1. Introduction
A protective coating is utilized to protect a material from various deterioration factors. A typical example of this is protective coatings for cementitious composites such as concrete and mortar that provide barrier protection against the intrusion of water, chloride ion, carbon dioxide, and other corrosive substances. However, if the protective coating is damaged by microcracking or scratching, those substances would penetrate through damaged holes. This causes the deterioration of the cementitious materials, leading to significant reduction of their serviceability. If the protective coating can automatically heal the damaged region, it would very effectively protect the materials from deterioration. Self-healing technology would provide improved public safety and reduced maintenance cost [
1,
2,
3,
4,
5]. Although many studies of self-healing anticorrosive coatings for metal protection have appeared [
6,
7,
8,
9], reports on self-healing coatings for cementitious composites are very rare [
10,
11].
Song et al. previously reported a microcapsule-type protective coating system for concrete application having a sunlight-induced self-healing capability [
10]. When the coating system is damaged, a healing agent is released from ruptured microcapsules and fills the damaged region. The healing agent generates a hard solid through photo-cross-linking, resulting in the recovery of the sealing function. However, the coating system has a limitation, in that it cannot be healed without UV light irradiation. A new concrete coating system that can be self-healed in a dark place is needed for wider application. Another limitation of the previous system is that the healing agent turns into a hard solid through the healing reaction. When microcracking occurs again in the healed region, the self-healing cannot be repeated—not only because the microcapsules are depleted, but also because the solidified healing agent is a hard solid with no additional self-healing ability.
Self-healing protective coatings on bridges, tunnels, and buildings can be exposed to intense vibration induced by heavy traffic. For example, it has been shown that civil engineering structures such as bridges are usually exposed to traffic-induced vibration of 2–10 Hz [
12]. The vibrations can cause damage such as microcracking and crazing. After the first self-healing event, secondary damage may occur in the healed region under continuous vibration, probably inside the solidified healing agent or in boundary between the solidified healing agent and coating matrix. Yang et al. reported a self-healing anticorrosive coating for metal application having secondary damage-preventing capability utilizing a healing agent that turns into a viscoelastic organogel material [
13]. It is known that vibration energy effectively dissipates in a viscoelastic material because of its viscosity and elasticity, leading to the maintenance of its shape [
14].
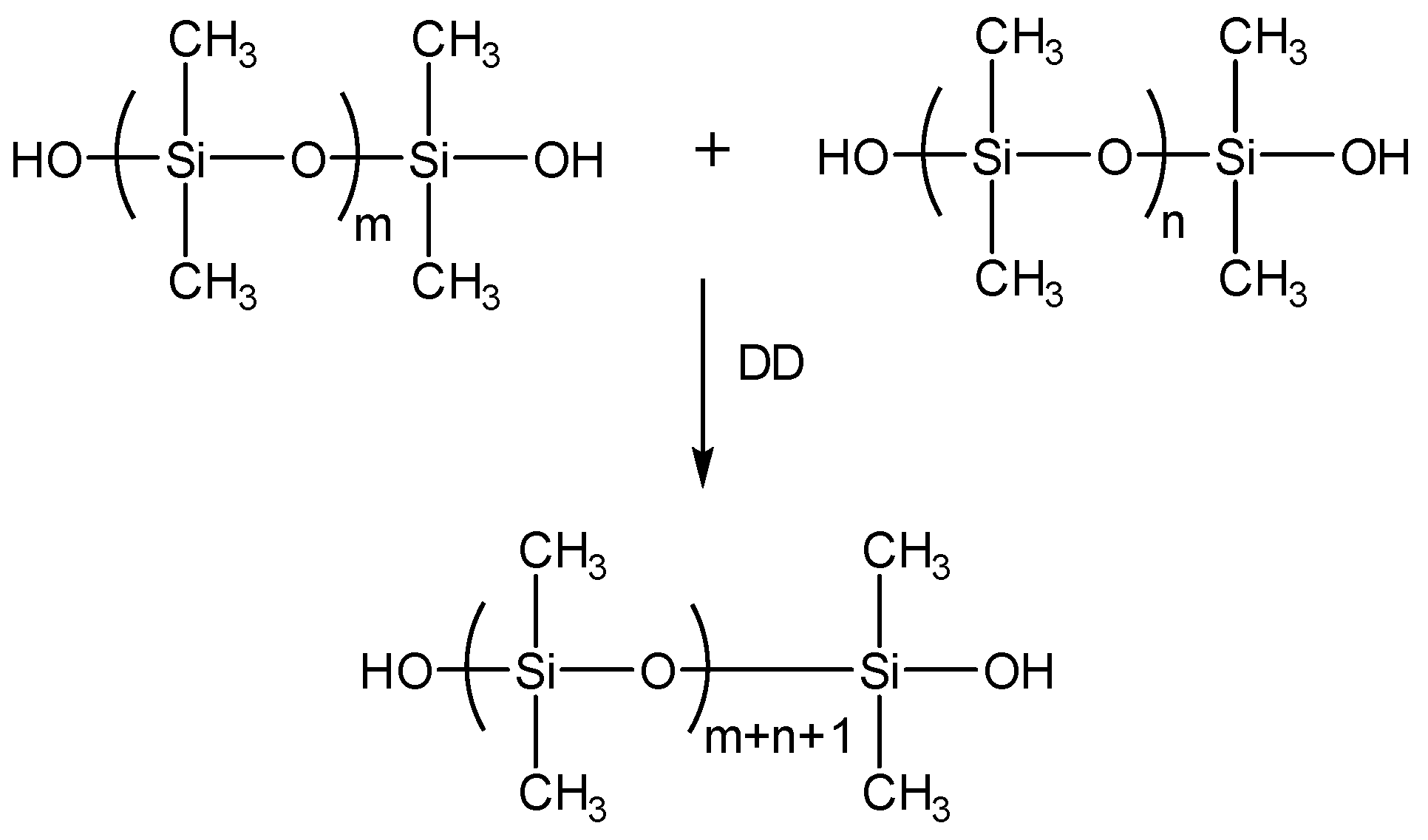
