Effects of Precipitant and pH on Coprecipitation of Nanosized Co-Cr-V Alloy Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
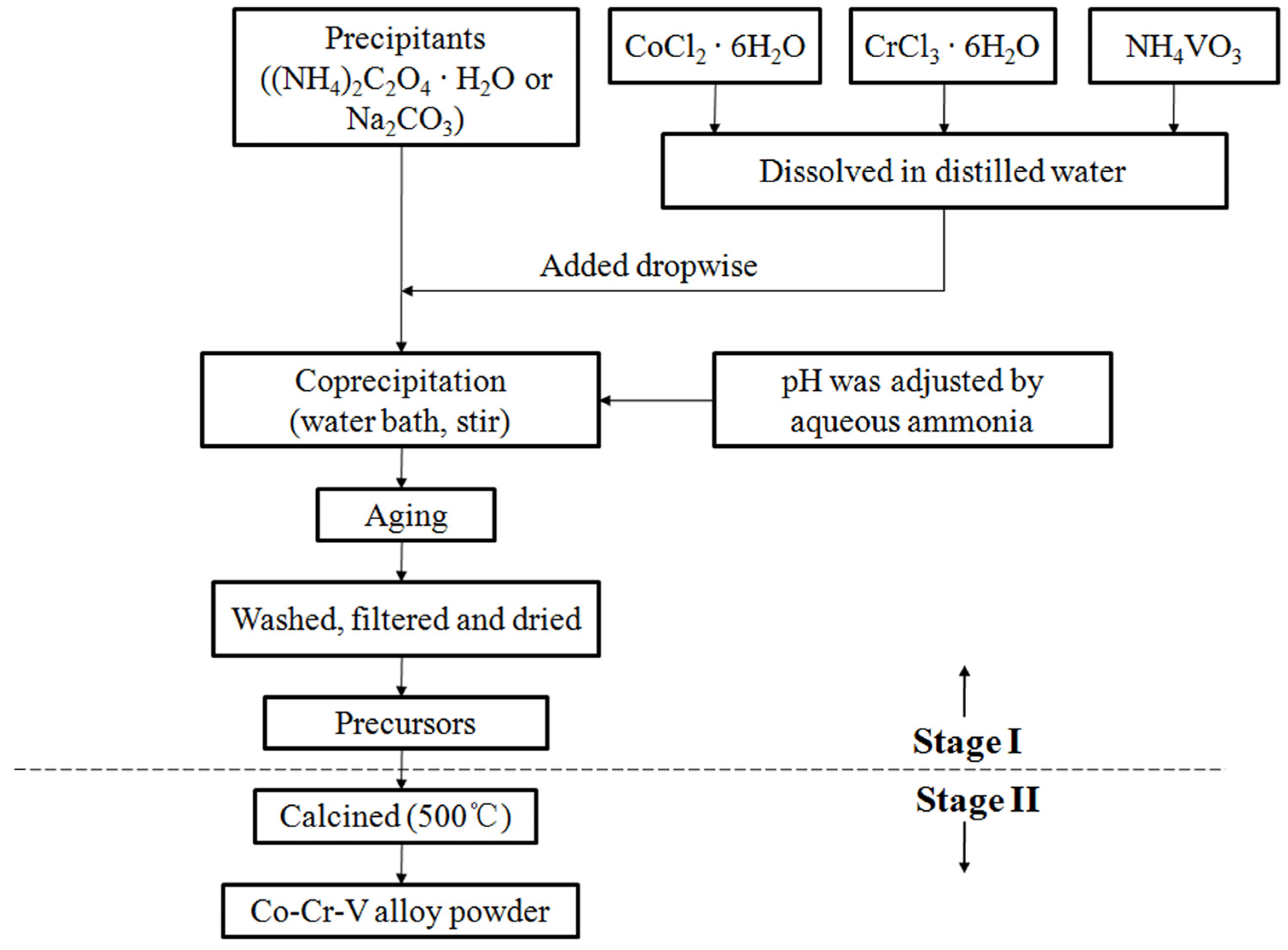
2.2. Synthesis
- (1)
- CoCl2·6H2O, CrCl3·6H2O and NH4VO3 were dissolved in distilled water at molar ratio of Co:Cr:V = 8:1:1 to prepare mixed salt solution of 0.5 mol/L with addition of 2 mol % PVP as dispersant.
- (2)
- The above solution was equally divided into six parts for later use.
- (3)
- Each part (of the solution) was dropped (4 mL/min) into aqueous solution of precipitant (1 mol/L (NH4)2C2O4·H2O or Na2CO3 solution) at designated pH value via constant pressure funnel. Excessive precipitant by 20 mol % was used to ensure fully precipitation of metallic ions.
- (4)
- Coprecipitation (i.e., Step 3) was thermostated in water bath at 50 °C for 1 h with continuous magnetic stirring. pH value of the solution was monitored (by pH meter) and maintained as constant by adding aqueous ammonia.
- (5)
- Solution with precipitates after coprecipitation was aged in open air for 12 h at room temperature.
- (6)
- As-prepared precipitates were washed with deionized water by filtrating (filtrates were collected for subsequent testing), dried at 80 °C overnight to obtain precursors denoted with PO for oxalates and PC for basic carbonates.
