1. Introduction
Separation and recovery of heavy mental ions have always been a significant topic because they are non renewable. There have been some technologies for ions removal from wastewater, such as chemical precipitation, membrane filtration, electrochemistry, adsorption, and so on, in which adsorption has generally been used as an attractive method for industry wastewater treatment. Chen [
1] prepared a kind of adsorbent based on copper hexacyanoferrate, which were chemically deposited on the electrode as a film. Metal ions could be adsorbed on the film under the electric power. Li [
2] synthesized Cd(II) ion-imprinted polymer with imprinting technology by using allylthiourea as a functional monomer and cadmium chloride as the template. It has higher selectivity for the separation of Cd(II) ions from solutions. However, obvious disadvantages appeared with respect to these methods, such as larger energy consumption, complex processing, and giving rise to the transfer of metal ions into water in the process of preparation and use. Thus, it was necessary to develop a novel adsorbent which was low cost, environmental friendly, simple to prepare, and have good performance for separating metal ions.
Potassium tetratitanate whiskers (PTW) are a kind of crystal with a chainlike and open-stratified structure. It has attracted more attention because it is environmentally friendly, non-toxic, and has strong mechanical properties. The interlayer potassium ions can play an important role in ion exchange with other positive ions, such as Pb
2+, Cr
2+, Cu
2+, and so on [
3,
4,
5,
6]. Additionally, PTW has powerful reactivity to be surface modified. Ce
3+-imprinted functionalized PTW sorbent was prepared using surface imprinted technology [
7] and the surface imprinted polymer composites (MIP/K
2Ti
4O
9) were prepared using dibenzothiophene (DBT) as the template, 4-vinylpyridine as the functional monomer, and potassium tetratitanate whiskers as the carrier [
8]; Ogawa et al. intercalated alkylmmonium cations into the layer of titanate [
9,
10,
11,
12]. These reports all illustrated that surface modification could promote the ion exchange properties of PTW.
Chitin is a building block of the skeletons of crustaceans, insects, and diatoms. Chitin/chitosan-based materials have been widely applied as adsorbents for heavy metal ions or radioactive elements from wastewater [
13,
14,
15,
16]. The high adsorption property of chitin/chitosan-based materials could be attributed to: (i) high hydrophilicity due to the large number of hydroxyl groups of glucose units; (ii) the presence of a large number of functional groups; (iii) high chemical reactivity of these groups; and (iv) the flexible structure of the polymer chain [
17]. Gomes [
18] studied the interaction between metal cations and chitin/chitosan by means of density functional theory. For chitin, compounds with Cu
2+ were more stable than others, and Cu
2+ preferred to combine with the oxygen atom of the amide group than the nitrogen atom of the amide group. Wysokowski [
19] proposed in their review that chitin-based inorganic-organic materials could be obtained under hydrothermal conditions, such as the formation of chitin/SiO
2, chitin/ZrO
2, and chitin/ZnO due to hydrogen bonds. Chitin whiskers (ChW) are widely applied as reinforcing agents because of its nontoxicity, biodegradability, and excellent mechanical behavior [
20,
21], can be obtained by removing the amorphous domains of chitin through acid hydrolysis [
22,
23,
24,
25]. Meanwhile, ChW has similar reactivity and adsorbability with chitin because they both have plenty of hydroxyl groups and acetyl amino groups [
20,
26,
27,
28,
29]. For example, Kalaprasad [
30] described the surface chemical modification of hydroxyl on ChW with various reagents to improve applied properties. ChW showed higher adsorption capability for crystal violet compared to other adsorbents [
31]. Blending products of ChW and layered rectorite could enhance physicochemical properties of chitosan film due to cooperation effects. Moreover chitin hydrogel could be reinforced with TiO
2 nanoparticles to enhance the adsorption of arsenic [
32].
