Investigation of Pyrolysis Kinetic Triplet, Thermodynamics, Product Characteristics and Reaction Mechanism of Waste Cooking Oil Biodiesel under the Influence of Copper Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Pyrolysis Products Analysis and Characterization
2.3. Yield of Pyrolysis Products
2.4. Kinetic Triplet and Thermodynamics
3. Results and Discussion
3.1. Physicochemical Properties of WCO-Biodiesel
3.2. Thermal Decomposition Properties
3.3. Kinetic Triplet and Thermodynamics
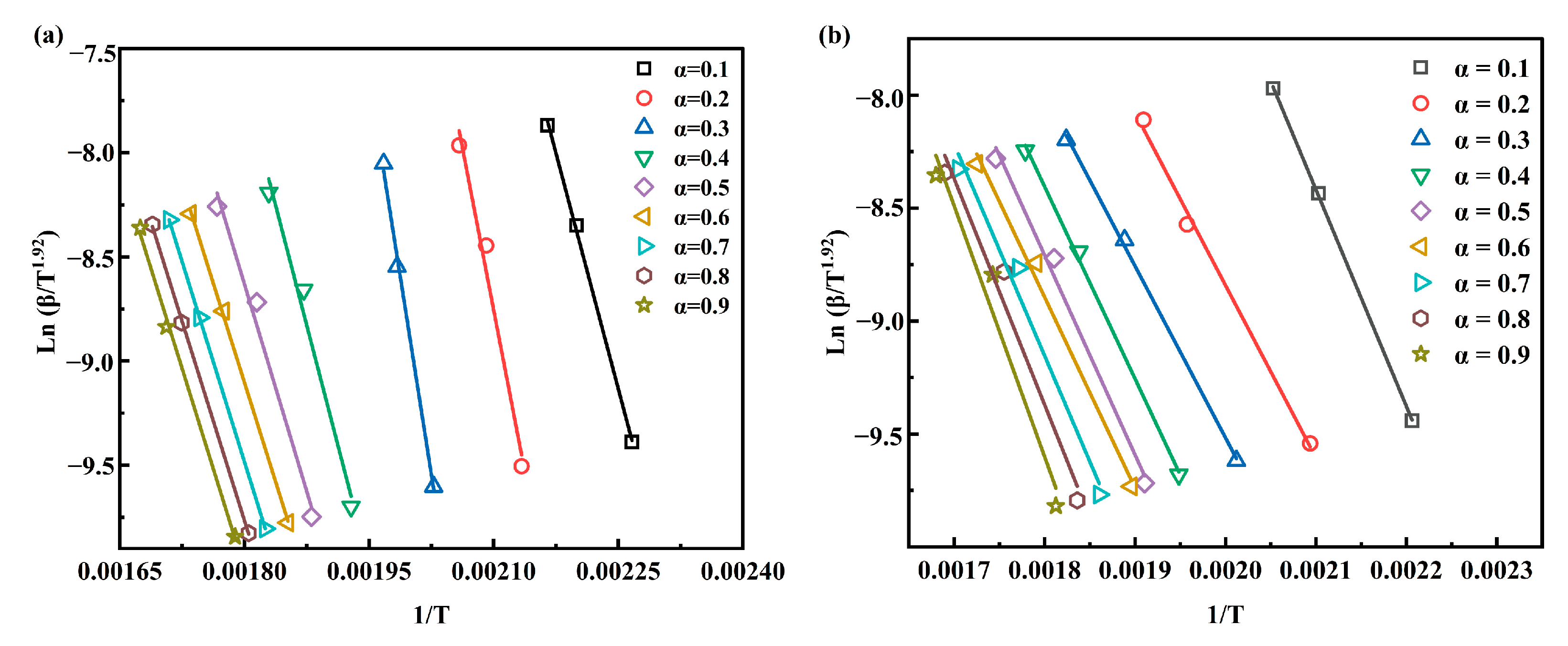
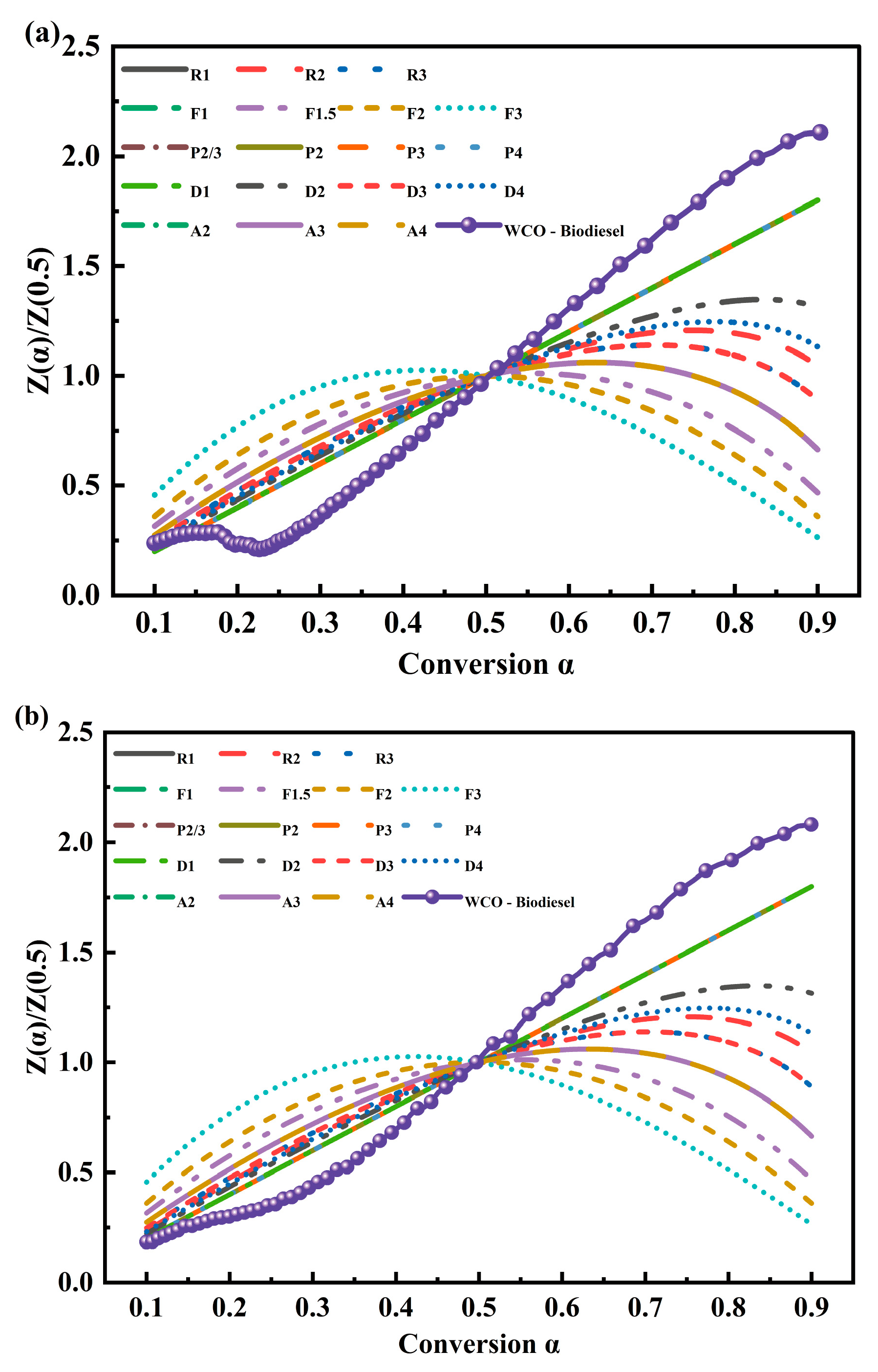
3.3.1. Kinetic Triplet
3.3.2. Thermodynamics
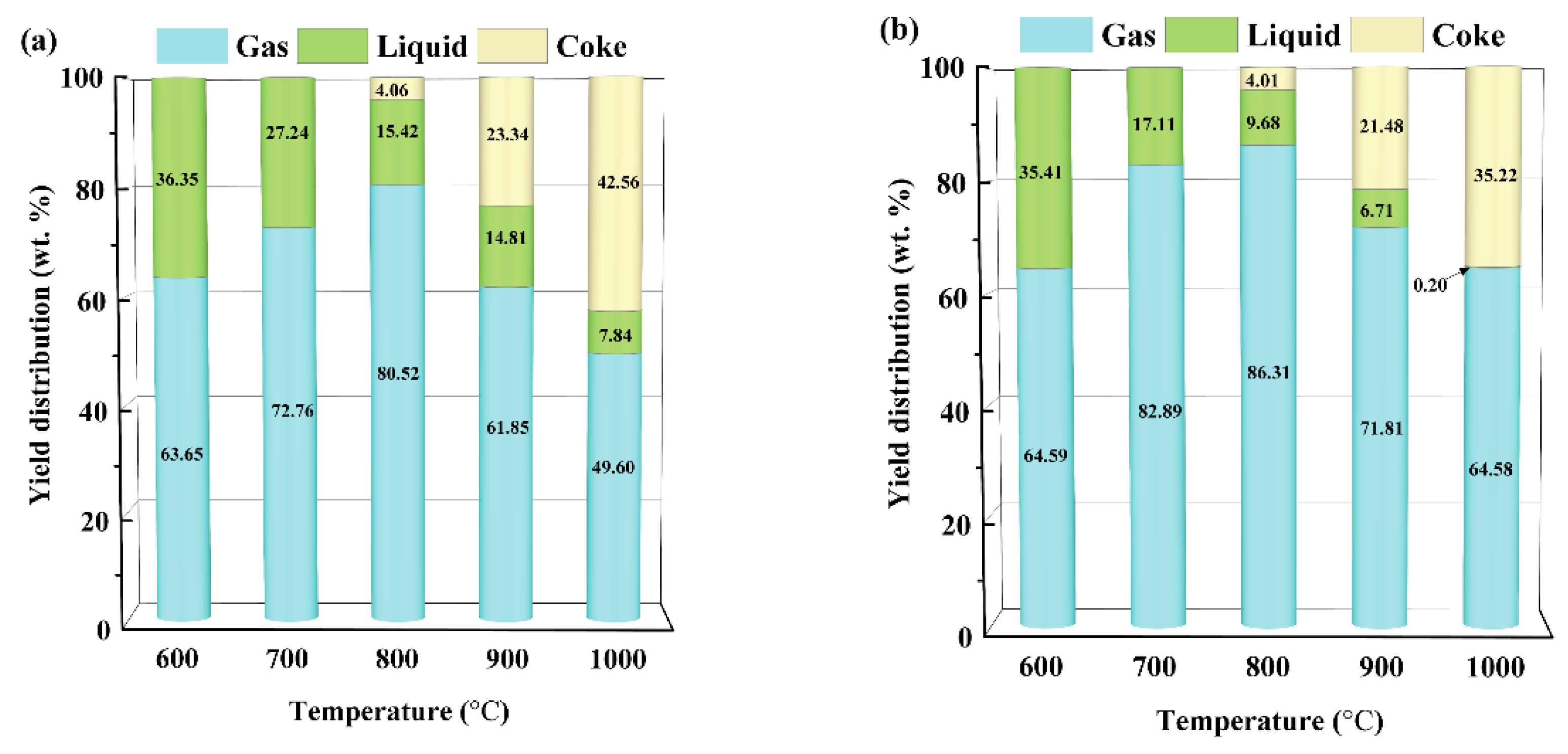
3.4. Distribution of Pyrolysis Products
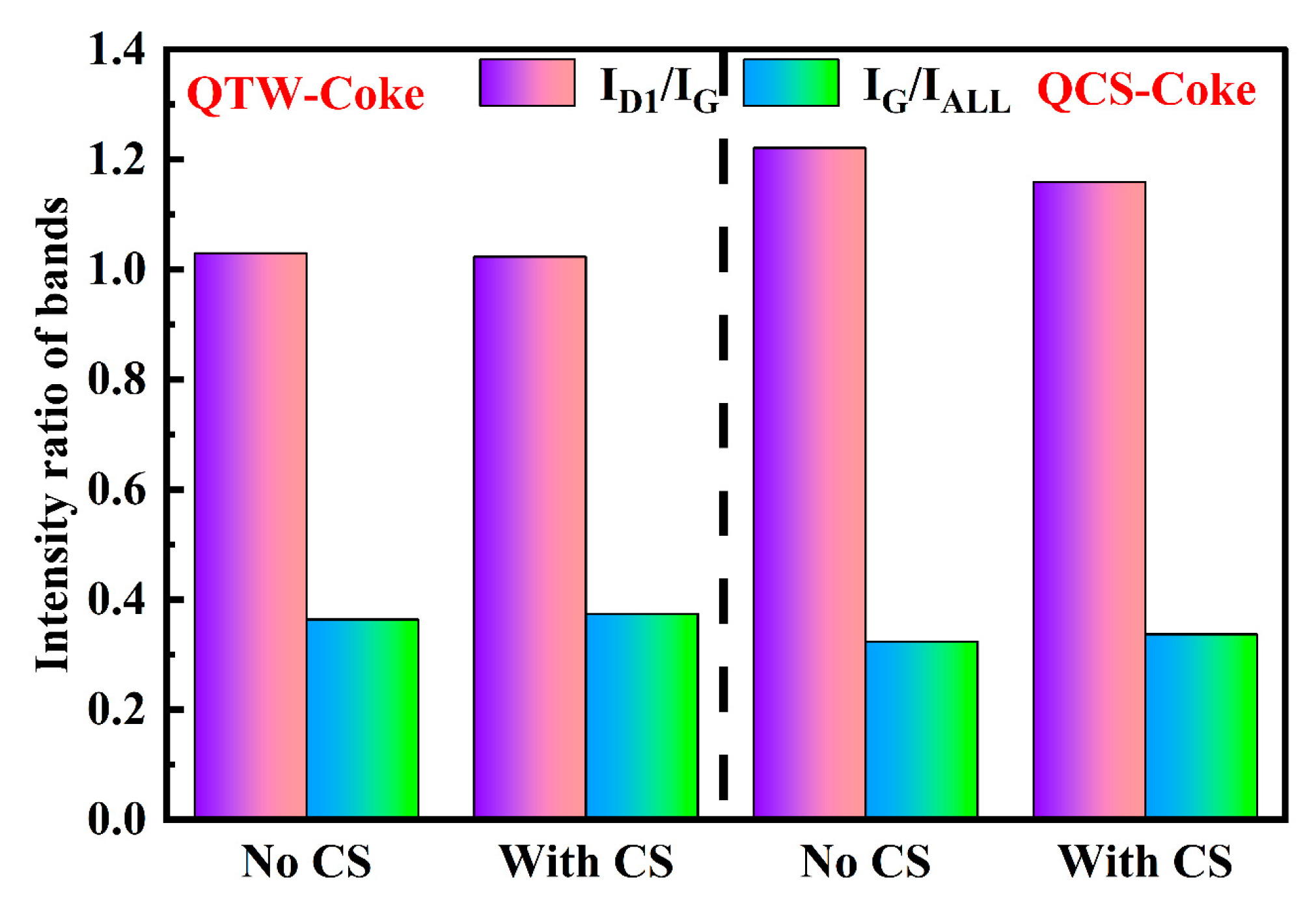
3.5. Characterization of Coke
3.6. Effect of CS on the Gaseous Products of WCO-Biodiesel
3.7. Liquid Products Analysis of WCO-Biodiesel
3.8. WCO-Biodiesel Pyrolysis Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H. Analysis of potential energy conservation and CO2 emissions reduction in China’s non-ferrous metals industry from a technology perspective. Int. J. Greenh. Gas Control 2014, 28, 45–56. [Google Scholar] [CrossRef]
- Rajendran, N.; Kang, D.; Han, J.; Gurunathan, B. Process optimization, economic and environmental analysis of biodiesel production from food waste using a citrus fruit peel biochar catalyst. J. Clean. Prod. 2022, 365, 132712. [Google Scholar] [CrossRef]
- Kurczyński, D.; Wcisło, G.; Łagowski, P. Experimental Study of Fuel Consumption and Exhaust Gas Composition of a Diesel Engine Powered by Biodiesel from Waste of Animal Origin. Energies 2021, 14, 3472. [Google Scholar] [CrossRef]
- Guo, M.; Jiang, W.; Ding, J.; Lu, J. Highly active and recyclable CuO/ZnO as photocatalyst for transesterification of waste cooking oil to biodiesel and the kinetics. Fuel 2022, 315, 123254. [Google Scholar] [CrossRef]
- Orlinski, P.; Wojs, M. Influence of the use of Bioethanol Additive on Waste Cooking Oil Methyl Ester on the Parameters of the CI Engine and Emissions of Toxic Exhaust Components. In Proceedings of the 23rd International Conference Transport Means 2019, Palanga, Lithuania, 2–4 October 2019. [Google Scholar]
