Electrical Performance and Carbon Deposition Differences between the Bi-Layer Interconnector and Conventional Straight Interconnector Solid Oxide Fuel Cell
Abstract
:1. Introduction
2. Physical Model
2.1. Geometric Model


2.2. Mathematical Model











| Component of inlet fuel | Inlet molar fraction | Basic case inlet molar fraction |
|---|---|---|
| H2O | 0.171 | 0.5 |
| CH4 | 0.263 | 0.5 |
| CO | 0.493 | 0 |
| CO2 | 0.029 | 0 |
| H2 | 0.044 | 0 |

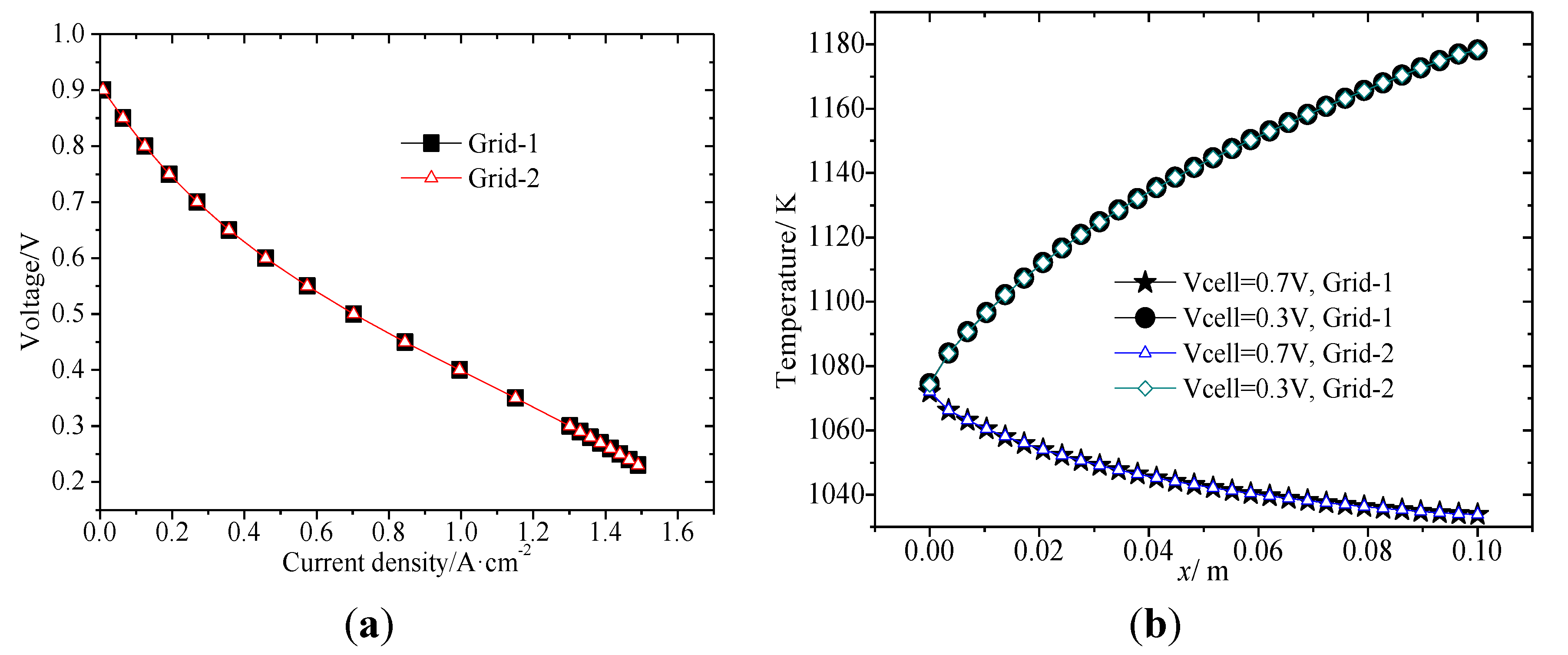
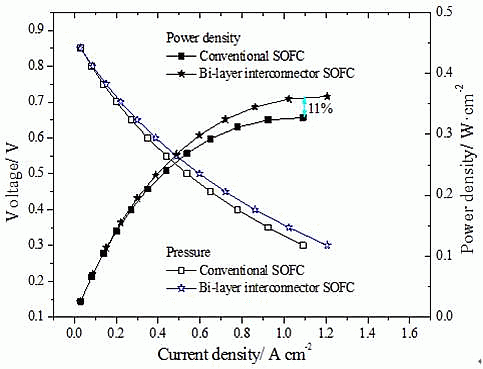
3. Results and Discussion
3.1. Original Steady State Electrical Properties
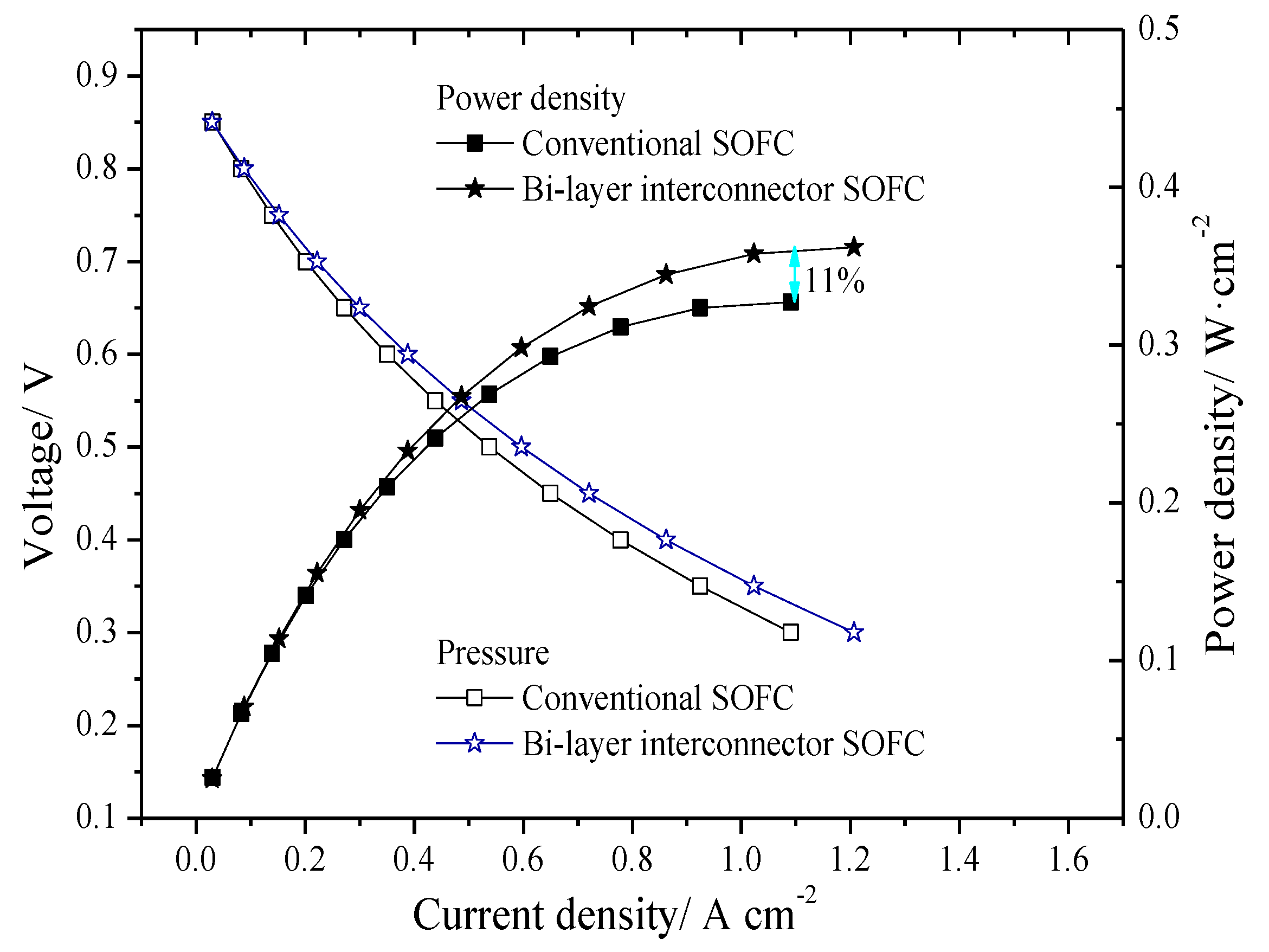
3.2. Unsteady State Performance of Models with Different Interconnectors




4. Conclusions
Acknowledgments
Author Contributions
Nomenclature
| cC | Molar concentration of carbon (mol·m−3) |
| cpi | Specific heat of species i at constant pressure (J·mol−1·K−1) |
| Dij | Binary diffusion coefficient (m2·s−1) |
| F | Faraday’s constant (96,487 C·mol−1) |
| J | Current density (A·m−2) |
| k | Thermal conductivity (W·m−1·K−1) |
| ki | Thermal conductivity of pure component i (W·m−1·K−1) |
| M | Molecular weight of species i (kg·mol−1) |
| mi | Mass of species i (kg) |
| Ni | Molar flux of species i (mol·m−2·s−1) |
| n | Moles of electrons transferred per mole reactant |
| ni | Moles of species i (mol) |
| p | Pressure (Pa) |
| pi | Partial pressure of species i (Pa) |
| Rg | Universal gas constant (8.3143 J·mol−1·K−1) |
| rC | Carbon deposition rate (mol·m−3·s−1) |
| Si | Source term of component i |
| T | Temperature (K) |
