Conjugated Polymer Nanoparticles for Bioimaging
Abstract
:1. Introduction
2. Recent Developments in Bioimaging with CPNPs
2.1. Preparation of Conjugated Polymer Nanoparticles
2.2. Strategies to Implement CPNPs for Bioimaging
2.3. Improving the Photoluminescence Quantum Yield
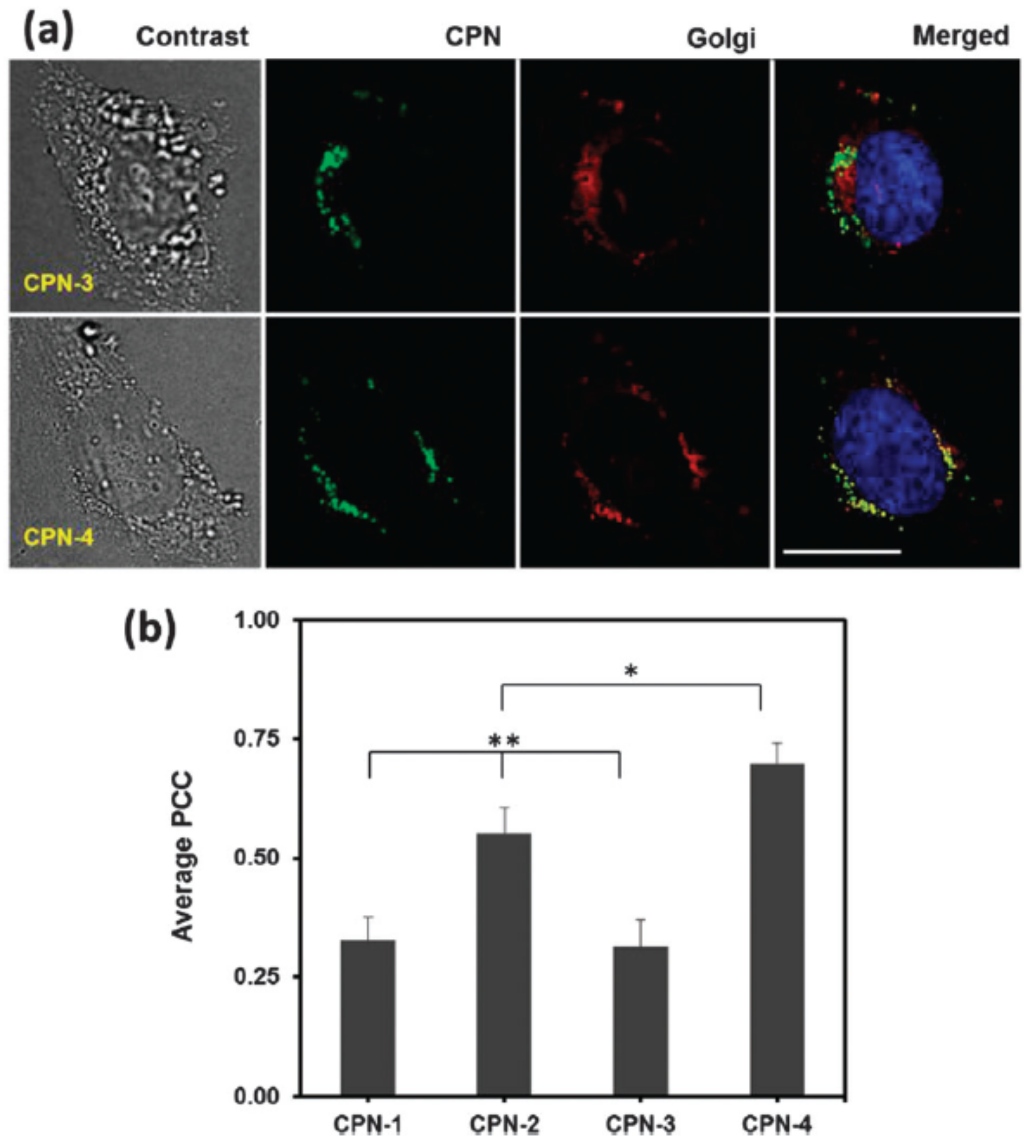
2.4. Surface Functionalization
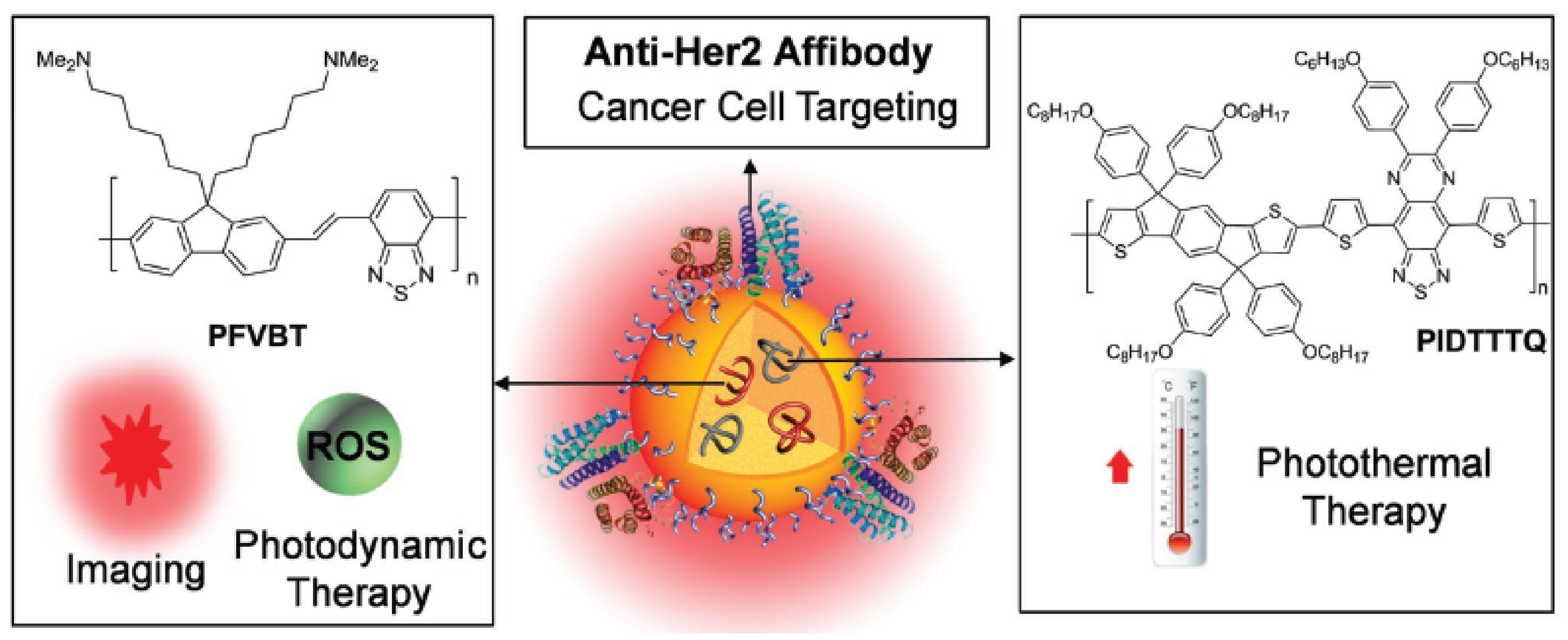
2.5. Theranostic CPNPs
3. Conclusions
Acknowledgements
Conflicts of Interest
References
- Wu, C.; Chiu, D.T. Highly Fluorescent Semiconducting Polymer Dots for Biology and Medicine. Angew. Chem. Int. Ed. 2013, 52, 3086–3109. [Google Scholar] [CrossRef] [PubMed]
- Carril, M. Activatable Probes for Diagnosis and Biomarker Detection by MRI. J. Mater. Chem. B 2017, 5, 4332–4347. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Paefgen, V.; Doleschel, D.; Kiessling, F. Evolution of Contrast Agents for Ultrasound Imaging and Ultrasound-Mediated Drug Delivery. Front. Pharmacol. 2015, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Lameka, K.; Farwell, M.D.; Ichise, M. Handbook of Clinical Neurology. Positron Emiss. Tomogr. 2016, 35, 209–227. [Google Scholar]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, Biology, and Medicine of Fluorescent Nanomaterials and Related Systems: New Insights into Biosensing, Bioimaging, Genomics, Diagnostics, and Therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative Tumor-Specific Fluorescence Imaging in Ovarian Cancer by Folate Receptor-α Targeting: First In-Human Results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Meth. 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bull, B.; Szymanski, C.; Christensen, K.; McNeill, J. Multicolor Conjugated Polymer Dots for Biological Fluorescence Imaging. ACS Nano 2008, 2, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; He, X.; Wang, K.; Tan, W.; Wang, Y.; Liu, Y. Noninvasive Monitoring of Intracellular pH Change Induced by Drug Stimulation Using Silica Nanoparticle Sensors. Anal. Bioanal. Chem. 2007, 388, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-S.; Stolwijk, J.A.; Sun, L.-N.; Wegener, J.; Wolfbeis, O.S. A Nanogel for Ratiometric Fluorescent Sensing of Intracellular pH Values. Angew. Chem. Int. Ed. 2010, 122, 4342–4345. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Kairdolf, B.A.; Smith, A.M.; Stokes, T.H.; Wang, M.D.; Young, A.N.; Nie, S. Semiconductor Quantum Dots for Bioimaging and Biodiagnostic Applications. Annu. Rev. Anal. Chem. 2013, 6, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Khanbeigi, R.; Abelha, T.F.; Woods, A.; Rastoin, O.; Harvey, R.D.; Jones, M.