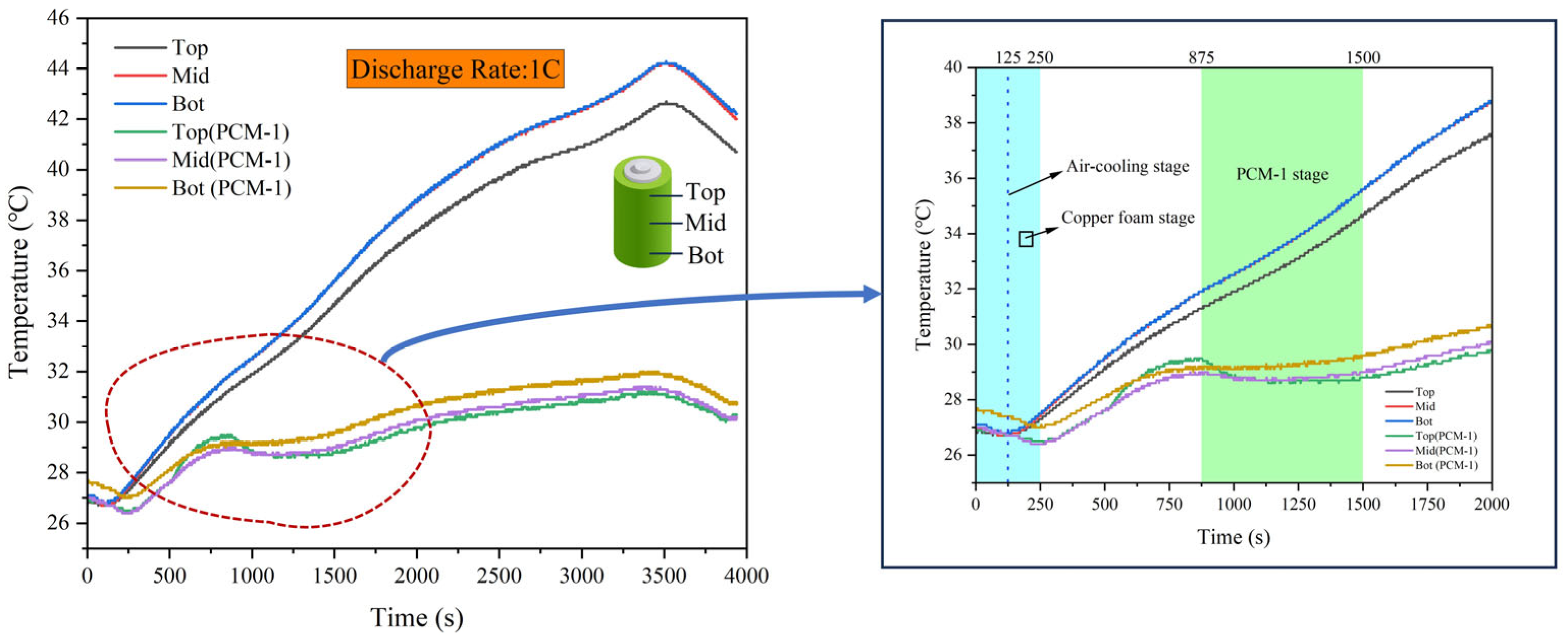
4.2. Temperature Distribution within the Batteries
4.3. Effect of Different Discharge Rates
4.4. Temperature Response under Dynamic Load Conditions
4.5. PCM-Based Battery-Cooling Mechanism
5. Conclusions and Future Directions
- (1)
- Based on the experimental feedback, we developed a consistent phase-change material battery thermal management system specifically designed for 18650 model lithium batteries. Furthermore, we proposed an innovative battery heat dissipation strategy that is extensively suitable for practical commercial applications.
- (2)
- The optimal ratio of polyethylene glycol (PEG) and ethylene glycol (EG) was determined, resulting in a significant enhancement in the material’s thermal conductivity while ensuring its enthalpy value. Furthermore, various tests revealed that PEG1500 and low-mass-percentage EG exhibited compatibility in terms of both physical and chemical properties.