To sum up, it is feasible for ChW to combine with PTW as a novel composite material. According to the earlier experimental data, adsorption capacities of PTW for metal ions are much larger than that of ChW. When ChW film is attached to the surface of the PTW, ChW film outside will be like a barrier to block other impurity ions, and pure Pb2+ will enter inside and be adsorbed by PTW. There is almost no literature referring to ion separation by such an organic-inorganic compound adsorbent.
The purpose of the present study was to discuss the separation property and mechanism of the novel ion separating agent, named ChW-PTW, prepared by clothing ChW on the surface of PTW under heating treatment. Lead is one of the most toxic heavy metals and is used in industry widely. It is exhausted quickly with economic development. Thus, it was significant to choose Pb2+ as the subject. Firstly, a batch of adsorption experiments of ChW and PTW on Pb2+ and Cu2+ were conducted to determine the adsorption isotherm pattern and adsorption kinetics model, on which adsorption affinity, adsorption rate constant, and diffusion rate constant could be estimated. Then the separation capability and mechanism of ChW-PTW for Pb2+ from Cu2+-Pb2+ mixed solution was discussed based on the difference of adsorption processes between ChW and PTW on Cu2+ or Pb2+.
3. Results and Discussions
3.1. Morphological and Structural Characteristics
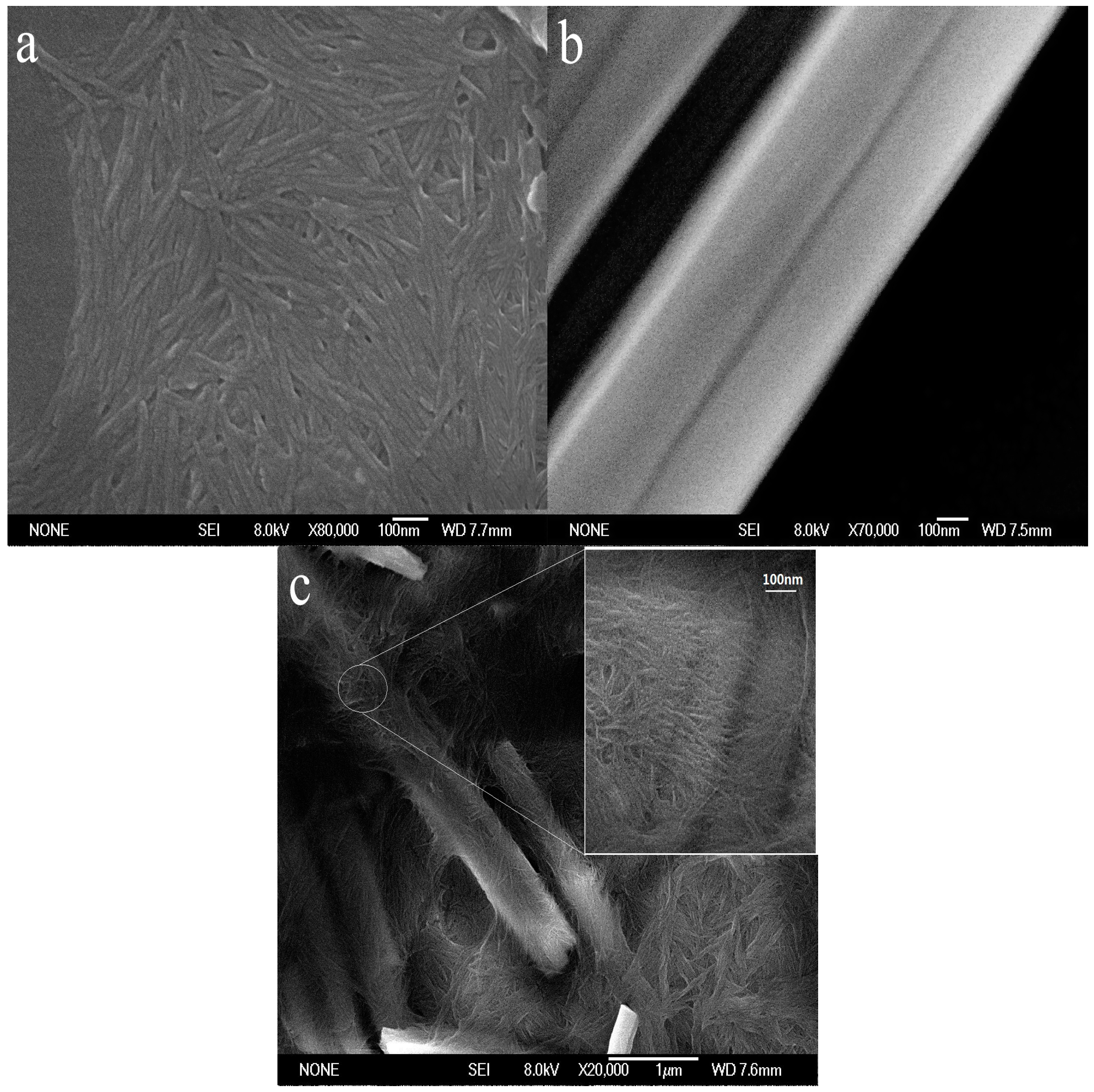
The morphology of ChW, PTW, and ChW-PTW are shown in the
Figure 1. It can be revealed in
Figure 1a that the length of the ChW is about 80 nm to 300 nm. It is well distributed and takes on a membrane shape. It can be seen from the
Figure 1b that PTW has a smoother and larger surface than ChW, and it presents stiff rod-like. In the