- Jiang, X.; Long, F.; Cao, X.; Zhao, J.; Liu, P.; Xu, J. Catalytic cracking of waste cooking oil followed with hydro-isomerization for high-quality biofuel production. J. Clean. Prod. 2022, 345, 131027. [Google Scholar] [CrossRef]
- Bahadorizadeh, O.; Sobati, M.A.; Shahnazari, S. Emission characteristics of a semi-industrial boiler fueled by waste cooking oil biodiesel containing different metal oxide nanoparticles. Process. Saf. Environ. Prot. 2022, 158, 199–209. [Google Scholar] [CrossRef]
- Gad, M.; Ismail, M.A. Effect of waste cooking oil biodiesel blending with gasoline and kerosene on diesel engine performance, emissions and combustion characteristics. Process. Saf. Environ. Prot. 2021, 149, 1–10. [Google Scholar] [CrossRef]
- Jarosiński, W.; Wiśniowski, P. Verifying the Efficiency of a Diesel Particulate Filter Using Particle Counters with Two Different Measurements in Periodic Technical Inspection of Vehicles. Energies 2021, 14, 5128. [Google Scholar] [CrossRef]
- Simbi, I.; Aigbe, U.O.; Oyekola, O.O.; Osibote, O.A. Chemical and quality performance of biodiesel and petrol blends. Energy Convers. Manag. X 2022, 15, 100256. [Google Scholar] [CrossRef]
- Kurczyński, D.; Wcisło, G.; Leśniak, A.; Kozak, M.; Łagowski, P. Production and Testing of Butyl and Methyl Esters as New Generation Biodiesels from Fatty Wastes of the Leather Industry. Energies 2022, 15, 8744. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, D.; Pan, J.; Zhang, F. Innovative methodology for comprehensive and harmless utilization of waste copper slag via selective reduction-magnetic separation process. J. Clean. Prod. 2018, 187, 910–922. [Google Scholar] [CrossRef]
- Pinto, R.; Yaremchenko, A.; Baptista, M.; Tarelho, L.; Frade, J. Synthetic fayalite Fe2SiO4 by kinetically-controlled reaction between hematite and silicon carbide. J. Am. Ceram. Soc. 2019, 102, 5090–5102. [Google Scholar] [CrossRef]
- Mishra, G. Synthesis of Fayalite Fe2SiO4 as a Tar Removal Catalyst for Biomass Gasification. In Proceedings of the Materiai’s 2017, Aveiro, Portugal, 9–12 April 2017. [Google Scholar]