In this paper, a new microcapsule-type self-healing protective coating for concrete application has been developed: the coating system can be self-healed without UV irradiation, and can prevent secondary cracking. A silanol-terminated polydimethylsiloxane (STP)/dibutyltin dilaurate (DD) combination was used as a healing agent, because the two components react to give a viscoelastic substance. The new STP/DD-based self-healing system can offer an additional advantage of minimized loss of healing agent during the healing event, while the self-healing of the previous organogel-based system occurs by evaporation of a relatively large amount of solvent [
13]. STP and DD were separately microencapsulated, and the resulting microcapsules were embedded in a coating matrix to prepare a dual-capsule self-healing coating. The self-healing and secondary damage-preventing ability of the new coating system was demonstrated, and its preliminary evaluation was carried out for mortar specimens.
2. Materials and Methods
2.1. Materials
Urea, formaldehyde solution (37 wt %), poly(ethylene-alt-maleic anhydride) (EMA), resorcinol, 1-octanol, ammonium chloride, tolylene 2, 4-diisocyanate (TDI), 1,4-butanediol (BD), ethylene glycol (EG), gum arabic, cyclohexanone, and chlorobenzene were used in microencapsulation process. Silanol-terminated polydimethylsiloxane (STP) (DMS-S12) and dibutyltin dilaurate (DD) were used as core materials. Gum arabic dispersed in de-ionized water was used as surfactant. An enamel paint formulation (KCI 720, white) and an epoxy coating formulation (KU-420K40) were used as coating materials. All solvents were purified using calcium sulfate as dry agent. Mortar specimens were prepared with a mass ratio of cement:sand:water = 2:6:1 according to a KSF2476 standard method. Mortar paste was first cured in a mold for 48 h at room temperature. Each mortar was further cured for 5 days in water, and then finally cured for 7 days under ambient conditions.
2.2. Instruments
Gel permeation chromatography (GPC) analysis was carried out using a doubly-connected Showa Denko Shodex KF-806L column at 100 °C and an eluent of N-methyl-2-pyrrolidone (NMP) at a flow rate of 1.0 mL/min; the results were calibrated with respect to polystyrene standards. A mechanical stirrer (NZ-1000, Eyela, Tokyo, Japan) was used in microencapsulation. A microscope (BX-51, Olympus, Tokyo, Japan) was used to take pictures of the microcapsules. The microcapsule size was analyzed using a CCD camera (CC-12, Olympus, Tokyo, Japan) equipped in the microscope. Image analysis software (analySIS TS, Olympus, Tokyo, Japan) IR spectra were recorded on a Fourier transform infrared (FT-IR) spectrophotometer (Spectrum One B, Perkin Elmer Co., Waltham, MA, USA). A field emission scanning electron microscope (FE-SEM) (SU-70, Hitachi, Quanta FEG 250, FEI, Tokyo, Japan) was used to examine the morphology of the microcapsules and coating surface. A potentiostat/galvanostat (263A, Ametek, Berwyn, PA, USA) was used to examine the conductivity of the coated substrate. A biaxial simultaneous vibration tester (BF-50UD, IDEX, Lake Forest, IL, USA) was used to apply vibration to coating specimens. An advanced Rheometric Expansion System (ARES, Rheometric Scientific, Piscataway, NJ, USA) was used to examine the viscoelasticity of the reacted STP. Thermal analysis was carried out on a Shimadzu TGA-50 (Shimadzu Corp., Kyoto, Japan) at a heating rate of 10 °C/min under nitrogen.