Figure 1c, it shows the morphology of ChW-PTW from an overall perspective and a partially enlarged view. Obvious coalescence between ChW and PTW occurs. ChW is even-distributed on the surface of PTW and clads PTW as a film.
ChW has enough activity binding sites, such as hydroxyl, acetyl, and amidogen, and there is powerful electronegativity on the surface of PTW due to Ti-O. When PTW was mixed with ChW emulsion completely and operated by heating treatment, a new composite material was obtained by hydrogen bonding between ChW and PTW.
3.2. The Adsorption Property of ChW and PTW
The optimal adsorption conditions were determined, when 0.1 g of adsorbent was added into 20 mL solution with a concentration of 1.0 mmol/L, optimal pH of the solution was 5–6, the suitable temperature was 25 °C, and the time needed to reach equilibrium was 2–3 h. At this time, the adsorption capacities and comparison with other adsorbents were listed in
Table 1.
It can be seen from
Table 1 that adsorption capacities of PTW for Cu
2+ and Pb
2+ are both much larger than that of ChW. This difference will contribute to the ion separation. To analyze the separation process of the composite, it is necessary to research each removal and diffusion pattern of ChW and PTW.
Compared with other adsorbents, the adsorption capacities are lower, possibly because that: (1) adsorbability of ChW itself is weaker than chitin; (2) before preparing of the composite material, ChW and PTW were both unground. The composite material was also unground, because the structure of the composite was very important to assure the separation degree. As contrast samples, ChW and PTW were unground when batch of experiments were carried out for isotherm and kinetics.
The symbols used in adsorption isotherm and kinetics were listed in
Table 2.