2.3. Characterization
3. Results and Discussion
3.1. XRD Analysis
3.2. Thermal Behavior of Precursors
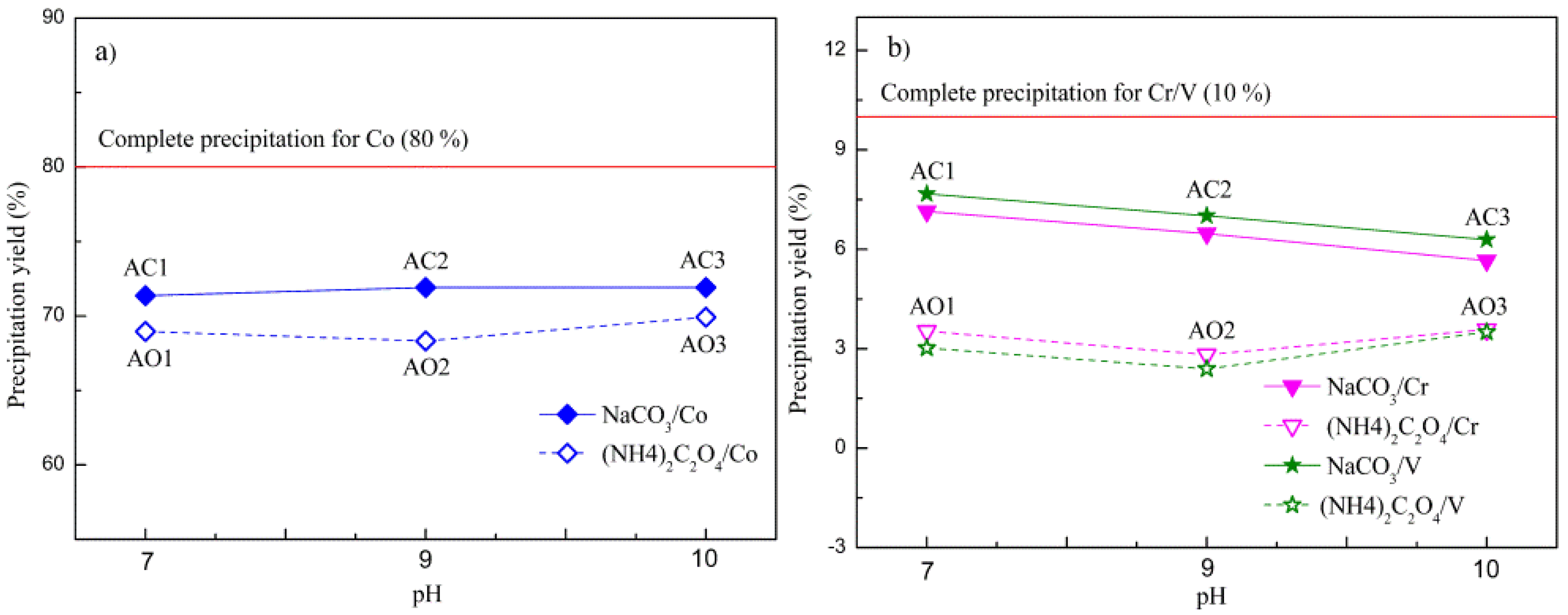
3.3. Composition Analysis of Precursors and Co-Cr-V Alloy
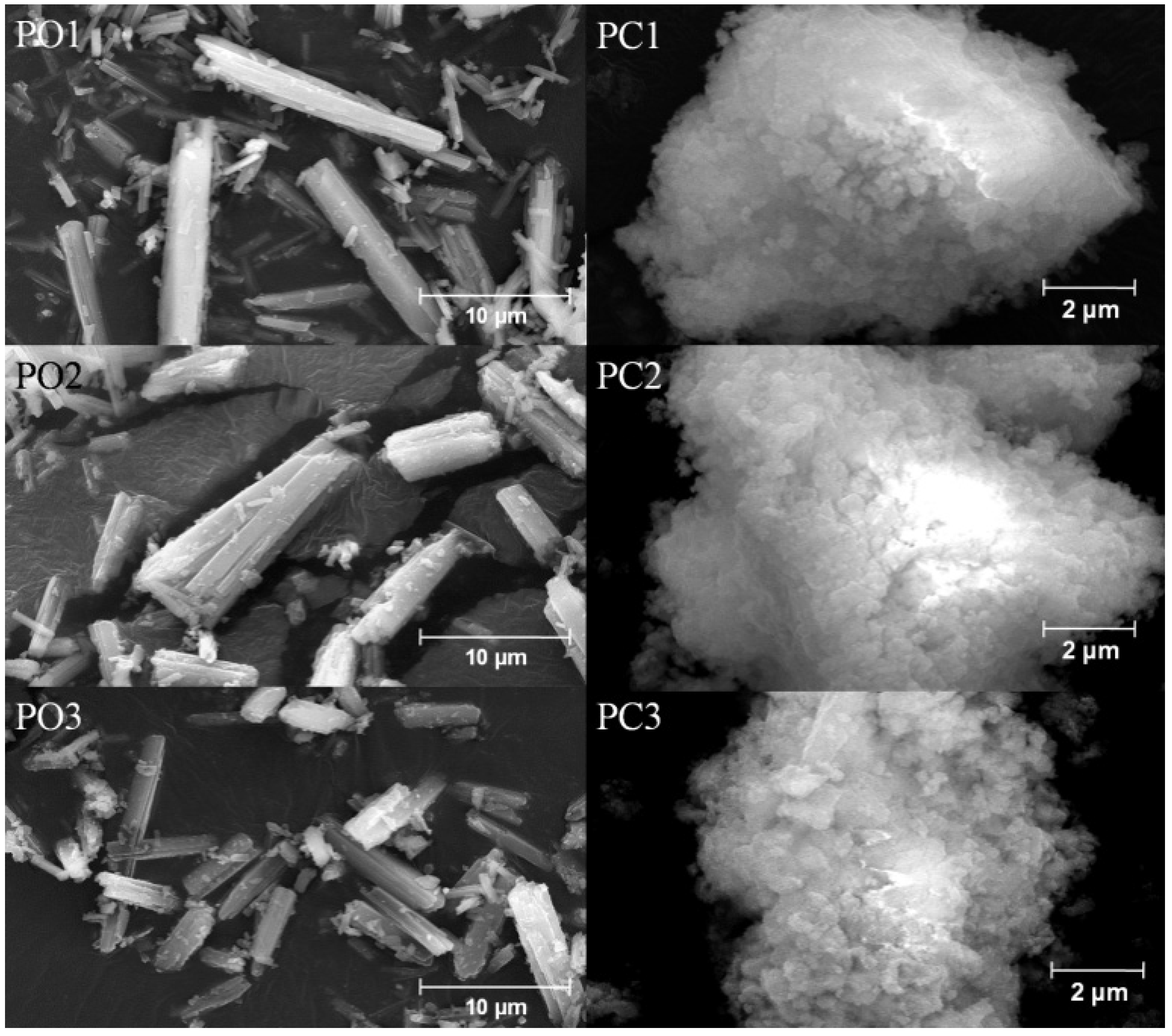
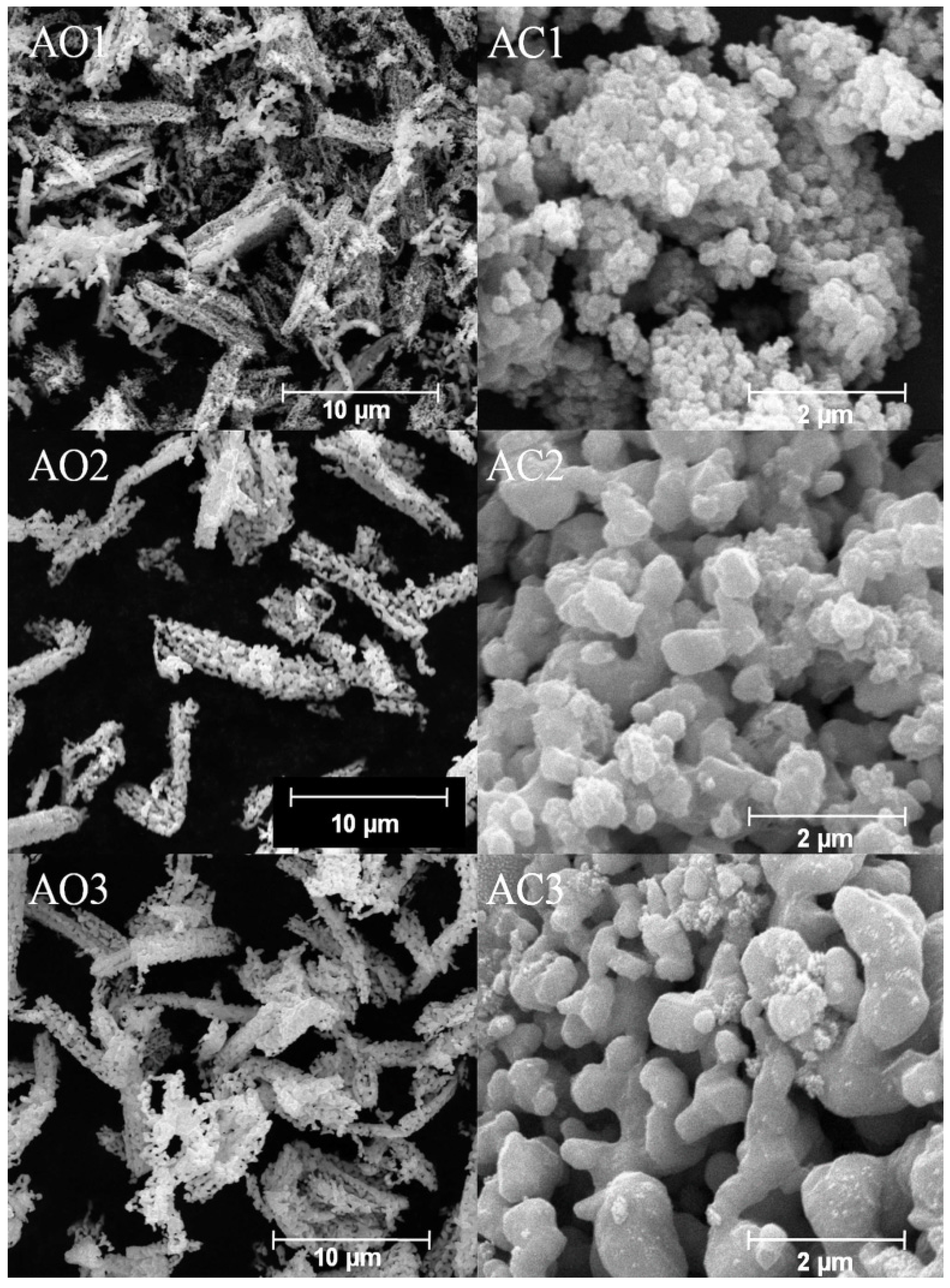
3.4. Morphology Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Knezevic, M.; Carpenter, J.S.; Lovato, M.L.; Mccabe, R.J. Deformation behavior of the cobalt-based superalloy Haynes 25: Experimental characterization and crystal plasticity modeling. Acta Mater. 2014, 63, 162–168. [Google Scholar] [CrossRef]
- Galerie, A. 1.23—High Temperature Corrosion of Chromia-forming Iron, Nickel and Cobalt-base Alloys. In Shreir’s Corrosion; Richardson, T.J.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 583–605. [Google Scholar]
- Mori, M.; Yamanaka, K.; Sato, S.; Tsubaki, S.; Satoh, K.; Kumagai, M.; Imafuku, M.; Shobu, T.; Chiba, A. Strengthening of biomedical Ni-free Co-Cr-Mo alloy by multipass “low-strain-per-pass” thermomechanical processing. Acta Biomater. 2015, 28, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, S.; Yu, G.; Chen, Y. Oxidation behaviour and mechanism of a cobalt based superalloy between 1050 °C and 1250 °C. Appl. Surf. Sci. 2013, 283, 590–598. [Google Scholar] [CrossRef]
- Aronson, J.K. (Ed.) Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Oxford, UK, 2016; pp. 490–491. [Google Scholar]
- Zhong, D.K.; Gamelin, D.R. Photoelectrochemical Water Oxidation by Cobalt Catalyst (“Co-Pi”)/α-Fe2O3 Composite Photoanodes: Oxygen Evolution and Resolution of a Kinetic Bottleneck. J. Am. Chem. Soc. 2010, 132, 4202–4207. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhang, H.; Xu, D.; Ma, L.; Yi, B. Hydrogen generation utilizing alkaline sodium borohydride solution and supported cobalt catalyst. J. Power Sources 2007, 164, 544–548. [Google Scholar] [CrossRef]