- Du, J.; Zhang, F.; Hu, J.; Yang, S.; Liu, H.; Wang, H. Pyrolysis of rubber seed oil over high-temperature copper slag: Gas and mechanism of coke formation. Renew. Energy 2022, 185, 1209–1220. [Google Scholar] [CrossRef]
- Supriyatna, Y.; Zulhan, Z.; Triapriani, Y. The ferromanganese production using Indonesian low-grade manganese ore using charcoal and palm kernel shell as reductant in mini electric arc furnace. IOP Conf. Ser. Mater. Sci. Eng. 2018, 285, 012022. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H. Cleaner recycling of iron from waste copper slag by using walnut shell char as green reductant. J. Clean. Prod. 2019, 217, 423–431. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wang, H.; Wei, Y.; Hu, J. Smelting reduction and kinetics analysis of magnetic iron in copper slag using waste cooking oil. Sci. Rep. 2017, 7, 2406. [Google Scholar] [CrossRef] [Green Version]
- Muravyev, N.V.; Vyazovkin, S. The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be. Thermo 2022, 2, 435–452. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, F.; Yang, S.; Liu, H.; Wang, H.; Hu, J. Investigation of pyrolysis kinetics, thermodynamics, product characteristics and reaction mechanism of rubber seed oil. Energy Convers. Manag. 2021, 244, 114535. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Chen, F.; Zhang, F.; Yang, S.; Liu, H.; Wang, H.; Hu, J. A comprehensive evaluation on pyrolysis kinetics, thermodynamics, product properties and formation pathways of jatropha oil for high-value utilization. Fuel 2022, 313, 122982. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Ji, Y.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y. Thermal decomposition of castor oil, corn starch, soy protein, lignin, xylan, and cellulose during fast pyrolysis. Bioresour. Technol. 2019, 278, 287–295. [Google Scholar] [CrossRef]
- Criado, J. Kinetic analysis of DTG data from master curves. Thermochim. Acta 1978, 24, 186–189. [Google Scholar] [CrossRef]