-C.; Forbes, B.; Green, M.A.; Collins, H.; Dailey, L.A. Surface Chemistry of Photoluminescent F8BT Conjugated Polymer Nanoparticles Determines Protein Corona Formation and Internalization by Phagocytic Cells. Biomacromolecules 2015, 16, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, D.; Demir, H.V. Conjugated Polymer Nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Boudreault, P.L.-T.; Najari, A.; Leclerc, M. Processable Low-Bandgap Polymers for Photovoltaic Applications. Chem. Mater. 2011, 23, 456–469. [Google Scholar]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the Biological Windows: Current Perspectives on Fluorescent Bioprobes Emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Klingstedt, T.; Nilsson, K.P.R. Conjugated Polymers for Enhanced Bioimaging. Biochim. Biophys. Acta 2011, 1810, 286–296. [Google Scholar] [PubMed]
- Kwon, N.Y.; Kim, D.; Jang, G.; Lee, J.H.; So, J.-H.; Kim, C.-H.; Kim, T.H.; Lee, T.S. Highly Selective Cysteine Detection and Bioimaging in Zebrafish through Emission Color Change of Water-Soluble Conjugated Polymer-Based Assay Complex. ACS Appl. Mater. Interfaces 2012, 4, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, Z.; Vázquez-Guilló, R.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. New Red-Emitting Conjugated Polyelectrolyte: Stabilization by Interaction with Biomolecules and Potential Use as Drug Carriers and Bioimaging Probes. ACS Appl. Mater. Interfaces 2016, 8, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Lei, M.; Tan, X.; Tan, F.; Li, N. Theranostic Liposomes Containing Conjugated Polymer Dots and Doxorubicin for Bio-imaging and Targeting Therapeutic Delivery. RSC Adv. 2016, 6, 1945–1957. [Google Scholar] [CrossRef]
- Yu, J.; Rong, Y.; Kuo, C.-T.; Zhou, X.-H.; Chiu, D.T. Recent Advances in the Development of Highly Luminescent Semiconducting Polymer Dots and Nanoparticles for Biological Imaging and Medicine. Anal. Chem. 2017, 89, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Wu, P.-J. Semiconducting Polymer Nanoparticles as Fluorescent Probes for Biological Imaging and Sensing. Part. Part. Syst. Charact. 2015, 32, 11–28. [Google Scholar] [CrossRef]
- Pecher, J.; Mecking, S. Nanoparticles of Conjugated Polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.K.; Kim, D.Y.; Norris, B.C.; Miller, W.L.; Barbara, P.F. Size-Dependent Spectroscopic Properties of Conjugated Polymer Nanoparticles. J. Phys. Chem. B 2006, 110, 25568–25572. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Hickey, R.J.; Park, S.-J. Encapsulating Light-Emitting Polymers in Block Copolymer Micelles. Langmuir 2010, 26, 7540–7543. [Google Scholar] [CrossRef] [PubMed]