- Lu, X.B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef] [PubMed]
- Panpranot, J.; Kaewkun, S.; Praserthdam, P.; Goodwin, J.G. Effect of Cobalt Precursors on the Dispersion of Cobalt on MCM-41. Catal. Lett. 2003, 91, 95–102. [Google Scholar] [CrossRef]
- Xi, X.; Nie, Z.; Jiang, Y.; Xu, X.; Zuo, T. Preparation and characterization of ultrafine cobalt powders and supported cobalt catalysts by freeze-drying. Powder Technol. 2009, 191, 107–110. [Google Scholar] [CrossRef]
- Klarstrom, D.; Crook, P.; Mridha, S. Cobalt Alloys: Alloy Designation System. In Reference Module in Materials Science and Materials Engineering; Elsevier: Oxford, UK, 2016; pp. 1279–1280. [Google Scholar]
- Plumer, M.L.; Ek, J.V.; Weller, D. (Eds.) The Physics of Ultra-High-Density Magnetic Recording, the Physics of Ultra-High-Density Magnetic Recording; Springer: Berlin, Germany, 2001; pp. 1–32. [Google Scholar]
- Vajpai, S.K.; Sawangrat, C.; Yamaguchi, O.; Ciuca, O.P.; Ameyama, K. Effect of bimodal harmonic structure design on the deformation behaviour and mechanical properties of Co-Cr-Mo alloy. Mater. Sci. Eng. C 2016, 58, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H. Co-V (Cobalt-Vanadium). J. Phase Equilib. Diffus. 2007, 28, 314. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, Y.; Liu, X.; Wang, C.P. Experimental determination of phase equilibria in the Co-Cr-V ternary system. J. Phase Equilib. Diffus. 2012, 33, 189–194. [Google Scholar] [CrossRef]