| t | Time (s) |
| u | Velocity vector (m·s−1) |
| Vi | Volume of species i (m3) |
| wi | Mass fraction of species i (%) |
| xi | Molar fraction of species i |
Greek symbols
| α | Catalyst activity (%) |
| σ | Electronic conductivity (S·m−1) |
| φel | Electronic potential (V) |
| φio | Ionic potential (V) |
| ε | Porosity |
| ηact | Electrode activation over-potential (V) |
| ρi | Density of species I (kg·m−3) |
| κ | Permeability |
| η | Dynamic viscosity |
| τ | Tortuosity |
Subscripts
| act | Activity |
| an | Anode |
| B | Boudouard reaction |
| C | Methane cracked reaction |
| ca | Cathode |
| cell | Fuel cell |
| pore | Porous media |
| R | Methane steam reforming reaction |
| re | Electrode reaction layer |
| S | CO water-gas shift reaction |
| st | Electrode support layer |
| total | Total amount |
| TPB | Triple phase boundary, the interface between anode and electrolyte |
| io | Ionic |
| el | Eelctronic |
Superscripts
| eff | Effective |
| T | Transposed matrix |
Conflicts of Interest
References
- Najafi, B.; Shirazi, A.; Aminyavari, M.; Rinaldi, F.; Taylor, R.A. Exergetic, economic and environmental analyses and multi-objective optimization of an SOFC-gas turbine hybrid cycle coupled with an MSF desalination system. Desalination 2014, 334, 46–59. [Google Scholar] [CrossRef]
- Shirazi, A.; Aminyavari, M.; Najafi, B.; Rinaldi, F.; Razaghi, M. Thermal-economic-environmental analysis and multi-objective optimization of an internal-reforming solid oxide fuel cell-gas turbine hybrid system. Int. J. Hydrog. Energy 2012, 37, 19111–19124. [Google Scholar] [CrossRef]