- Brédas, J.L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-transfer and Energy-transfer Processes in Pi-conjugated Oligomers and Polymers: A Molecular Picture. Chem. Rev. 2004, 104, 4971–5003. [Google Scholar] [CrossRef] [PubMed]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence Quenching by Photoinduced Electron Transfer: A Reporter for Conformational Dynamics of Macromolecules. ChemPhysChem 2009, 10, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Klauk, H. Organic Electronics: Materials, Manufacturing and Applications; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Szymanski, C.; Wu, C.; Hooper, J.; Salazar, M.A.; Perdomo, A.; Dukes, A.; McNeill, J. Single Molecule Nanoparticles of the Conjugated Polymer MEH-PPV, Preparation and Characterization by Near-Field Scanning Optical Microscopy. J. Phys. Chem. B 2005, 109, 8543–8546. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Peng, H.; Jiang, Y.; McNeill, C. Energy Transfer Mediated Fluorescence from Blended Conjugated Polymer Nanoparticles. J. Phys. Chem. B 2006, 110, 14148–14154. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Su, S.-G.; Fryd, M.; Saven, J.G.; Park, S.-J. Highly Tunable Photoluminescent Properties of Amphiphilic Conjugated Block Copolymers. J. Am. Chem. Soc. 2010, 132, 9931–9933. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated Polymer Nanoparticles: Preparation, Properties, Functionalization and Biological Applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kwag, J.; Shin, T.J.; Park, J.; Lee, Y.M.; Lee, Y.; Park, J.; Heo, J.; Joo, C.; Park, T.J.; et al. Nanoparticles of Conjugated Polymers Prepared from Phase-Separated Films of Phospholipids and Polymers for Biomedical Applications. Adv. Mater. 2014, 26, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y.; et al. Ultrafast Fluorescence Imaging In Vivo with Conjugated Polymer Fluorophores in the Second Near-Infrared Window. Nat. Commun. 2014, 5, 4206. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.; Webb, W. Two-Photon Laser Scanning Fluorescence Microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Szymanski, C.; Cain, Z.; McNeill, J. Conjugated Polymer Dots for Multiphoton Fluorescence Imaging. J. Am. Chem. Soc. 2007, 129, 12904–12905. [Google Scholar] [CrossRef] [PubMed]
- Pecher, J.; Huber, J.; Winterhalder, M.; Zumbusch, A.; Mecking, S. Tailor-Made Conjugated Polymer Nanoparticles for Multicolor and Multiphoton Cell Imaging. Biomacromolecules 2010, 11, 2776–2780. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, P.; Ding, H.; Wu, Y.; Yan, Y.; Liu, H.; Wang, X.; Huang, F.; Zhao, Y.; Tian, Z. Conjugated Polymer-Based Hybrid Nanoparticles with Two-Photon Excitation and Near-Infrared Emission Features for Fluorescence Bioimaging within the Biological Window. ACS Appl. Mater. Interfaces 2015, 7, 20640–20648. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Zaquen, N.; D’Olieslaeger, L.; Bové, H.; Vanderzande, D.; Hellings, N.; Junkers, T.; Ethirajan, A. PPV-Based Conjugated Polymer Nanoparticles as a Versatile Bioimaging Probe: A Closer Look at the Inherent Optical Properties and Nanoparticle−Cell Interactions. Biomacromolecules 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Wu, C.; Yu, J.; Zhang, X.; Ye, F.; Zeigler, M.; Gallina, M.E.; Wu, I.-C.; Zhang, Y.; Chan, Y.-H.; et al. Multicolor Fluorescent Semiconducting Polymer Dots with Narrow Emissions and High Brightness. ACS Nano 2013, 7, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-P.; Huang, Y.-C.; Liou, S.-Y.; Wu, P.-J.; Kuo, S.-Y.; Chan, Y.-H. Near-Infrared Fluorescent Semiconducting Polymer Dots with High Brightness and Pronounced Effect of Positioning Alkyl Chains on the Comonomers. ACS Appl. Mater. Interfaces 2014, 6, 21585–21595. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Wu, P.-J.; Kuo, S.-Y.; Chen, C.-P.; Chang, E.-H.; Wu, C.-Y.; Chan, Y.-H. Quinoxaline-Based Polymer Dots with Ultrabright Red to Near-Infrared Fluorescence for In Vivo Biological Imaging. J. Am. Chem. Soc. 2015, 137, 10420–10429. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, G.; Ding, D.; Liu, B. Bright Far-Red/Near-Infrared Fluorescent Conjugated Polymer Nanoparticles for Targeted Imaging of HER2-Positive Cancer Cells. Polym. Chem. 2013, 4, 4326–4334. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Liu, B. Far-Red/Near-Infrared Conjugated Polymer Nanoparticles for Long-Term In Situ Monitoring of Liver Tumor Growth. Adv. Sci. 2015, 5, 1500008. [Google Scholar] [CrossRef] [PubMed]
- D’Olieslaeger, L.; Braeken, Y.; Cheruku, S.; Smits, J.; Ameloot, M.; Vanderzande, D.; Maes, W.; Ethirajan, A. Tuning the Optical Properties of Poly(p-phenylene ethynylene) Nanoparticles as Bio-Imaging Probes by Side Chain Functionalization. J. Colloid Interface Sci. 2017, 504, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, J.; Feng, G.; Li, K.; Hu, Y.; Liu, B. Bright Far-Red/Near-Infrared Conjugated Polymer Nanoparticles for In Vivo Bioimaging. Small 2013, 9, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, H.; Zhang, Y.; Xu, Z.; Wang, X.; Cao, B.; Wang, M. Hydrophobic-Sheath Segregated Macromolecular Fluorophores: Colloidal Nanoparticles of Polycaprolactone-Grafted Conjugated Polymers with Bright Far-Red/Near-Infrared Emission for Biological Imaging. Biomacromolecules 2016, 17, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.-I.; Jin, Y.-J.; Lee, W.-E.; Byun, D.J.; Yu, R.; Park, S.J.; Kim, H.; Song, K.-H.; Jang, S.-Y.; Kwak, G. Highly Fluorescent, Photostable, Conjugated Polymer Dots with Amorphous, Glassy-State, Coarsened Structure for Bioimaging. Adv. Opt. Mater. 2015, 3, 78–86. [Google Scholar] [CrossRef]