2.3. Synthesis of Prepolymer
TDI (11.497 g, 0.066 mol) was dissolved in cyclohexanone (70 mL) under nitrogen atmosphere in a 150-mL round-bottom flask. The flask was suspended and heated in an oil bath, and BD (2.704 g, 0.030 mol) was slowly added at 55 °C. The resultant mixture was allowed to react at 80 °C for 24 h. The reaction mixture was evaporated at 80 °C for 3–4 h and then at 100 °C for 1 h in a vacuum oven to remove cyclohexanone, water, and excess TDI. A prepolymer was obtained as a viscous yellow oil.
2.4. Microencapsulation of DD
A gum arabic (3.10 g) surfactant was dissolved in deionized water (20 mL) in a 100-mL beaker to obtain Solution A. On the other hand, the prepolymer (3.00 g) was dissolved in chlorobenzene (32.00 g) and DD (1.00 g) was then added. The resultant mixture was slowly poured into Solution A to give an emulsion, which was then heated. Ethylene glycol (3.00 g) as a chain extender was slowly added to the emulsion at 50 °C. When the temperature reached 70 °C, the reaction was stopped. After filtration, the microcapsules were rinsed with de-ionized water and air-dried for 72 h (yield = 92%).
2.5. Microencapsulation of STP and Methacryloxypropyl-Terminated Polydimethylsiloxane (MTP)
In a 100-mL beaker, water (20 mL) and a 2.5 wt % aqueous solution of EMA (5 mL) were added. The beaker was placed in a temperature-controlled water bath equipped with a mechanical stirrer. Under agitation at 300 rpm, urea (0.503 g), ammonium chloride (0.050 g), and resorcinol (0.050 g) were added. The pH of the resulting mixture was adjusted to 3.5 using a 10% NaOH solution. The mixture was agitated at 1000 rpm, and two drops of 1-octanol were added. STP (8 mL) was added, and the resultant mixture was maintained for 15 min to form a stable emulsion. After adding 37% formaldehyde (1.456 g) solution, the temperature of the mixture was increased to 55 °C and maintained for 4.5 h. The reaction mixture was cooled to room temperature, and the microcapsules were isolated by vacuum filtration. The microcapsules were washed with water and THF, and air-dried for 48 h (yield = 87%). MTP-loaded microcapsules were prepared according to a previously reported method [
10].
2.6. Measurement of Microcapsule Core/Shell Ratio
After weighing, the STP-containing microcapsules were crushed by pressing them in a 5-mL vial and washed with ethanol twice. The ruptured shell material was dried under ambient conditions and weighed. In the case of DD-loaded microcapsules, additional thermogravimetric analysis (TGA) study was performed to investigate the mass ratio of chlorobenzene/DD.