3.2.1. Adsorption Isotherm
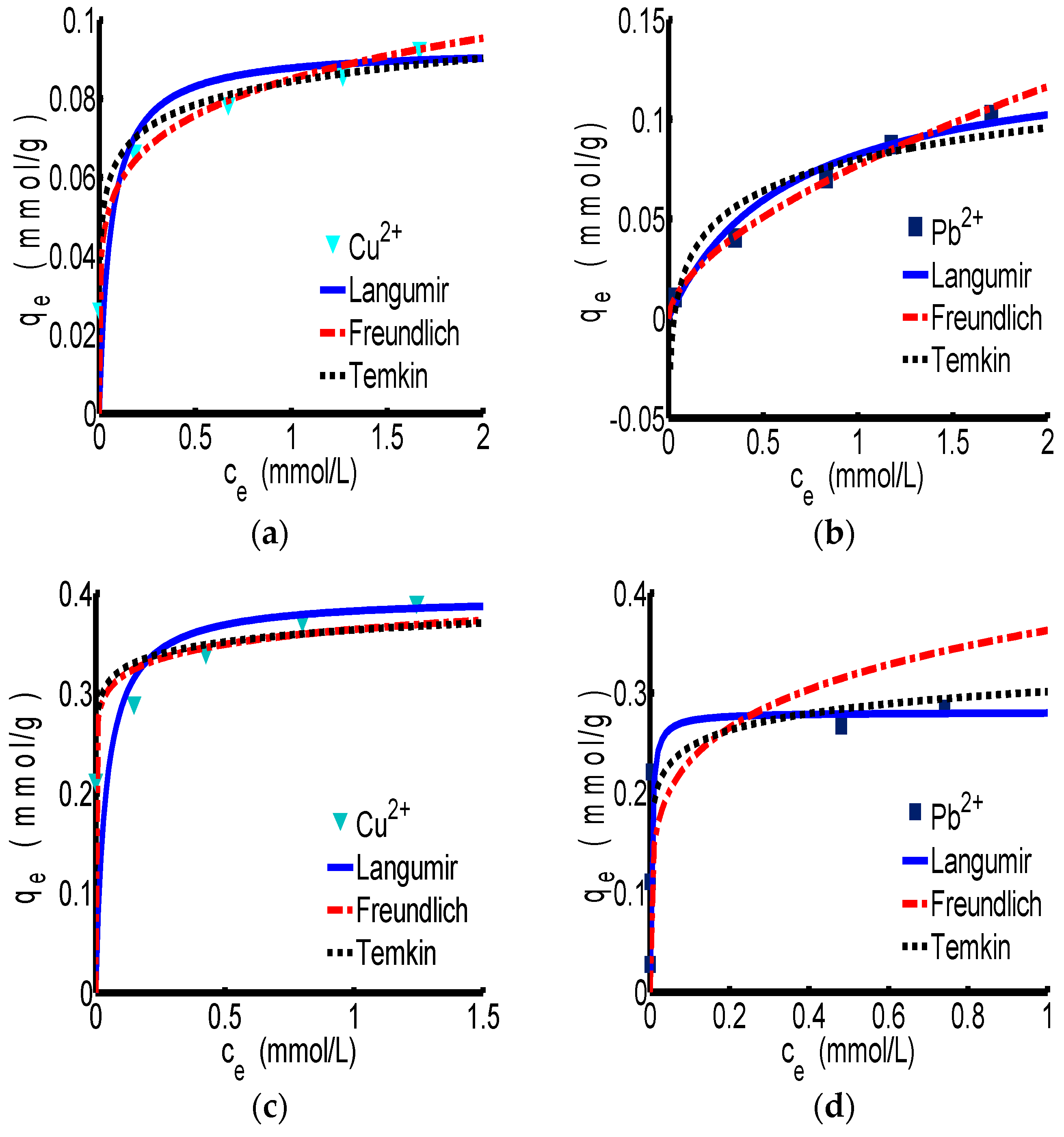
The adsorption isotherm curves of Langmuir, Freundlich and Temkin for ChW and PTW are revealed in
Figure 2.
Figure 2a,b correspond to the adsorption process of ChW on Cu
2+ and Pb
2+, respectively. According to the parameters in
Table 3, the correlation coefficient (R
2) of the Freundlich model for ChW is 0.998 for Cu
2+ and 0.999 for Pb
2+, which is slightly larger than that of Langmuir and Temkin, indicating that the absorption pattern of ChW for Cu
2+ and Pb
2+ is more inclined to the Freundlich adsorption model. In this case, k
F was 0.085 g
−1 for Cu
2+ and 0.077 g
−1 for Pb
2+, that is to say, binding activity of Cu
2+ on ChW is stronger than that of Pb
2+.