- Behrendt, J.M.; Esquivel Guzman, J.A.; Purdie, L.; Willcock, H.; Morrison, J.J.; Foster, A.B.; O’Reilly, R.K.; McCairn, M.C.; Turner, M.L. Scalable Synthesis of Multicolour Conjugated Polymer Nanoparticles via Suzuki-Miyaura Polymerisation in a Miniemulsion and Application in Bioimaging. React. Funct. Polym. 2016, 107, 69–77. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, J.; Wu, C.; Jin, Y.; Rong, Y.; Ye, F.; Chiu, D.T. Importance of Having Low-Density Functional Groups for Generating High-Performance Semiconducting Polymer Dots. ACS Nano 2012, 6, 5429–5439. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Morton, S.W.; Hammond, P.T.; Swager, T.M. Fluorescent Multiblock π-Conjugated Polymer Nanoparticles for In Vivo Tumor Targeting. Adv. Mater. 2013, 25, 4504–4510. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cui, Q.; Wang, C.; Wang, X.; Yang, Y.; Li, L. Preparation of Sialic Acid-Imprinted Fluorescent Conjugated Nanoparticles and Their Application for Targeted Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2017, 9, 3006–3015. [Google Scholar] [CrossRef] [PubMed]
- Mendez, E.; Moon, J.H. Side Chain and Backbone Structure-Dependent Subcellular Localization and Toxicity of Conjugated Polymer Nanoparticles. Chem. Commun. 2013, 49, 6048–6050. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Liu, B.; Tomczak, N. Highly Emissive PEG-Encapsulated Conjugated Polymer Nanoparticles. Nanoscale 2012, 4, 5694–5702. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, L.; Lv, F.; Bazan, G.C.; Wang, S. Preparation and Biofunctionalization of Multicolor Conjugated Polymer Nanoparticles for Imaging and Detection of Tumor Cells. Adv. Mater. 2014, 26, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Lin, M.; Chen, G.; Jiang, M. Modification of Polyfluorene Nanoparticles via Inclusion Complexation based on Cyclodextrin for Lectin Sensing and Cell Imaging. Sci. China Chem. 2016, 59, 1616–1620. [Google Scholar] [CrossRef]
- Li, M.; Nie, C.; Feng, L.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated Polymer Nanoparticles for Cell Membrane Imaging. Chem. Asian J. 2014, 9, 3121–3124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Geng, J.; Wang, G.; Liu, J.; Liu, B. Facile Synthesis of Stable and Water-Dispersible Multihydroxy Conjugated Polymer Nanoparticles with Tunable Size by Dendritic Cross-Linking. ACS Macro. Lett. 2012, 1, 927–932. [Google Scholar] [CrossRef]