- Li, P.W.; Chen, S.P.; Chyu, M.K. To achieve the best performance through optimization of gas delivery and current collection in solid oxide fuel cells. ASME J. Fuel Cell Sci. Technol. 2006, 3, 188–194. [Google Scholar] [CrossRef]
- Yuan, J.L.; Rokni, M.; Sundén, B. Simulation of fully developed laminar heat and mass transfer in fuel cell ducts with different cross-sections. Int. J. Heat Mass Transf. 2001, 44, 4047–4058. [Google Scholar] [CrossRef]
- Kong, W.; Gao, X.; Liu, S.; Su, S.; Chen, D. Optimization of the interconnect ribs for a cathode-supported solid oxide fuel cell. Energies 2014, 7, 295–313. [Google Scholar] [CrossRef]
- Liu, H.; Akhtar, Z.; Li, P.W.; Wang, K. Mathematical modeling analysis and optimization of key design parameters of proton-conductive solid oxide fuel cells. Energies 2014, 7, 173–190. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Craig, R.H. Method of Fabricating a Monolithic Solid Oxide Fuel Cell. U.S. Patent No. 5,290,642 A1, 1 March 1994. [Google Scholar]
- Bedogni, S.; Campanari, S.; Iora, P.; Montelatici, L.; Silva, P. Experimental analysis and modeling for a circular-planar type IT-SOFC. J. Power Sources 2007, 171, 617–625. [Google Scholar] [CrossRef]
- Koh, J.H.; Yoo, Y.S.; Park, J.W.; Lim, H.C. Carbon deposition and cell performance of Ni-YSZ anode support SOFC with methane fuel. Solid State Ion. 2002, 149, 157–166. [Google Scholar] [CrossRef]
- Xu, Z.R.; Fu, X.Z.; Luo, J.L.; Chuang, K.T. Carbon deposition on vanadium-based anode catalyst for SOFC using syngas as fuel. J. Electrochem. Soc. 2010, 157, B1556–B1560. [Google Scholar] [CrossRef]