- (3)
- The synthesis of the PEG/EG/CF material led to the preparation of a novel fixed phase-change material with enhanced adsorption capabilities, superior thermal conductivity, and high latent heat. Furthermore, the non-flammable nature of PEG1500, coupled with the flame-retardant properties of EG at room temperature, significantly enhanced the overall safety performance of the system.
- (4)
- The findings reveal that the negative electrode of the 18650 battery generated a greater amount of heat than the positive electrode when the discharge time was extended at a low discharge rate. Conversely, under high-rate discharge conditions (4C), the positive electrode temperature of the 18650 battery surpassed that of the negative electrode. This phenomenon merits further investigation and corresponding enhancements in the thermal management system.
- (5)
- Compared to the prevailing liquid cooling system, the battery thermal management system based on fixed phase-change materials effectively ensures uniformity of battery temperature, significantly reduces the temperature variation of individual batteries, enhances heat dissipation efficiency, and safeguards the overall safety performance of the battery.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, H. Thermal characteristics of power battery module with composite phase change material and external liquid cooling. Int. J. Heat Mass Transf. 2020, 156, 119820. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Wang, M.; Xi, H. Experimental investigation on reciprocating air-cooling strategy of battery thermal management system. J. Energy Storage 2023, 58, 106406. [Google Scholar] [CrossRef]
- Hakeem Akinlabi, A.A.; Solyali, D. Configuration, design, and optimization of air-cooled battery thermal management system for electric vehicles: A review. Renew. Sustain. Energy Rev. 2020, 125, 109815. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- He, L.; Jing, H.; Zhang, Y.; Li, P.; Gu, Z. Review of thermal management system for battery electric vehicle. Energy Storage 2023, 59, 106443. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium-ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Panchal, S.; Gudlanarva, K.; Tran, M.-K.; Herdem, M.S.; Panchal, K.; Fraser, R.; Fowler, M. Numerical Simulation of Cooling Plate Using K-Epsilon Turbulence Model to Cool Down Large-Sized Graphite/LiFePO4 Battery at High C-Rates. World Electr. Veh. J. 2022, 13, 138. [Google Scholar] [CrossRef]
- Liang, K.; Wang, M.; Gao, C.; Dong, B.; Feng, C.; Zhou, X.; Liu, J. Advances and challenges of integrated thermal management technologies for pure electric vehicles. Sustain. Energy Technol. Assess. 2021, 46, 101319. [Google Scholar] [CrossRef]
- Mei, J.; Shi, G.; Liu, H.; Wang, Z.; Chen, M. Investigation on the optimization strategy of phase change material thermal management system for lithium-ion battery. J. Energy Storage 2022, 55, 105365. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Zhang, H. A review of air-cooling battery thermal management systems for electric and hybrid electric vehicles. J. Power Sources 2021, 501, 230001. [Google Scholar] [CrossRef]
- Qin, P.; Liao, M.; Mei, W.; Sun, J.; Wang, Q. The experimental and numerical investigation on a hybrid battery thermal management system based on forced-air convection and internal finned structure. Appl. Therm. Eng. 2021, 195, 117212. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, P.; He, Y.; Li, S. Cooling performance optimization of air-cooling lithium-ion battery thermal management system based on multiple secondary outlets and baffle. J. Energy Storage 2022, 52, 104678. [Google Scholar] [CrossRef]