- Koner, A.L.; Krndija, D.; Hou, Q.; Sherratt, D.J.; Howarth, M. Hydroxy-Terminated Conjugated Polymer Nanoparticles Have Near-Unity Bright Fraction and Reveal Cholesterol-Dependence of IGF1R Nanodomains. ACS Nano 2013, 7, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.S.; Herrmann, I.K.; Howes, P.D.; Stevens, M.M. Tailoring Cellular Uptake of Conjugated Polymer Nanoparticles Using Modular Amphiphilic Peptide Capping Ligands. Chem. Mater. 2015, 27, 6879–6889. [Google Scholar] [CrossRef]
- Joshi, P.B.; Zhang, P. Facile Capture of Conjugated Polymer Nanodots in Silica Nanoparticles to Facilitate Surface Modification. J. Mater. Sci. 2015, 50, 3597–3603. [Google Scholar] [CrossRef]
- Chen, T.; Xu, W.; Huang, Z.; Peng, H.; Ke, Z.; Lu, X.; Yan, Y.; Liu, R. Poly(phenylene ethynylene) Nanoparticles: Preparation, Living Cell Imaging and Potential Application as Drug Carriers. J. Mater. Chem. B 2015, 3, 3564–3572. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, R.; Yang, M.; Fan, Q.; Hu, W.; Zhang, L.; Yang, Z.; Deng, W.; Shen, Q.; Huang, Y.; et al. Monodispersed Grafted Conjugated Polyelectrolyte Stabilized Magnetic Nanoparticles as Multifunctional Platform for Cellular Imaging and Drug Delivery. J. Mater. Chem. B 2014, 2, 376–386. [Google Scholar] [CrossRef]
- Yang, C.; Huang, S.; Wang, X.; Wang, M. Theranostic Unimolecular Micelles of Highly Fluorescent Conjugated Polymer Bottlebrushes for Far Red/Near Infrared Bioimaging and Efficient Anticancer Drug Delivery. Polym. Chem. 2016, 7, 7455–7468. [Google Scholar] [CrossRef]
- Muthuraj, B.; Mukherjee, S.; Patra, C.R.; Iyer, P.K. Amplified Fluorescence from Polyfluorene Nanoparticles with Dual State Emission and Aggregation Caused Red Shifted Emission for Live Cell Imaging and Cancer Theranostics. ACS Appl. Mater. Interfaces 2016, 8, 32220–32229. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Fang, Y.; Liu, J.; Geng, J.; Ding, D.; Liu, B. Multifunctional Conjugated Polymer Nanoparticles for Image-Guided Photodynamic and Photothermal Therapy. Small 2017, 13, 1602807. [Google Scholar] [CrossRef] [PubMed]
- Doshi, M.; Copik, A.; Gesquiere, A.J. Development and Characterization of Conducting Polymer Nanoparticles for Photodynamic Therapy in Vitro. Photodiagn. Photodyn. Ther. 2015, 12, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Doshi, M.; Krienke, M.; Khederzadeh, S.; Sanchez, H.; Copik, A.; Oyer, J.; Gesquiere, A.J. Conducting Polymer Nanoparticles for Targeted Cancer Therapy. RSC Adv. 2015, 5, 37943–37956. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Gong, X.; Heeger, A.J. Low Bandgap Semiconducting Polymers for Polymeric Photovoltaics. Chem. Soc. Rev. 2016, 45, 4825–4846. [Google Scholar] [CrossRef] [PubMed]















| Polymer | NP/Hybrid | Hybrid Material | Preparation Method | Particle Size (nm) | PLQY (%) | Other Applications | Ref. |
|---|---|---|---|---|---|---|---|