2.7. Viscoelasticity Test
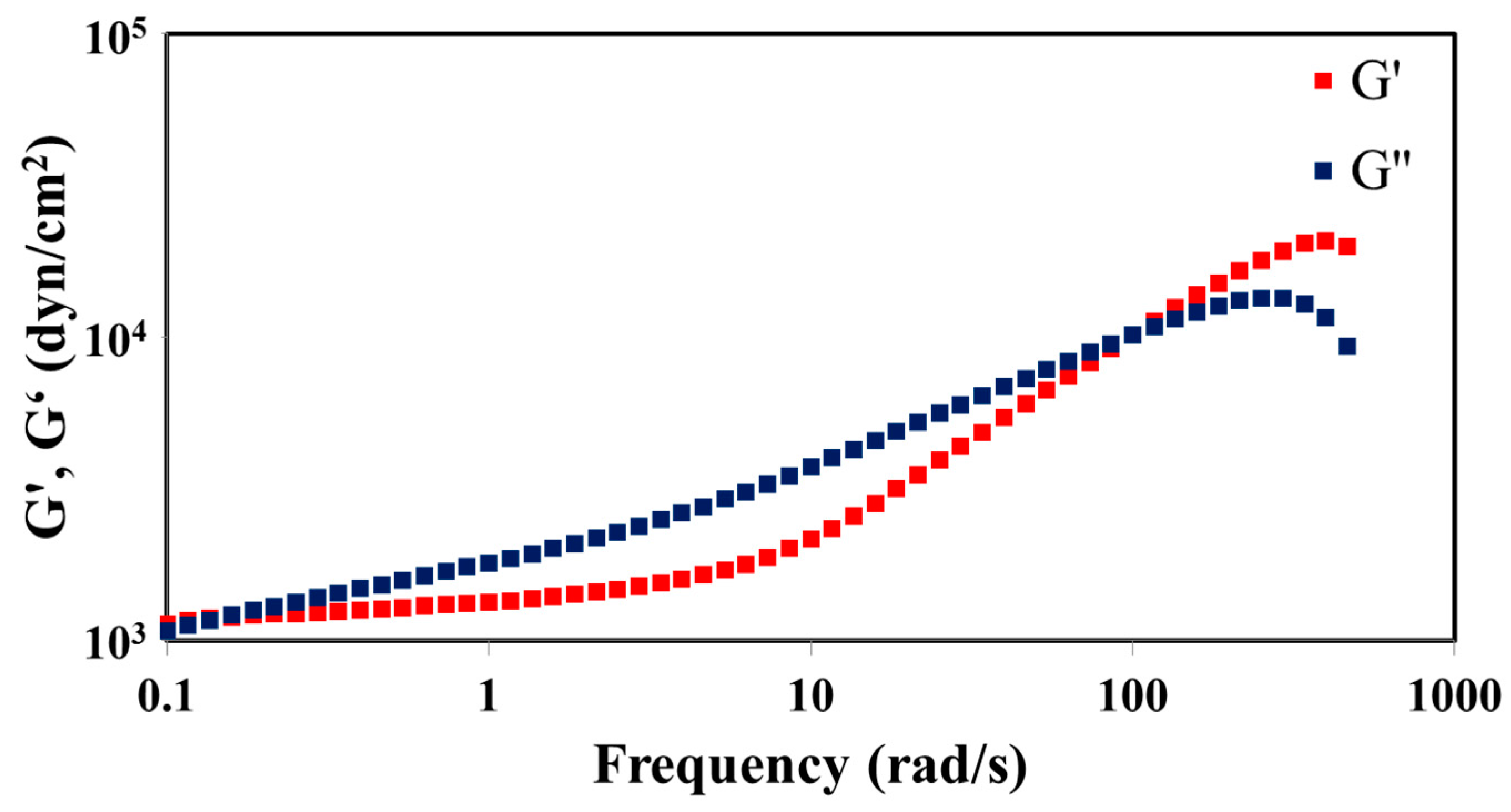
The STP and DD were mixed with a mass ratio of 10:1, and the mixture was poured in molds with 25-mm diameter and 1-mm height and allowed to react for 4 weeks. Disk-like specimens of the reacted STP were separated from the molds. The viscoelasticity of the reacted STP was examined using ARES. This experiment was conducted under conditions where strain and temperature were held constant, while frequency was varied from 0.1 to 510.0 rad/s. Then, experimental results were plotted by G’ and G” versus frequency.