- Sumi, H.; Puengjinda, P.; Muroyama, H.; Matsui, T.; Eguchi, K. Effects of crystal structure of yttria- and scandia-stabilized zirconia in nickel-based SOFC anodes on carbon deposition and oxidation behavior. J. Power Sources 2011, 196, 6048–6054. [Google Scholar] [CrossRef]
- Maček, J.; Novosel, B.; Marinšek, M. Ni–YSZ SOFC anodes—Minimization of carbon deposition. J. Eur. Ceram. Soc. 2007, 27, 487–491. [Google Scholar] [CrossRef]
- Bae, G.; Bae, J.; Kim-Lohsoontorn, P.; Jeong, J. Performance of SOFC coupled with n-C4H10 autothermal reformer: Carbon deposition and development of anode structure. Int. J. Hydrog. Energy 2010, 35, 12346–12358. [Google Scholar] [CrossRef]
- Kan, H.; Lee, H. Sn-doped Ni/YSZ anode catalysts with enhanced carbon deposition resistance for an intermediate temperature SOFC. Appl. Catal. B Environ. 2010, 97, 108–114. [Google Scholar] [CrossRef]
- Singh, D.; Hernández-Pacheco, E.; Hutton, P.N.; Patel, N.; Mann, M.D. Carbon deposition in an SOFC fueled by tar-laden biomass gas: A thermodynamic analysis. J. Power Sources 2005, 142, 194–199. [Google Scholar] [CrossRef]
- Girona, K.; Laurencin, J.; Fouletier, J.; Lefebvre-Joud, F. Carbon deposition in CH4/CO2 operated SOFC: Simulation and experimentation studies. J. Power Sources 2012, 210, 381–391. [Google Scholar] [CrossRef]
- Eveloy, V.; Daoudi, M. Numerical Investigation of the Effect of Fuel Recycling on the Susceptibility of a Direct Internal Methane Reforming SOFC to Carbon Deposition. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Boston, MA, USA, 31 October–6 November 2008.