- Tabal, A.; Barakat, A.; Aboulkas, A.; El Harfi, K. Pyrolysis of ficus nitida wood: Determination of kinetic and thermodynamic parameters. Fuel 2021, 283, 119253. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Cheng, C.K.; Omar, R.; Chong, C.T.; Chase, H.A. Recovery of diesel-like fuel from waste palm oil by pyrolysis using a microwave heated bed of activated carbon. Energy 2016, 115, 791–799. [Google Scholar] [CrossRef]
- Almazrouei, M.; Janajreh, I. Model-fitting approach to kinetic analysis of non-isothermal pyrolysis of pure and crude glycerol. Renew. Energy 2020, 145, 1693–1708. [Google Scholar] [CrossRef]
- Ordonez-Loza, J.; Chejne, F.; Jameel, A.G.A.; Telalovic, S.; Arrieta, A.A.; Sarathy, S.M. An investigation into the pyrolysis and oxidation of bio-oil from sugarcane bagasse: Kinetics and evolved gases using TGA-FTIR. J. Environ. Chem. Eng. 2021, 9, 106144. [Google Scholar] [CrossRef]
- Li, Y.; Yellezuome, D.; Liu, R.; Cai, J.; Gao, Y. Investigation of product selectivity and kinetics of poplar sawdust catalytic pyrolysis over bi-metallic Iron-Nickel/ZSM-5 catalyst. Bioresour. Technol. 2022, 349, 126838. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Naqvi, M.; Chen, W.; Ceylan, S.; Rashid, H.; Ahmad, J.; Taqvi, S.A.; et al. Pyrolysis of high ash sewage sludge: Kinetics and thermodynamic analysis using Coats-Redfern method. Renew Energy 2019, 131, 854–860. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Liu, P.; Xu, G.-S.; He, Z.-X.; Ji, H.-S.; Wang, Q. Behenic acid pyrolysis to produce diesel-like hydrocarbons. Energy Convers. Manag. 2017, 138, 393–399. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Chen, F.; Yang, S.; Hu, J.; Xiong, Q. n Situ Catalytic Pyrolysis of Municipal Sewage Sludge under Calcined Copper Slag: Thermokinetic Analysis and Real-Time Monitoring of Evolved Gases. ACS Sustain. Chem. Eng. 2022, 10, 14381–14390. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Pérez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- Bhuyan, N.; Choudhury, N.D.; Dutta, B.K.; Upadhyaya, K.; Saikia, N.; Kataki, R. Assessment of kinetic parameters, mechanisms and thermodynamics of Tithonia diversifolia pyrolysis. Biomass-Convers. Biorefinery 2021, 13, 2703–2718. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, J.; Liu, J.; Buyukada, M.; Evrendilek, F.; Liang, G. Pyrolytic behaviors, kinetics, decomposition mechanisms, product distributions and joint optimization of Lentinus edodes stipe. Energy Convers. Manag. 2020, 213, 112858. [Google Scholar] [CrossRef]
- Shahbeig, H.; Nosrati, M. Pyrolysis of municipal sewage sludge for bioenergy production: Thermo-kinetic studies, evolved gas analysis, and techno-socio-economic assessment. Renew. Sustain. Energy Rev. 2020, 119, 109567. [Google Scholar] [CrossRef]
- Niu, H.; Luo, J.; Li, C.; Wang, B.; Liang, C. Transfer Hydrogenation of Biomass-Derived Furfural to 2-Methylfuran over CuZnAl Catalysts. Ind. Eng. Chem. Res. 2019, 58, 6298–6308. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Z.; Song, W.; Li, S. Aldol condensation of furfural with acetone over Ca/ZSM-5 catalyst with lower dosages of water and acetone. J. Taiwan Inst. Chem. Eng. 2020, 108, 16–22. [Google Scholar] [CrossRef]
- Chen, J.; Sun, J.; Wang, Y. Catalysts for Steam Reforming of Bio-oil: A Review. Ind. Eng. Chem. Res. 2017, 56, 4627–4637. [Google Scholar] [CrossRef]
- Lu, Q.; Yuan, S.; Wang, X.; Zhao, Y.; Xie, X.; Liu, H.; Liu, J. Coking behavior and syngas composition of the char supported Fe catalyst of biomass pyrolysis volatiles reforming. Fuel 2021, 298, 120830. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Zhang, J.; Yu, J. Formation of hollow carbon nanofibers on bio-char during microwave pyrolysis of palm kernel shell. Energy Convers. Manag. 2017, 148, 583–592. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Mubarak, N.; Sahu, J.; Abdullah, E.; Jayakumar, N.; Ganesan, P. Single stage production of carbon nanotubes using microwave technology. Diam. Relat. Mater. 2014, 48, 52–59. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Zhang, S.; Li, B.; Wang, H.; Yang, Y.; Barati, M. Reduction of copper smelting slag using waste cooking oil. J. Clean. Prod. 2019, 236, 117668. [Google Scholar] [CrossRef]