2.8. Preparation of the Coating Samples
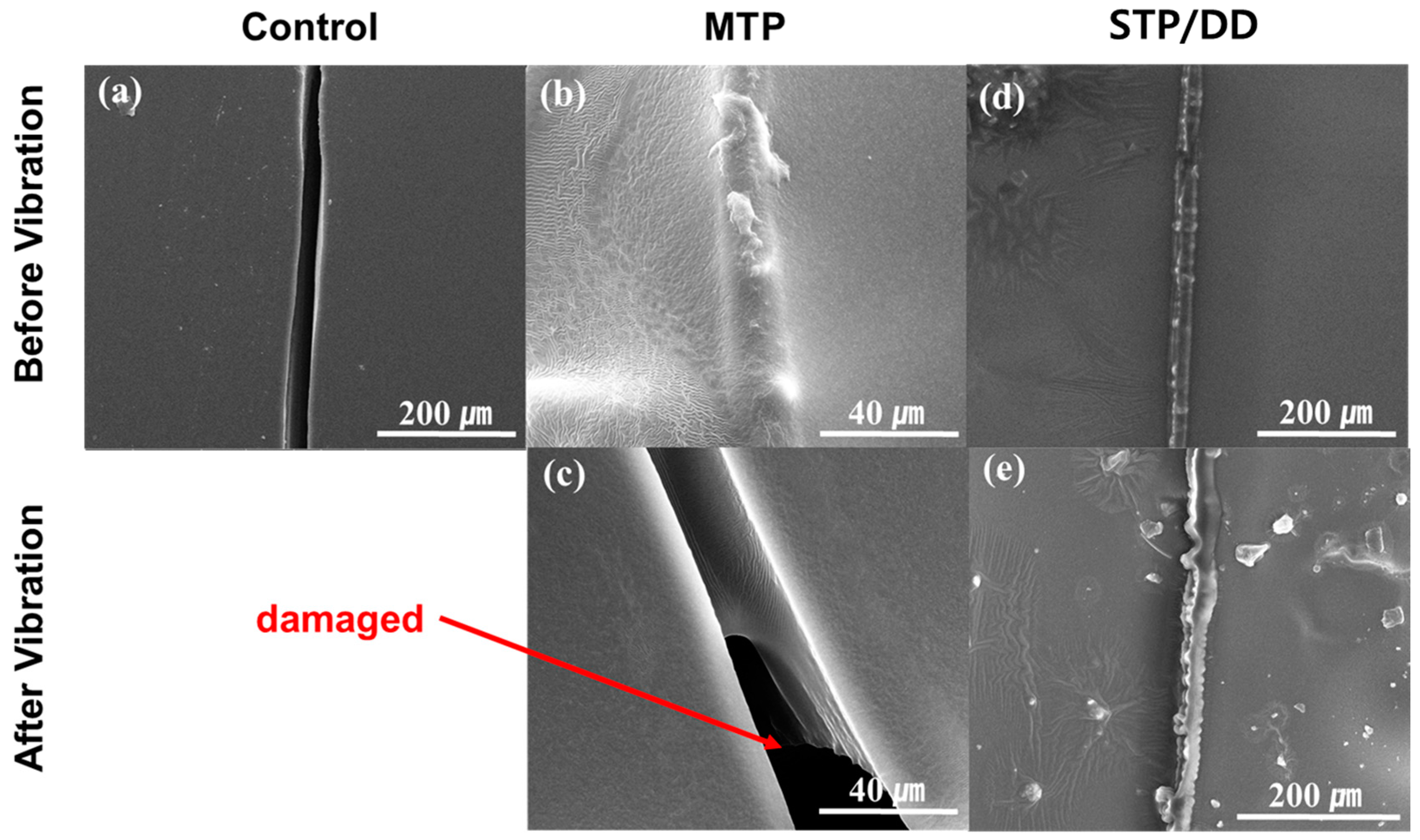
The STP- and DD-containing microcapsules were mixed into the commercial enamel paint (or epoxy coating) formulation with a mass ratio of STP capsules:DD capsules:paint of 19:6:75. The resulting formulations were applied to the surface of the silicon wafer, steel panel, or mortar specimen, and then dried for 3–4 days at room temperature. Control coating samples were prepared in a similar fashion without microcapsules. MTP-based self-healing coatings were prepared by mixing the MTP-loaded microcapsules and the commercial enamel paint (or epoxy coating) formulation with a mass ratio of 1:4. A scratch was generated in the coated surface with a razor blade. The scratched STP/DD-based samples were allowed to heal for 4 h. The scratched MTP-based coatings were photoirradiated with a xenon lamp for 1.5 h.
2.9. Application of Vibration
The coated silicon wafers and steel plates were attached on the top surface of the vibration tester. Vibration was applied in transportation mode with a frequency range of 10–30 Hz. Sweep time and cycle were 15 min and 4 cycles, respectively.