- Qin, D.; Wang, C.; Sun, Q.; Li, H.L. The effects of annealing on the structure and magnetic properties of CoNi patterned nanowire arrays. Appl. Phys. A 2002, 74, 761–765. [Google Scholar] [CrossRef]
- Thongmee, S.; Pang, H.; Yi, J.; Ding, J.; Lin, J.Y.; Van, L.H. Unique nanostructures in NiCo alloy nanowires. Acta Mater. 2009, 57, 2482–2487. [Google Scholar] [CrossRef]
- Li, X.Z.; Wei, X.W.; Ye, Y. Template electrodeposition to cobalt-based alloys nanotube arrays. Mater. Lett. 2009, 63, 578–580. [Google Scholar] [CrossRef]
- Vahid, S.; Mirzaei, A.A. An investigation of the kinetics and mechanism of Fischer-Tropsch synthesis on Fe-Co-Ni supported catalyst. J. Ind. Eng. Chem. 2014, 20, 2166–2173. [Google Scholar] [CrossRef]
- Wu, K.-L.; Wei, X.-W.; Zhou, X.-M.; Wu, D.-H.; Liu, X.-W.; Ye, Y.; Wang, Q. NiCo2 alloys: Controllable synthesis, magnetic properties, and catalytic applications in reduction of 4-nitrophenol. J. Phys. Chem. C 2011, 115, 16268–16274. [Google Scholar] [CrossRef]
- Zhu, L.P.; Xiao, H.M.; Fu, S.Y. Surfactant-Assisted Synthesis and Characterization of Novel Chain-Like CoNi Alloy Assemblies. Eur. J. Inorg. Chem. 2007, 25, 3947–3951. [Google Scholar] [CrossRef]
- Ung, D.; Viau, G.; Ricolleau, C.; Warmont, F.; Gredin, P.; Fiévet, F. CoNi nanowires synthesized by heterogeneous nucleation in liquid polyol. Adv. Mater. 2005, 17, 338–344. [Google Scholar] [CrossRef]
- Wen, M.; Wang, Y.F.; Zhang, F.; Wu, Q.S. Nanostructures of Ni and NiCo amorphous alloys synthesized by a double composite template approach. J. Phys. Chem. C 2009, 113, 5960–5966. [Google Scholar] [CrossRef]