- Qin, P.; Sun, J.; Yang, X.; Wang, Q. Battery thermal management system based on the forced-air convection: A review. Transportation 2021, 7, 100097. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Li, C. An up-to-date review on the design improvement and optimization of the liquid-cooling battery thermal management system for electric vehicles. Appl. Therm. Eng. 2023, 219, 119626. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Li, M.; Ren, F.; Feng, Y. A manifold channel liquid cooling system with low-cost and high temperature uniformity for lithium-ion battery pack thermal management. Therm. Sci. Eng. Prog. 2023, 41, 101857. [Google Scholar] [CrossRef]
- He, P.; Lu, H.; Fan, Y.; Ruan, H.; Wang, C.; Zhu, Y. Numerical investigation on a lithium-ion battery thermal management system utilizing a double-layered I-shaped channel liquid cooling plate exchanger. Int. J. Therm. Sci. 2023, 187, 108200. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Xu, Z.; Zhou, X.; Mei, X. A systematic review and comparison of liquid-based cooling system for lithium-ion batteries. eTransportation 2023, 17, 100242. [Google Scholar] [CrossRef]
- Weragoda, D.M.; Tian, G.; Burkitbayev, A.; Lo, K.-H.; Zhang, T. A comprehensive review on heat pipe-based battery thermal management systems. Appl. Therm. Eng. 2023, 224, 120070. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Quan, Z.; Liang, J. Investigation of thermal management of lithium-ion battery based on micro heat pipe array. J. Energy Storage 2021, 39, 102624. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, J.; Yin, X.; Wang, D. A novel heat pipe assisted separation type battery thermal management system based on phase change material. Appl. Therm. Eng. 2020, 165, 114571. [Google Scholar] [CrossRef]
- Nasajpour-Esfahani, N.; Garmestani, H.; Rozati, M.; Smaisim, G.F. The role of phase change materials in lithium-ion batteries: A brief review on current materials, thermal management systems, numerical methods, and experimental models. J. Energy Storage 2023, 63, 107061. [Google Scholar] [CrossRef]
- Rao, Z.; Huo, Y.; Liu, X.; Zhang, G. Experimental investigation of battery thermal management system for electric vehicle based on paraffin/copper foam. J. Energy Inst. 2015, 88, 241–246. [Google Scholar] [CrossRef]
- Yang, X.; Deng, G.; Cai, Z.; Li, H.; Zeng, J.; Yang, H. Experimental study on novel composite phase change materials with room-temperature flexibility and high-temperature shape stability in a battery thermal management system. Int. J. Heat Mass Transf. 2023, 206, 123953. [Google Scholar] [CrossRef]
- Li, C.; Ding, Y.; Zhou, Z.; Jin, Y.; Ren, X.; Cao, C.; Hu, H. Parameter optimization and sensitivity analysis of a Lithium-ion battery thermal management system integrated with composite phase change material. Appl. Therm. Eng. 2023, 228, 120530. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, H.; Zuo, H.; Zuo, Q.; He, X.; Chen, W.; Wei, R. EG@Bi-MOF derived porous carbon/lauric acid composite phase change materials for thermal management of batteries. Energy 2023, 272, 127180. [Google Scholar] [CrossRef]
- Yang, W.; Lin, R.; Li, X.; Li, C.; Wu, Y.; Zhang, G.; Liu, X.; Li, S.; Wang, Y. High thermal conductive and anti-leakage composite phase change material with halloysite nanotube for battery thermal management system. J. Energy Storage 2023, 66, 107372. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, Y.; Zhang, C.; Whitehouse, J.; Zhou, Q.; Xu, H.; Wang, C. Experimental study of battery passive thermal management system using copper foam-based phase change materials. Int. J. Thermofluids 2023, 17, 100255. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y. Experimental study of a passive thermal management system using copper foam-paraffin composite for lithium-ion batteries. Energy Procedia 2017, 142, 2403–2408. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, M.; Wu, S.; Liu, Q.; Wan, J. Preparation of expanded graphite/polyethylene glycol composite phase change material for thermoregulation of asphalt binder. Constr. Build. Mater. 2018, 169, 513–521. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Han, X. Analysis of the heat generation of lithium-ion battery during charging and discharging considering different influencing factors. J. Therm. Anal. Calorim. 2014, 116, 1001–1010. [Google Scholar] [CrossRef]
- Sheng, L.; Su, L.; Zhang, H.; Fang, Y.; Xu, H.; Ye, W. An improved calorimetric method for characterizations of the specific heat and the heat generation rate in a prismatic lithium-ion battery cell. Energy Convers. Manag. 2019, 180, 724–732. [Google Scholar] [CrossRef]
- Bian, H.; Wang, Z.; Jiang, J.; Yang, Y.; Wang, H.; Chen, S. Thermal runaway hazard characteristics and influencing factors of Li-ion battery packs under high-rate charge condition. Fire Mater. 2019, 44, 189–201. [Google Scholar] [CrossRef]
- Pan, C.; He, P.; Wu, J.; Chen, N.; Wei, J.; Xu, T.; Shi, E.; Wang, A.; Jia, H. Copper foam effectively improves the thermal performance of graphene-aerogel composite phase-change materials for thermal storage. J. Energy Storage 2022, 51, 104485. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P. Thermal property measurement and heat transfer analysis of acetamide and acetamide/expanded graphite composite phase change material for solar heat storage. Sol. Energy Mater. Sol. Cells 2011, 95, 2246–2254. [Google Scholar] [CrossRef]



| Advantage | Disadvantages | |
|---|---|---|
| Air cooling | Simple structure; low cost; large volume | Low thermal conductivity of gas |
| Liquid cooling | High heat-exchange efficiency; moderate volume | Complex structure; large weight; liquid leakage hazards |
| Heat pipe | Good temperature uniformity; small size | Potential liquid leakage hazards |
| Phase change | Simple structure; high heat-exchange efficiency | Low thermal conductivity of materials |
| Properties | Value |
|---|---|
| Melting point | 64~66 °C |
| Boiling point | >250 °C |
| Density | 1.27 g/mL |
| Solubility | 50 mg/mL (water) |
| Stability | Stable, but easily oxidized by strong oxidants |
| Samples | PCM Percentage | EG Percentage |
|---|---|---|
| PCM-1 | 99% | 1% |
| PCM-3 | 97% | 3% |
| PCM-5 | 95% | 5% |
| PCM-8 | 92% | 8% |
| Apparatus | Formula | Uncertainty |
|---|---|---|
| Thermocouples | (1) | ±0.053 °C |
| Electronic balance | (2) | ±0.56 mg |
| TGA | (2) | ±0.56% |
| DSC | (2) | ±0.56% |
| Samples | Melting | Crystallization | ||
|---|---|---|---|---|
| ΔHm (J/g) | Tm (°C) | ΔHc (J/g) | Tc (°C) | |
| PEG | 157.65 | 46.2 | 157.49 | 29.1 |
| PCM-1 | 155.74 | 46.0 | 156.31 | 34.6 |
| PCM-3 | 150.82 | 47.5 | 155.56 | 34.9 |
| PCM-5 | 144.89 | 45.9 | 144.62 | 33.6 |
| PCM-8 | 140.78 | 45.6 | 135.75 | 33.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Li, C.; Yu, H.; Wang, Z. Experimental Study of a Passive Thermal Management System Using Expanded Graphite/Polyethylene Glycol Composite for Lithium-Ion Batteries. Energies 2023, 16, 7786. https://doi.org/10.3390/en16237786
Xia Z, Li C, Yu H, Wang Z. Experimental Study of a Passive Thermal Management System Using Expanded Graphite/Polyethylene Glycol Composite for Lithium-Ion Batteries. Energies. 2023; 16(23):7786. https://doi.org/10.3390/en16237786
Chicago/Turabian StyleXia, Zhenggang, Chaoen Li, Hang Yu, and Zhirong Wang. 2023. "Experimental Study of a Passive Thermal Management System Using Expanded Graphite/Polyethylene Glycol Composite for Lithium-Ion Batteries" Energies 16, no. 23: 7786. https://doi.org/10.3390/en16237786