2.10. Electrochemical Test
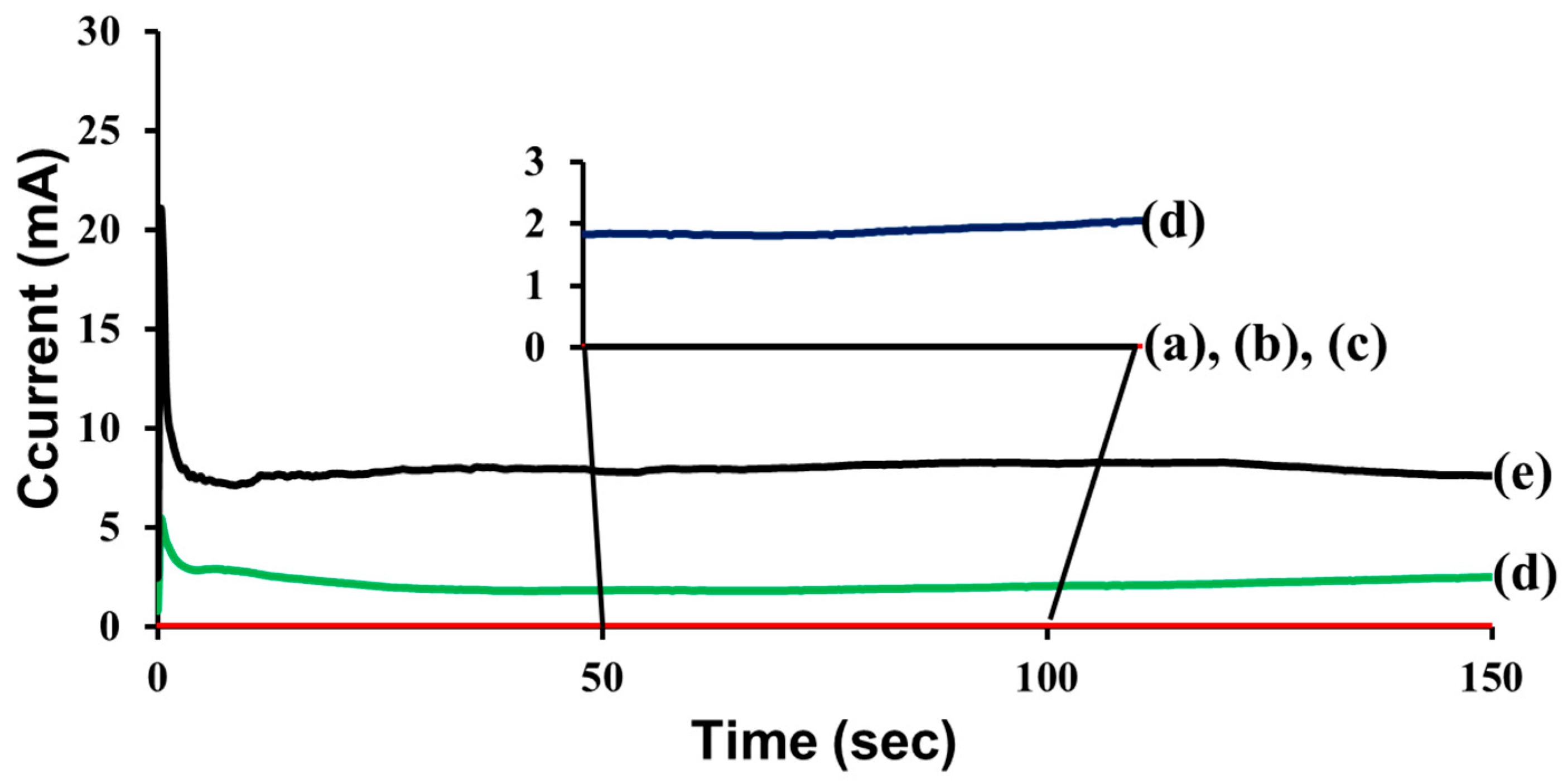
Self-healing coatings and control coatings were prepared on steel panels. Electrochemical tests were conducted by using a computer-controlled potentiostat/galvanostat (model 263A, Advanced Measurement Technology) in a conventional three-electrode electrochemical cell equipped with a platinum counter electrode, an Ag/AgCl electrode in a saturated NaCl aqueous solution as the reference electrode, and the coated steel panel as the working electrode (
Figure S1). The steady-state conduction between the coated metal substrate and a platinum electrode held at 3 V through an aqueous electrolyte (1 mol/L NaCl) was measured. The current passing through the specimen was recorded by using Electrochemistry PowerSuite software (Advanced Measurement Technology, Oak Ridge, TN, USA). For each kind of sample, at least five specimens were used, and an average of the measured values was taken.