- Domínguez-Crespo, M.; Plata-Torres, M.; Torres-Huerta, A.; Arce-Estrada, E.M.; Hallen-Lopez, J.M. Kinetic study of hydrogen evolution reaction on Ni30Mo70, Co30Mo70, Co30Ni70 and Co10Ni20 Mo70 alloy electrodes. Mater. Charact. 2005, 55, 83–91. [Google Scholar] [CrossRef]
- Bahlawane, N.; Premkumar, P.A.; Tian, Z.Y.; Hong, X.; Qi, F.; Kohse-Höinghaus, K. Nickel and nickel-based nanoalloy thin films from alcohol-assisted chemical vapor deposition. Chem. Mater. 2009, 22, 92–100. [Google Scholar] [CrossRef]
- Chow, G.; Ding, J.; Zhang, J.; Lee, K.Y.; Surani, D. Magnetic and hardness properties of nanostructured Ni-Co films deposited by a nonaqueous electroless method. Appl. Phys. Lett. 1999, 74, 1889–1891. [Google Scholar] [CrossRef]
- Behrens, M. Coprecipitation: An excellent tool for the synthesis of supported metal catalysts-from the understanding of the well known recipes to new materials. Catal. Today 2015, 246, 46–54. [Google Scholar] [CrossRef]
- Buschow, K.H.J. Handbook of Magnetic Materials; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Noce, R.D.; Barelli, N.; Sumodjo, P.T.A.; Comejo, D.R.; Benedetti, A.V. Effect of the bath pH on the electrodeposition of nanocrystalline Pd-Co alloys and their magnetic properties. J. Magn. Magn. Mater. 2006, 306, 199–203. [Google Scholar] [CrossRef]
- Wu, K.H.; Huang, W.C.; Wang, G.P.; Wu, T.R. Effect of pH on the magnetic and dielectric properties of SiO2/NiZn ferrite nanocomposites. Mater. Res. Bull. 2005, 40, 1822–1831. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Jin, W.; Xu, N. Effect of pH on synthesis and properties of perovskite oxide via a citrate process. AIChE J. 2006, 52, 769–776. [Google Scholar] [CrossRef]
- Thu, T.N.T.; Thi, N.N.; Quang, V.T.; Hong, K.N.; Minh, T.N.; Hoai, N.L.T. Synthesis, characterisation, and effect of pH on degradation of dyes of copper-doped TiO2. J. Exp. Nanosci. 2016, 11, 226–238. [Google Scholar]