| 30, 31 | NP | / | Solvent exchange | 4–264 | 14–26 | / | [52] |
| 7–9, 32 | NP | / | Solvent exchange | 23 | 1–36 | / | [43] |
| 38–41 | NP | / | Solvent exchange | 58–87 | / | / | [55] |
| 47 | NP | / | Solvent exchange | 42–57 | / | / | [58] |
| 10–12, 36 | NP | / | Solvent exchange | 22 | 47 | / | [44] |
| 5, 6 | NP | / | Solvent exchange | 16 | 13, 19 | / | [42] |
| 20 | Hybrid NP | PEG | Solvent exchange | 80 | 27 | / | [48] |
| 52 | Hybrid NP | Silica | Solvent exchange | 5–50 | 1.5 | / | [63] |
| 23–26 | NP | / | Solvent exchange | 50–100 | 76 | / | [50] |
| 16 | Hybrid NP | PEG | Solvent exchange | 56 | / | / | [46] |
| 2 | Hybrid NP | PEG and dye | Solvent exchange | 45 | 45 | / | [40] |
| 21, 22 | Hybrid NP | PCL-b-POEGMA | Solvent exchange | 50–500 | 26 | / | [49] |
| 14, 15 | Hybrid NP | PEG | Solvent exchange | 28 | 14 | / | [45] |
| 50, 51 | Hybrid NP | PS-PEG | Solvent exchange | 13 | 57 | / | [61] |
| 43–46 | Hybrid NP | PSMA | Solvent exchange | 30 | 3–78 | / | [57] |
| 57, 58 | Hybrid NP | DSPE-PEG | Solvent exchange | 30 | 23 | Theranostic | [68] |
| 37 | NP | / | Solvent exchange | 30 | 14 | / | [54] |
| 30–32 | NP | / | Solvent exchange | 16–21 | 17–30 | / | [52] |
| 27–29 | NP | / | Emulsion polymerization | 25–73 | 56 | / | [51] |
| 53 | NP | / | Self-assembly | 117 | / | Theranostic | [64] |
| 56 | NP | / | Self-assembly | 24 | 3 | Theranostic | [67] |
| 1 | NP | / | Mini-emulsion | 2.9 | 1.7 | / | [36] |
| 3, 4 | NP | / | Mini-emulsion | 116, 117 | 3 | / | [41] |
| 17–19 | NP | / | Mini-emulsion | 78–188 | 8–13 | / | [47] |
| 42 | Hybrid NP | PEG | Mini-emulsion | 20 | 46 | / | [56] |
| 48 | Hybrid NP | Azide-funct. PEG | Mini-emulsion | 130 | 4 | / | [59] |
| 49 | Hybrid NP | HPG | Mini-emulsion | 40–210 | 23 | / | [60] |
| 50 | Hybrid NP | Peptide | Mini-emulsion | 40 | 37–42 | / | [61] |
| 54 | Hybrid NP | Fe3O4 | Ligand exchange on Fe3O4 | 26 | 21.5 | Theranostic | [65] |
| 55 | NP | / | 1 polymer brush/NP | 20–54 | 20–30 | Theranostic | [66] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated Polymer Nanoparticles for Bioimaging. Materials 2017, 10, 1420. https://doi.org/10.3390/ma10121420
Braeken Y, Cheruku S, Ethirajan A, Maes W. Conjugated Polymer Nanoparticles for Bioimaging. Materials. 2017; 10(12):1420. https://doi.org/10.3390/ma10121420
Chicago/Turabian StyleBraeken, Yasmine, Srujan Cheruku, Anitha Ethirajan, and Wouter Maes. 2017. "Conjugated Polymer Nanoparticles for Bioimaging" Materials 10, no. 12: 1420. https://doi.org/10.3390/ma10121420
APA StyleBraeken, Y., Cheruku, S., Ethirajan, A., & Maes, W. (2017). Conjugated Polymer Nanoparticles for Bioimaging. Materials, 10(12), 1420. https://doi.org/10.3390/ma10121420