The absorption pattern of PTW on Cu
2+ and Pb
2+ fits well to the Langmuir model, as shown in the
Figure 2c,d and
Table 3. At this time, the k
L was 25.85 L·mmol
−1 for Cu
2+ and 310.59 L·mmol
−1 for Pb
2+, indicating that Pb
2+ can be captured by PTW much more firmly than Cu
2+.
The adsorption pattern of ChW on Cu2+ and Pb2+ is in accordance with the Freundlich isotherm, and Cu2+ will be adsorbed more easily than Pb2+. The adsorption pattern of PTW on Cu2+ and Pb2+ can be represented by the Langmuir isotherm, and the adsorption of Pb2+ was much stronger than that of Cu2+.
3.2.2. Adsorption Kinetics
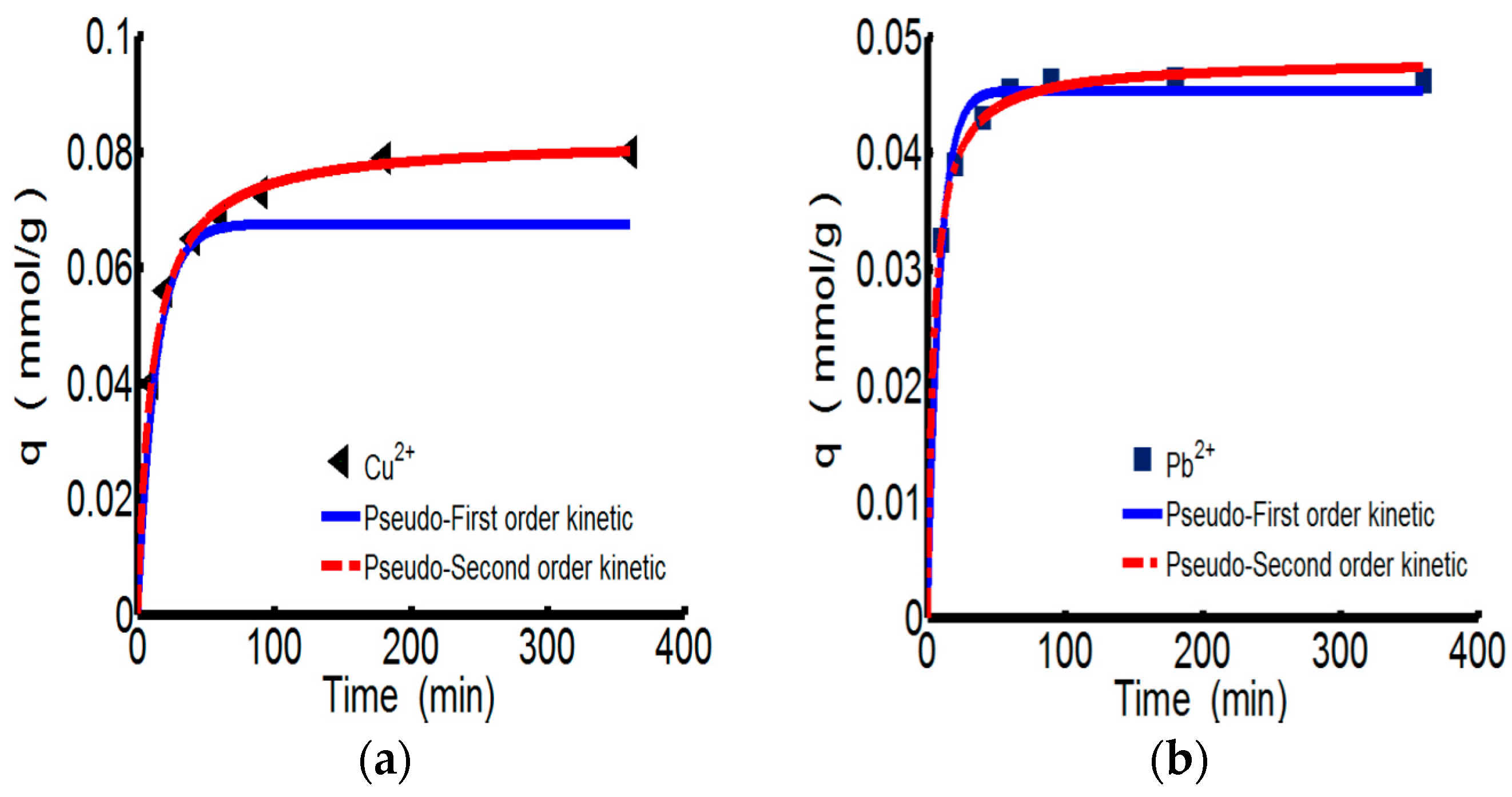
Figure 3 shows the adsorption kinetics curves of ChW or PTW on Cu
2+ and Pb
2+. Relevant parameters for pseudo-first-order and types 1–4 of pseudo-second-order were shown in the
Table 4. All of the models fit the experiment data very well. However considering the conformity of data and the calculated values expressed by the correlation coefficient (R
2), the pseudo-second-order kinetics are more suitable for describing the adsorption behaviors of both ChW and PTW. The results illustrate that ions can combine with active sites on ChW or PTW by covalent chemical bonds [
2].
As for the pseudo-second-order kinetics, k
2 and
qe can be calculated from the plots for the linear forms of pseudo-second-order kinetics models of types 1–4 [
34] given in