- Rong, Y. Phase transformations and phase stability in nanocrystalline materials. Curr. Opin. Solid State Mater. Sci. 2005, 9, 287–295. [Google Scholar] [CrossRef]
- Zhao, X.; Veintemillas-Verdaguer, S.; Bomati-Miguel, O.; Morales, M.P.; Xu, H.B. Thermal history dependence of the crystal structure of Co fine particles. Phys. Rev. B 2005, 71. [Google Scholar] [CrossRef]
- Kitakami, O.; Sato, H.; Shimada, Y.; Sato, F.; Tanaka, M. Size effect on the crystal phase of cobalt fine particles. Phys. Rev. B 1997, 56, 13849–13854. [Google Scholar] [CrossRef]
- Meng, Q.P.; Rong, Y.H.; Xu, Y.Z. The structural stability in nano-sized crystals of metals. Sci. China Ser. E Technol. Sci. 2002, 45, 485–494. [Google Scholar]
- Jing, Z.; He, Y.H.; Zhou, D.F.; Zhang, C.F. Thermodynamic analysis on synthesis of fibrous Ni-Co alloys precursor and Ni/Co ratio control. Trans. Nonferr. Met. Soc. China 2011, 21, 1141–1148. [Google Scholar]
- Jongen, N.; Bowen, P.; Lemaître, J.; Valmalette, J.C.; Hofmann, H. Precipitation of self-organized copper oxalate polycrystalline particles in the presence of hydroxypropylmethylcellulose (HPMC): Control of Morphology. J. Colloid Interface Sci. 2000, 226, 189–198. [Google Scholar] [CrossRef]
- Zhan, J.; Zhou, D.F.; Zhang, C.F. Shape-controlled synthesis of novel precursor for fibrous Ni-Co alloy powders. Trans. Nonferrous Met. Soc. China 2011, 21, 544–551. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 7th ed.; Wiley: New York, NY, USA, 2007. [Google Scholar]
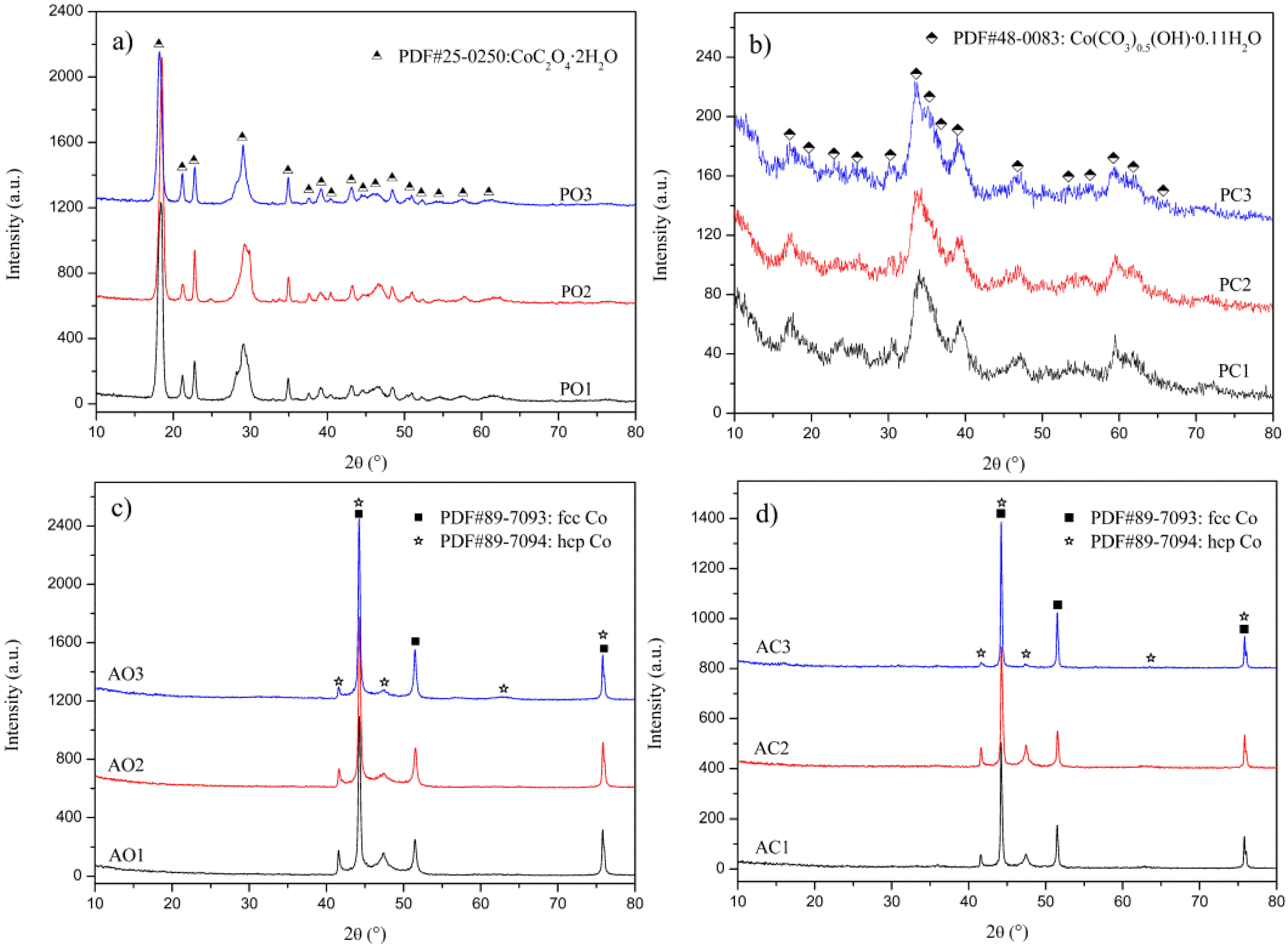
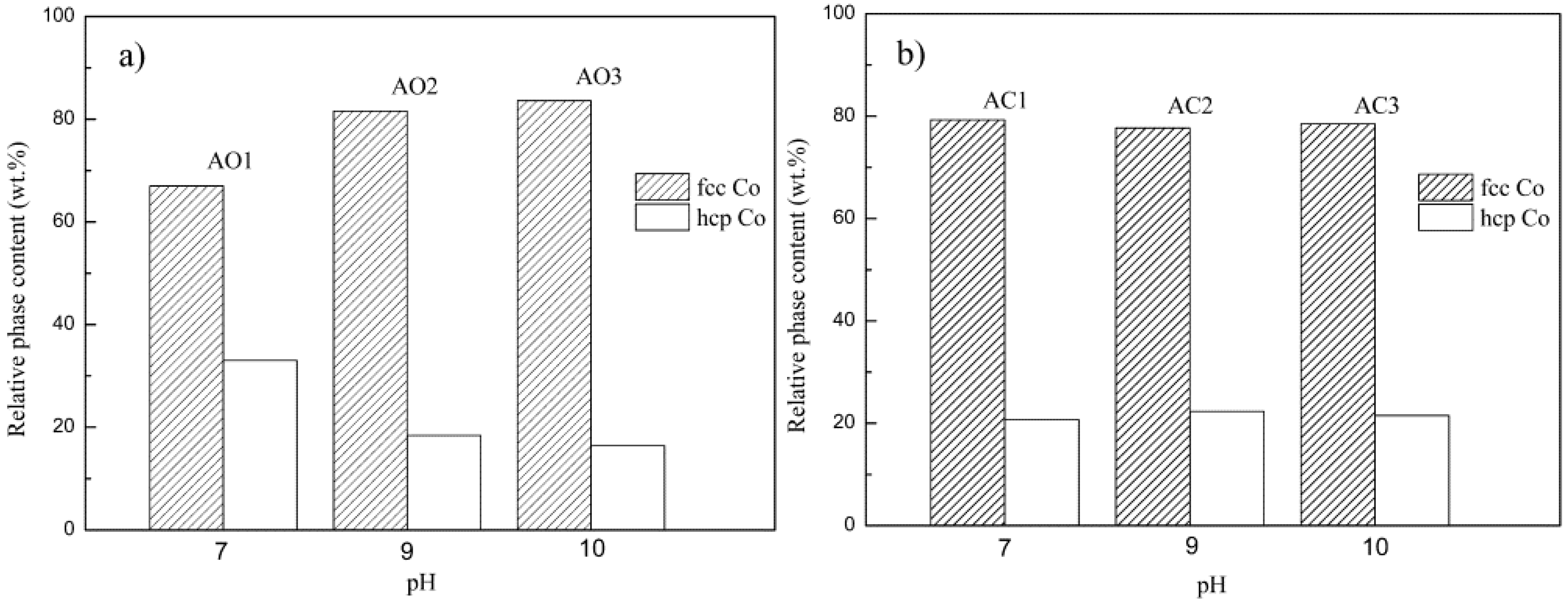
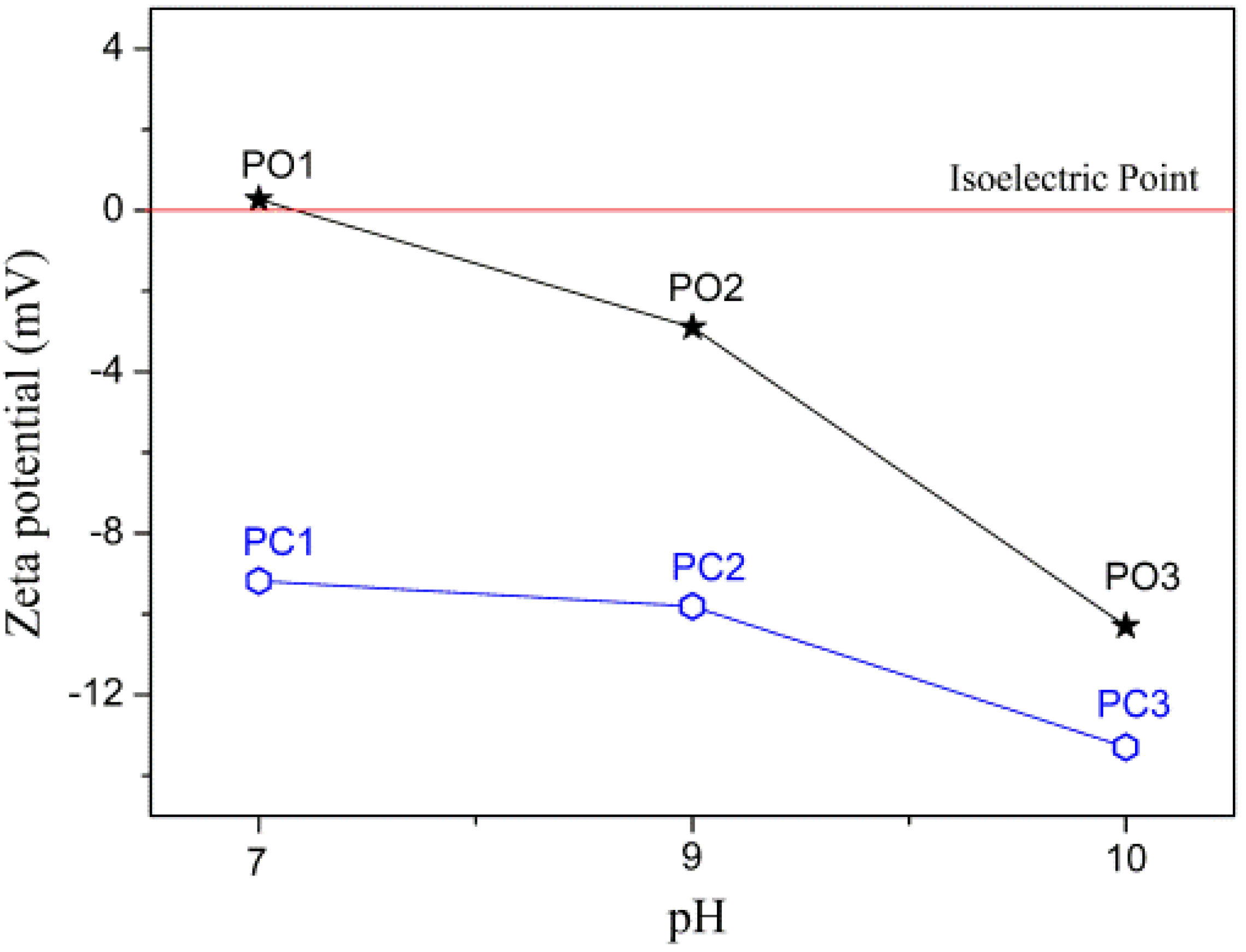