- Lee, W.Y.; Hanna, J.; Ghoniem, A.F. On the predictions of carbon deposition on the nickel anode of a SOFC and its impact on open-circuit conditions. J. Electrochem. Soc. 2013, 160, F94–F105. [Google Scholar] [CrossRef]
- Koh, J.H.; Kang, B.S.; Lim, H.C.; Yoo, Y.S. Thermodynamic analysis of carbon deposition and electrochemical oxidation of methane for SOFC anodes. Electrochem. Solid State Lett. 2001, 4, A12–A15. [Google Scholar] [CrossRef]
- Yan, M.; Zeng, M.; Chen, Q.Y.; Wang, Q.W. Numerical study on carbon deposition of SOFC with unsteady state variation of porosity. Appl. Energy 2012, 97, 754–762. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Wang, Q.W.; Zhang, J.; Yuan, J.L. Effect of bi-layer interconnector design on mass transfer performance in porous anode of solid oxide fuel cells. Int. J. Heat Mass Transf. 2011, 54, 1994–2003. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Zeng, M.; Zhang, J.; Wang, Q.W. Optimal design of bi-layer interconnector for SOFC based on CFD-Taguchi method. Int. J. Hydrog. Energy 2010, 35, 4292–4300. [Google Scholar] [CrossRef]
- Hussain, M.M.; Li, X.; Dincer, I. A general electrolyte-electrode-assembly model for the performance characteristics of planar anode-supported solid oxide fuel cells. J. Power Sources 2009, 189, 916–928. [Google Scholar] [CrossRef]
- Alazmi, B.; Vafai, K. Constant wall heat flux boundary conditions in porous media under local thermal non-equilibrium conditions. Int. J. Heat Mass Transf. 2002, 45, 3071–3087. [Google Scholar] [CrossRef]
- Kim, S.J.; Jang, S.P. Effects of the Darcy number, the Prandtl number, and the Reynolds number on local thermal non-equilibrium. Int. J. Heat Mass Transf. 2002, 45, 3885–3896. [Google Scholar] [CrossRef]
- Damm, D.L.; Fedorov, A.G. Local thermal non-equilibrium effects in porous electrodes of the hydrogen-fueled SOFC. J. Power Sources 2006, 159, 1153–1157. [Google Scholar] [CrossRef]
- Andersson, M.; Yuan, J.L.; Sundén, B. Review on modeling development for multiscale chemical reactions coupled transport phenomena in solid oxide fuel cells. Appl. Energy 2010, 87, 1461–1476. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, M.; Fu, P.; Chen, Q.; Wang, Q.; Zeng, M.; Pandit, J. Electrical Performance and Carbon Deposition Differences between the Bi-Layer Interconnector and Conventional Straight Interconnector Solid Oxide Fuel Cell. Energies 2014, 7, 4601-4613. https://doi.org/10.3390/en7074601
Yan M, Fu P, Chen Q, Wang Q, Zeng M, Pandit J. Electrical Performance and Carbon Deposition Differences between the Bi-Layer Interconnector and Conventional Straight Interconnector Solid Oxide Fuel Cell. Energies. 2014; 7(7):4601-4613. https://doi.org/10.3390/en7074601
Chicago/Turabian StyleYan, Min, Pei Fu, Qiuyang Chen, Qiuwang Wang, Min Zeng, and Jaideep Pandit. 2014. "Electrical Performance and Carbon Deposition Differences between the Bi-Layer Interconnector and Conventional Straight Interconnector Solid Oxide Fuel Cell" Energies 7, no. 7: 4601-4613. https://doi.org/10.3390/en7074601
APA StyleYan, M., Fu, P., Chen, Q., Wang, Q., Zeng, M., & Pandit, J. (2014). Electrical Performance and Carbon Deposition Differences between the Bi-Layer Interconnector and Conventional Straight Interconnector Solid Oxide Fuel Cell. Energies, 7(7), 4601-4613. https://doi.org/10.3390/en7074601