Table 5. The values of k
2,
qe,
h, and
qe, cal obtained from the four linear forms of pseudo-second-order equations were found to be different. Giving consideration to both correlation coefficients and differences between the experimental (
qe, exp) and calculated sorption capacities (
qe, cal), the adsorption of ChW based on Cu
2+ and Pb
2+ better fits type 1, while adsorption of PTW can be described by type 2. Pseudo-second-order models of types 3–4, although the high values of their correlation coefficients, cannot be taken into consideration because of the larger differences between the experimental and calculated sorption capacities.
The reaction rate constant (k2) of ChW on Cu2+ is 1.152, much lower than that of Pb2+ (6.518), while k2 of PTW on Cu2+ is 0.825, slightly smaller than that of Pb2+ (0.846). Demonstrating that Pb2+ can be more rapidly captured on both available adsorption sites of ChW and PTW than the adsorption of Cu2+.
Weber-Morris model is applied to determine the rate-limiting step in this adsorption system, which represents the time dependent intra-particle diffusion of the solvend. If the Weber-Morris curve of
q (mmol/g) against
t0.5 (min
0.5) gives a straight line, the adsorption process is diffusion controlled. If the plot is multi-linear and the lines do not pass through the origin, then a combination of two or more processes influence the adsorption [
36].