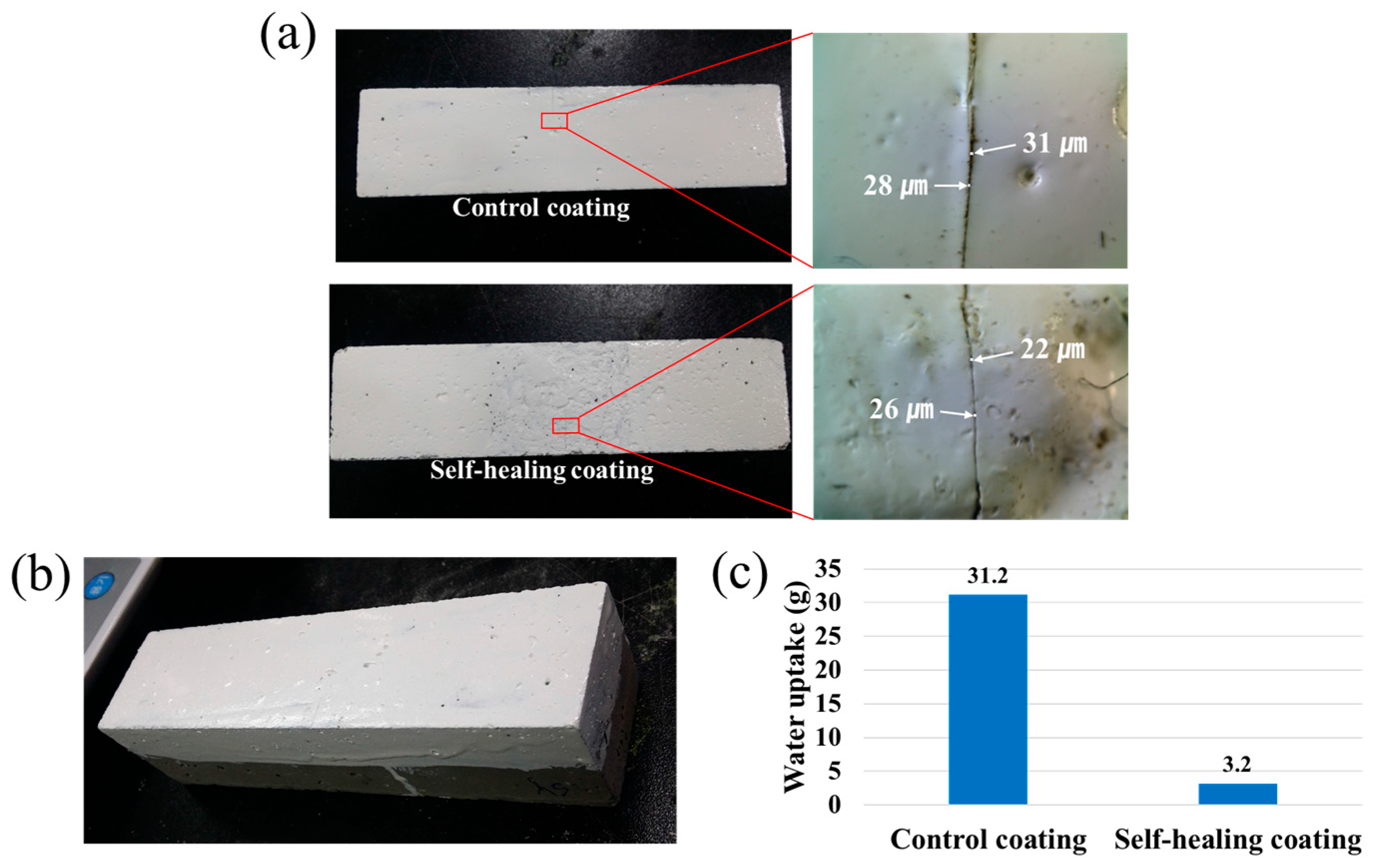
2.11. Water Permeability Test
The self-healing and control coatings were applied to one rectangular side of 40 mm × 40 mm × 130 mm square column mortars. Four side surfaces adjacent to the coated side were covered with epoxy resin to prevent water permeation through the proximate surfaces, and the scratched surface was brought into contact with water. After 48 h, the increase in mass due to water absorption was determined. Three specimens were tested for each kind of sample, and an average of the measured values was taken.