- Taufiqurrahmi, N.; Bhatia, S. Catalytic cracking of edible and non-edible oils for the production of biofuels. Energy Environ. Sci. 2011, 4, 1087–1112. [Google Scholar] [CrossRef]
- Meier, H.; Wiggers, V.; Zonta, G.; Scharf, D.; Simionatto, E.; Ender, L. A kinetic model for thermal cracking of waste cooking oil based on chemical lumps. Fuel 2015, 144, 50–59. [Google Scholar] [CrossRef]
- Li, S.; Guo, L. Stability and activity of a co-precipitated Mg promoted Ni/Al2O3 catalyst for supercritical water gasification of biomass. Int. J. Hydrogen Energy 2019, 44, 15842–15852. [Google Scholar] [CrossRef]
- Xu, J.; Long, F.; Jiang, J.; Li, F.; Zhai, Q.; Wang, F.; Liu, P.; Li, J. Integrated catalytic conversion of waste triglycerides to liquid hydrocarbons for aviation biofuels. J. Clean. Prod. 2019, 222, 784–792. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Zong, P.; Tian, Y.; Xu, F.; Li, D.; Li, F.; Tian, Y. Thermal behavior, kinetics and fast pyrolysis characteristics of palm oil: Analytical TG-FTIR and Py-GC/MS study. Energy Convers. Manag. 2019, 199, 111964. [Google Scholar] [CrossRef]
- Sui, M.; Li, F.; Wang, S.; Wang, H. Molecular dynamics simulation and experimental research on the oxidation reaction of methyl linoleate at low oxygen and high temperature. Fuel 2021, 305, 121478. [Google Scholar] [CrossRef]
- Maher, K.D.; Bressler, D.C. Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef]
- Beims, R.; Botton, V.; Ender, L.; Scharf, D.; Simionatto, E.; Meier, H.; Wiggers, V. Effect of degree of triglyceride unsaturation on aromatics content in bio-oil. Fuel 2018, 217, 175–184. [Google Scholar] [CrossRef]
- Kozliak, E.; Sulkes, M.; Alhroub, I.; Kubátová, A.; Andrianova, A.; Seames, W. Influence of early stages of triglyceride pyrolysis on the formation of PAHs as coke precursors. Phys. Chem. Chem. Phys. 2019, 21, 20189–20203. [Google Scholar] [CrossRef]
- Kubátová, A.; Št’Ávová, J.; Seames, W.S.; Luo, Y.; Sadrameli, S.M.; Linnen, M.J.; Baglayeva, G.V.; Smoliakova, I.P.; Kozliak, E.I. Triacylglyceride Thermal Cracking: Pathways to Cyclic Hydrocarbons. Energy Fuels 2012, 26, 672–685. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, L.; Peng, Y.; Yang, Q.; Du, Z.; Dai, L.; Zhou, N.; Liu, Y.; Fu, G.; Ruan, R.; et al. Characteristics of the catalytic fast pyrolysis of vegetable oil soapstock for hydrocarbon-rich fuel. Energy Convers. Manag. 2020, 213, 112860. [Google Scholar] [CrossRef]