| Samples | Parameters of Synthesis | ||
|---|---|---|---|
| Precursor | Alloy Powder | Precipitant | pH Value |
| PO1 | AO1 | (NH4)2C2O4.H2O | 7 |
| PO2 | AO2 | 9 | |
| PO3 | AO3 | 10 | |
| PC1 | AC1 | Na2CO3 | 7 |
| PC2 | AC2 | 9 | |
| PC3 | AC3 | 10 | |
| Samples | Lattice Parameters (Å) | ||
|---|---|---|---|
| fcc | hcp | ||
| A = b = c | a = b | c | |
| AO1 | 3.5493 ± 0.0010 | 2.5066 ± 0.0008 | 4.0853 ± 0.0007 |
| AO2 | 3.5482 ± 0.0003 | 2.5068 ± 0.0009 | 4.0886 ± 0.0009 |
| AO3 | 3.5463 ± 0.0009 | 2.5071 ± 0.0012 | 4.0924 ± 0.0017 |
| AC1 | 3.5448 ± 0.0007 | 2.5076 ± 0.0012 | 4.0989 ± 0.0029 |
| AC2 | 3.5455 ± 0.0001 | 2.5063 ± 0.0015 | 4.0913 ± 0.0036 |
| AC3 | 3.5461 ± 0.0006 | 2.5071 ± 0.0010 | 4.0929 ± 0.0023 |
| JCPDS No.89-7093 (fcc Co) | 3.5442 | - | - |
| JCPDS No.89-7094 (hcp Co) | - | 2.5074 | 4.0699 |
| Precursors | Grain Size (nm) | Alloy Powders | Grain Size (nm) | |
|---|---|---|---|---|
| fcc Co | hcp Co | |||
| PO1 | 13 | AO1 | 40 | 44 |
| PO2 | 15 | AO2 | 41 | 46 |
| PO3 | 39 | AO3 | 48 | 65 |
| PC1 | 3 | AC1 | 39 | 46 |
| PC2 | 4 | AC2 | 59 | 66 |
| PC3 | 6 | AC3 | 43 | 58 |
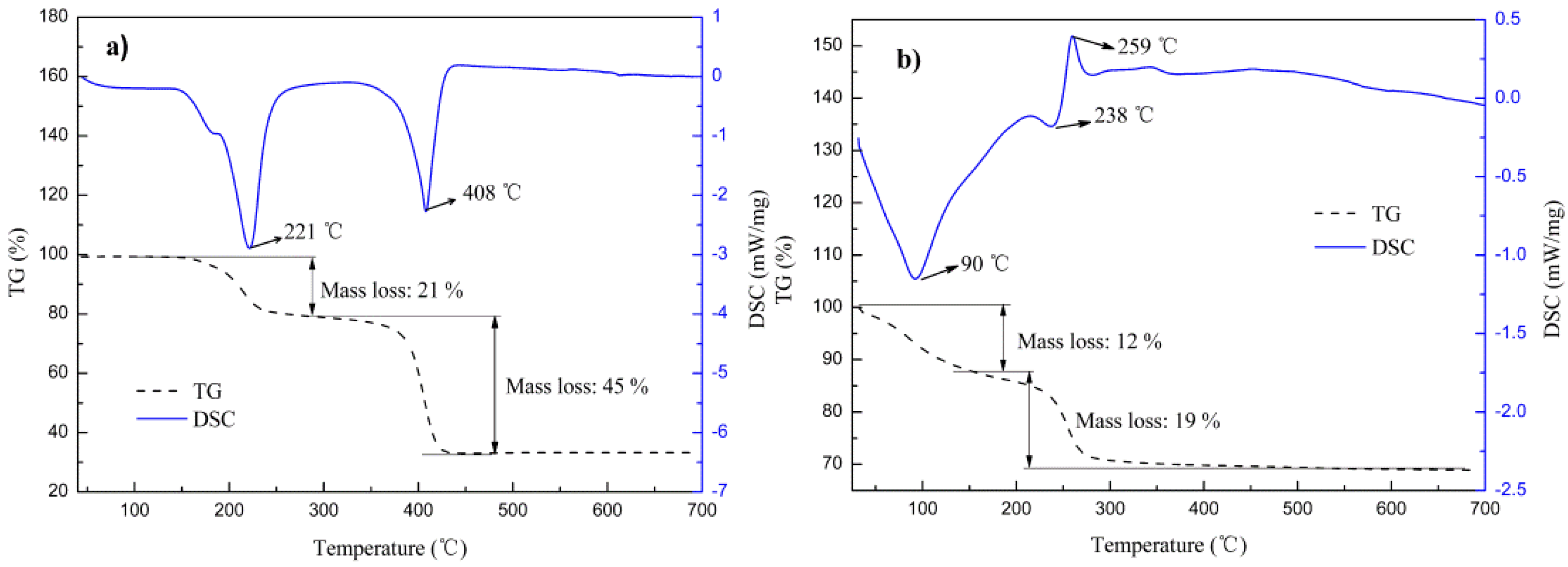
| Sample | Temperature (°C) | Massloss (wt %) | Phase Evolution | Thermal Effect | |
|---|---|---|---|---|---|
| Exp. | Theor. | ||||
| PO1 | 150~300 | 21 | 20 | CoC2O4·2H2O → CoC2O4 | Endoth./221 °C |
| 300~450 | 45 | 48 | CoC2O4 → Co | Endoth./408 °C | |
| PC1 | 80~150 | 12 | / | Co(CO3)0.5(OH)·xH2O → Co(CO3)0.5(OH) | Endoth./90 °C |
| 150~500 | 19 | 18 | Co(CO3)0.5(OH) → CoO + Co(OH)2 | Endoth./238 °C partial Exoth./259 °C | |
| Precursor Samples | Calculated Chemical Formula of Precursors | Alloy Samples | Composition of Alloy Powders | Molar Ratio of Co:Cr:V |