Multi-linear relationships that have three different linear regions with different slopes are shown in
Figure 4. The intra-particle diffusion rate constants (k
ind1, k
ind2, k
ind3) correspond with the diffusion rates which can be calculated from the slopes of the linear curves and changes in different adsorption stages. The diffusion rate constants are presented in
Table 6, along with the correlation coefficients. The initial region (0–10 min) is the sharpest and corresponds to the external adsorbate diffusion in the boundary layer. That is, ions could quickly diffuse to exposed adsorption sites on the surface, and k
ind1 of Pb
2+ towards PTW is significantly higher than that of Pb
2+ towards ChW. The second region (20–90 min) relates to the gradual adsorption stage in which the intra-particle diffusion is potentially the rate-limiting step. When the external surface is nearly saturated, the ions gradually pass through the surface pores and will be retained in the micropores of the particles. At this stage, the diffusion resistance increases and the diffusion rate decreases. k
ind2 of ChW-Pb
2+ decreases from 0.0106 mmol/g·min
0.5 to 0.0016 mmol/g·min
0.5, reduces to about 15%. In contrast, k
ind2 of PTW-Pb
2+ decreases from 0.0247 mmol/g·min
0.5 to 0.0114 mmol/g·min
0.5, reduces to about 46%, illustrating that after 20 min, the diffusion resistance of Pb
2+ on ChW will be far greater than on PTW. The third region (>90 min) is a plateau that represents the final equilibrium stage, k
ind3 especially of the adsorption ChW reduce to nearly zero. By contrast, PTW can absorb ions continuously at this stage (k
ind3 was 0.001 mmol/g·min
0.5 for both Cu
2+ and Pb
2+).
3.3. The Separation Effect of Double-Ion-Mixed Solution
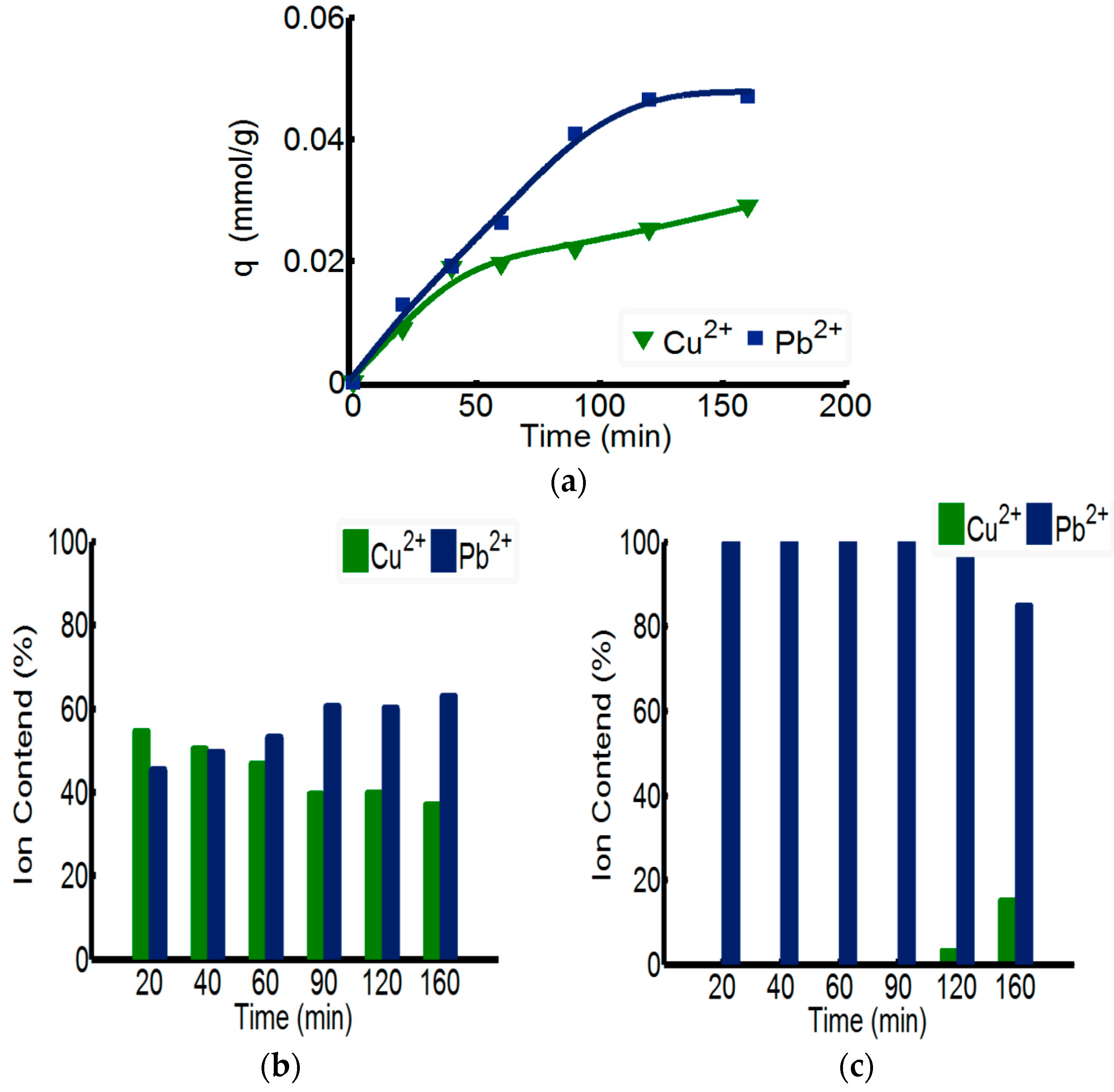
The separating capability of ChW-PTW on Pb
2+ from Cu
2+-Pb
2+ mixed solution is discussed in
Figure 5. As shown in the
Figure 5a, the overall adsorption rate of ChW-PTW on Pb
2+ is quicker than that of Cu
2+. When the time reaches 160 min, the adsorbance of Pb
2+ can be up to 0.046 mmol/g, more than that of Cu
2+. This outcome can be explained by the test data of adsorption kinetics, as the adsorption rate constant of Pb