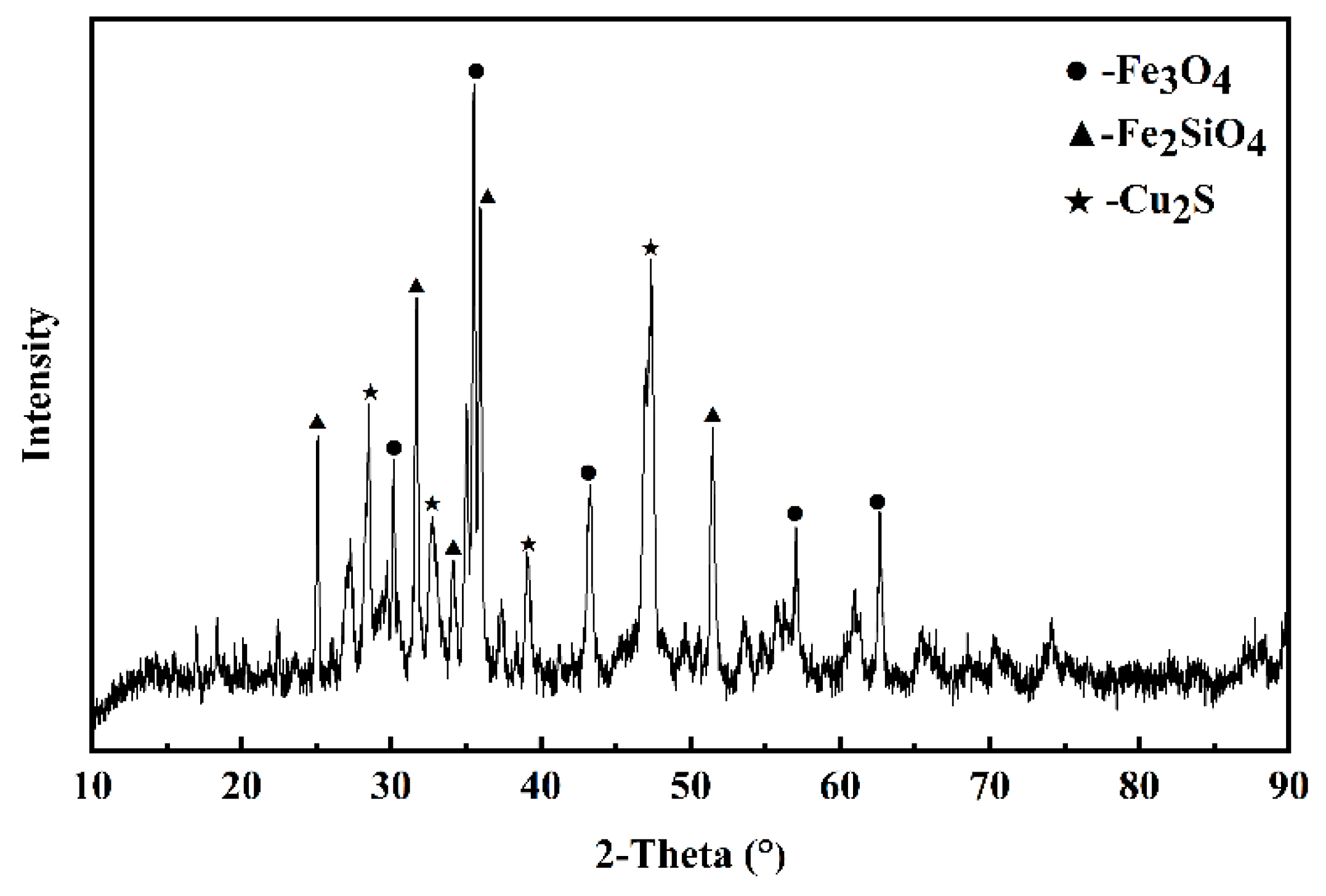






| Cu | SiO2 | Al2O3 | CaO | MgO | ZnO | S | Fe (Total) | Fe3O4 |
|---|---|---|---|---|---|---|---|---|
| 17.82 | 19.13 | 2.96 | 2.12 | 1.95 | 1.83 | 8.07 | 33.06 | 12.9 |
| Reaction Mechanism | Code | f(α) | g(α) | |
|---|---|---|---|---|
| Limiting surface reaction | One-dimensional | R1 | ||
| Two-dimensional | R2 | |||
| Three-dimensional | R3 | |||
| Order of reaction | First-order | F1 | ||
| 1.5-order | F1.5 | |||
| Second-order | F2 | |||
| Third-order | F3 | |||
| Exponential nucleation | Power law | P2/3 | ||
| Power law | P2 | |||
| Power law | P3 | |||
| Power law | P4 | |||
| Diffusional | 1-D diffusional | D1 | ||
| 2-D diffusional | D2 | |||
| 3-D diffusional | D3 | |||
| Ginstling-Brounshtein | D4 | |||
| Random nucleation and nuclei growth | Two-dimensional | A2 | ||
| Three-dimensional | A3 | |||
| Four-dimensional | A4 | |||
| Composition | Values | Composition | Values |
|---|---|---|---|
| Elemental compositions (wt%) | Fatty acid compositions (wt%) | ||
| C | 70.26 | Palmitic acid | 23.70 |
| H | 11.27 | Oleic acid | 42.27 |
| O a | 17.60 | Dinoleic acid | 29.37 |
| N | 0.87 | Linolenic acid | 2.32 |
| O/C (molar ratio) | 0.25 | Other acids | 2.34 |
| H/C (molar ratio) | 0.16 | Proximate analysis (wt%) | |
| Volatile matter | 99.91 | ||
| Higher heating value (HHV) b (MJ·kg−1) | 36.97 | Fixed carbon | 0.09 |
| Ash | 0 |
| α | No CS | With CS | |||||
|---|---|---|---|---|---|---|---|
| Eα (kJ/mol) | A (s−1) | R2 | Eα (kJ/mol) | A (s−1) | R2 | ||
| STR | 0.1 | 125.42 | 2.62 × 1011 | 0.99833 | 79.79 | 1.06 × 107 | 0.99965 |
| 0.2 | 172.26 | 8.68 × 1015 | 0.96171 | 63.50 | 2.45 × 105 | 0.99108 | |
| 0.3 | 210.87 | 4.36 × 1019 | 0.99387 | 63.21 | 2.29 × 105 | 0.99775 | |
| 0.4 | 127.79 | 4.46 × 1011 | 0.96577 | 70.78 | 1.33 × 106 | 0.99644 | |