|---|---|---|---|---|
| PO1 | (Co0.84Cr0.04V0.04□0.08)C2O4·2H2O | AO1 | Co0.91Cr0.05V0.04 | 8:0.41:0.35 |
| PO2 | (Co0.87Cr0.04V0.03□0.06)C2O4·2H2O | AO2 | Co0.93Cr0.04V0.03 | 8:0.33:0.28 |
| PO3 | (Co0.84Cr0.04V0.04□0.08)C2O4·2H2O | AO3 | Co0.91Cr0.05V0.04 | 8:0.41:0.40 |
| PC1 | (Co0.71Cr0.07V0.07□0.15)(CO3)0.5(OH)·0.11H2O | AC1 | Co0.83Cr0.08V0.09 | 8:0.80:0.86 |
| PC2 | (Co0.73Cr0.06V0.07□0.14)(CO3)0.5(OH)·0.11H2O | AC2 | Co0.84Cr0.08V0.08 | 8:0.72:0.78 |
| PC3 | (Co0.75Cr0.06V0.06□0.13)(CO3)0.5(OH)·0.11H2O | AC3 | Co0.86Cr0.07V0.07 | 8:0.63:0.70 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, Y.; Huang, L.; Zou, D.; Wu, E.; Liu, Y.; Xie, Y.; Yao, R.; Liao, S.; Wang, G.; et al. Effects of Precipitant and pH on Coprecipitation of Nanosized Co-Cr-V Alloy Powders. Materials 2017, 10, 1108. https://doi.org/10.3390/ma10101108
Chen X, Li Y, Huang L, Zou D, Wu E, Liu Y, Xie Y, Yao R, Liao S, Wang G, et al. Effects of Precipitant and pH on Coprecipitation of Nanosized Co-Cr-V Alloy Powders. Materials. 2017; 10(10):1108. https://doi.org/10.3390/ma10101108
Chicago/Turabian StyleChen, Xiaoyu, Yongxia Li, Lan Huang, Dan Zou, Enxi Wu, Yanjun Liu, Yuanyan Xie, Rui Yao, Songyi Liao, Guangrong Wang, and et al. 2017. "Effects of Precipitant and pH on Coprecipitation of Nanosized Co-Cr-V Alloy Powders" Materials 10, no. 10: 1108. https://doi.org/10.3390/ma10101108
APA StyleChen, X., Li, Y., Huang, L., Zou, D., Wu, E., Liu, Y., Xie, Y., Yao, R., Liao, S., Wang, G., & Zheng, F. (2017). Effects of Precipitant and pH on Coprecipitation of Nanosized Co-Cr-V Alloy Powders. Materials, 10(10), 1108. https://doi.org/10.3390/ma10101108