2+ on ChW or PTW is both larger than that of Cu
2+.
The relative percentage quantity of Cu
2+ and Pb
2+ in the ChW film on the surface of PTW is shown in
Figure 5b. The relative mass percent of Cu
2+ gradually reduces as time progresses, while that of Pb
2+ gradually increases.
Figure 5c shows the relative percentage quantity of Cu
2+ and Pb
2+ in the inner PTW layer. When adsorbed within 90 min, the mass percent of Pb
2+ was 100%, but when the time was prolonged to 120 min, Cu
2+ could pass through the ChW film and permeate into the PTW layer.
In summary, Pb2+ can be adsorbed more quickly on ChW-PTW than Cu2+ and is more inclined to be combined on the PTW layer. When adsorbing within 90 min, ions on the PTW are pure Pb2+. Thus, ChW-PTW will be a promising ion separating agent, and can play an important role to purify Pb2+.
3.4. The Possible Separation Mechanism of ChW-PTW
Based on the isotherm patten and kinetics model of ChW and PTW, (1) the reaction rate of Pb2+ is greater than that of Cu2+ on both ChW and PTW; (2) the adsorption affinity between Cu2+ and ChW is much larger than that of PTW; (3) PTW has greater advantage to adsorb Pb2+; (4) the diffusion resistance of Pb2+ in the intra-particle of PTW is much smaller than ChW in the second stage; and (5) the third stage begins after adsorbing 90–120 min. At this time, adsorption of Cu2+ on ChW is over, and Cu2+ can pass through the ChW film and permeate into the PTW layer.
When Cu
2+ and Pb
2+ contacted with the composite material ChW-PTW, the ChW film could catch Cu
2+ firmly and block Cu
2+ from entering into the PTW layer. Most of the chelation sites of ChW could be occupied by Cu
2+. On the other hand, Pb
2+ would adsorb on the ChW layer prior to Cu
2+, but the adsorption capability (k
F) and diffusion rate (k
ind2) of Pb
2+ on ChW was less than Cu
2+, moreover, the diffusion rate (k
ind2) of Pb
2+ on PTW was much larger than on ChW. Then partial Pb
2+ chelated on the residual active sites of external ChW film quickly, other Pb
2+ would continuously transfer into the interior-ChW-PTW and adsorbed on the PTW layer by ion exchange. The possible separation process and mechanism of the double-ion-mixed solution is revealed in the
Figure 6.