| 0.5 | 110.34 | 8.95 × 109 | 0.96795 | 73.47 | 2.48 × 106 | 0.98213 | |
| 0.6 | 105.62 | 3.10 × 109 | 0.99852 | 70.30 | 1.19 × 106 | 0.98577 | |
| 0.7 | 106.51 | 3.79 × 109 | 1 | 77.95 | 6.96 × 106 | 0.96176 | |
| 0.8 | 105.98 | 3.36 × 109 | 0.99937 | 83.21 | 2.33 × 107 | 0.94648 | |
| 0.9 | 106.62 | 3.88 × 109 | 0.99531 | 92.34 | 1.88 × 108 | 0.92555 | |
| average | 130.16 | 4.84 × 1018 | 0.98676 | 74.95 | 2.60 × 107 | 0.97629 | |
| α | No CS | With CS | |||||
|---|---|---|---|---|---|---|---|
| ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) | ||
| STR | 0.1 | 121.75 | 143.03 | −38.11 | 76.02 | 143.74 | −122.37 |
| 0.2 | 168.36 | 141.55 | 48.02 | 59.53 | 144.80 | −154.06 | |
| 0.3 | 206.77 | 140.61 | 118.49 | 59.08 | 144.82 | −154.90 | |
| 0.4 | 123.48 | 142.94 | −34.85 | 66.52 | 144.30 | −140.53 | |
| 0.5 | 105.92 | 143.62 | −67.53 | 69.12 | 144.12 | −135.52 | |
| 0.6 | 101.14 | 143.82 | −76.46 | 65.91 | 144.33 | −141.67 | |
| 0.7 | 101.95 | 143.78 | −74.92 | 73.48 | 143.85 | −127.14 | |
| 0.8 | 101.37 | 143.81 | −76.00 | 78.68 | 143.55 | −117.21 | |
| 0.9 | 101.97 | 143.78 | −74.88 | 87.75 | 143.07 | −99.95 | |
| average | 125.86 | 142.99 | −30.69 | 70.68 | 144.06 | −132.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, T.; Zhang, F.; Yang, S.; Wang, H.; Hu, J. Investigation of Pyrolysis Kinetic Triplet, Thermodynamics, Product Characteristics and Reaction Mechanism of Waste Cooking Oil Biodiesel under the Influence of Copper Slag. Energies 2023, 16, 2137. https://doi.org/10.3390/en16052137
Shen T, Zhang F, Yang S, Wang H, Hu J. Investigation of Pyrolysis Kinetic Triplet, Thermodynamics, Product Characteristics and Reaction Mechanism of Waste Cooking Oil Biodiesel under the Influence of Copper Slag. Energies. 2023; 16(5):2137. https://doi.org/10.3390/en16052137
Chicago/Turabian StyleShen, Tianhao, Fengxia Zhang, Shiliang Yang, Hua Wang, and Jianhang Hu. 2023. "Investigation of Pyrolysis Kinetic Triplet, Thermodynamics, Product Characteristics and Reaction Mechanism of Waste Cooking Oil Biodiesel under the Influence of Copper Slag" Energies 16, no. 5: 2137. https://doi.org/10.3390/en16052137




