1. Introduction
Granular activated carbon (GAC) has a remarkable adsorption capacity due to its extensively developed internal pore structure and large specific surface area [
1], making it an ideal medium for adsorbing impurities and purifying both aqueous solutions [
2,
3,
4] and flue gas [
5]. To recycle and conserve the limited resources, the GAC needs to be regenerated. Microwave thermal treatment for regenerating GAC is promising [
6,
7,
8]. For instance, Ania et al. [
9] studied the effect of different heating mechanisms (a conventional electric furnace versus microwave device) to regenerate the activated carbon exhausted with phenol. Regeneration time was considerably shortened in the microwave device compared to regeneration with the conventional electric furnace. Meanwhile, the porous structure of the regenerated AC using the microwave device was more efficient than that using the conventional electric furnace.
However, the conventional characterization method to evaluate the adsorption performance of the generated GAC is time-consuming. It usually takes dozens of hours to accomplish the test [
10]. This paper established the relationship between effective permittivity and pore volume of the GAC. Many studies indicate that either pore volume [
11] or pore structure [
12], especially pore size distribution (PSD) [
13,
14] is the most important adsorbent property for GAC. With the development of the microwave measurement method in complex permittivity, e.g., resonant cavity method [
15,
16,
17], perturbation method [
15,
18,
19], transmission and reflection method [
20,
21,
22], it is convenient to get the complex permittivity data of the GAC.
This paper proposes two equivalent models to establish the relationship between effective complex permittivity (εeff) and pore volume (vg) for a given commercial GAC. Brunauer–Emmett–Teller (BET) testing and microwave permittivity measurement with perturbation method are introduced below to verify the relationship between effective complex permittivity and pore volume.
2. Derivation
The Maxwell-Garnett (MG) effective medium theory [
23], originally derived by neglecting the density fluctuations of dipolar character of scatterers, is the most widely used theory to characterize the complex permittivity of medium [
24]. Li et al. [
25] applied it in characterizing complex permittivity of solids. It can predict the effective permittivity of a mixture based on the equation:
where ε
m, ε
h and ε
i are the complex permittivity of the mixture, host medium and inclusions, respectively, and δ
i is the volume fraction of the inclusions. Equation (1) is valid under the condition of low volume fractions. It is necessary to make sure that δ
i does not exceed 0.5.
In this particular case, we simply regard the pure GAC with no pore structure, namely the “the ideal pure GAC”, as the host medium. Its complex permittivity is characterized as . The value of should only exist in theory as it can not be measured directly. Similarly, we regard the pore as the inclusions. Thus, the whole GAC is composed of pure GAC and pores. The complex permittivity (εeff) of the GAC should be the mixture.
With several steps of elementary algebraic transformation, the MG effective permittivity represented by Equation (1) can be solved by:
We simply assume the pore is full of air, which means ε
i = 1. Taking the defining equation of δ
i into account, we obtain:
where
Vi is the volume of the inclusions and
Vtotal is the total volume of the whole GAC. Particularly, when it is for the unit mass of the GAC,
Vi turns to be a significant physical concept–the pore volume (
vg) in cm
3/g.
We introduce the granule density (ρg), which is defined as the mass (m0) divided by the volume (v0) in a single granule, i.e., ρg = m0/v0. This approach offers two ways to build our model to make the connection between εeff and vg.
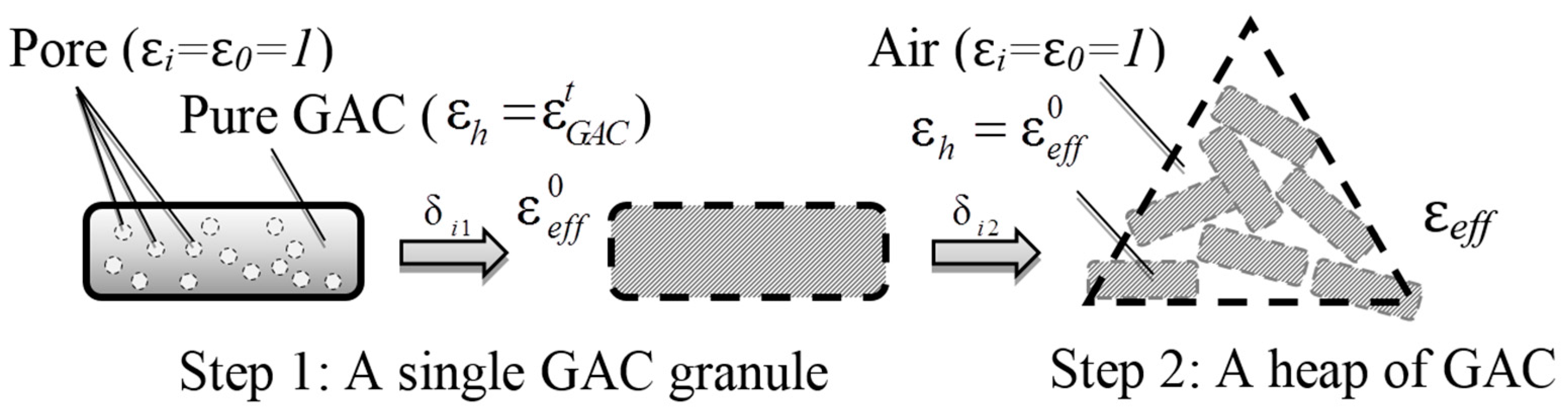
2.1. Model A
Step by step with applying the MGA Equation (3) twice. As is shown below (
Figure 1), it takes two steps to make the connection between ε
eff and
vg.
Step 1: Focusing on a single GAC granule.
Volume fraction δ
i of a single granule should be expressed as follows:
Substituting this relationship into Equation (3):
Step 2: Considering a heap of GAC.
Whereby the volume fraction is calculated as follows:
where
M and
V are the mass and the volume of the heap, respectively, and
Vhost is the volume of the host media (i.e., the total volume of the GAC granule in the heap).
Similarly, substituting Equation (6) into (3):
Equation (7) is simplified by multiplying the numerator and denominator with
V∙ρ
g:
where
M,
V, ρ
g, and
are constant.
M,
V, and ρ
g can be measured directly and conveniently. The independent variable
vg is implied in the expression of
in Equation (5).
2.2. Model B
Directly focus on a heap of GAC, applying MGA Equation (3) only once. In this particularly situation, we simply treat porosity both in the GAC granule (i.e., total pore volume) and between each GAC granules the same. They are all regarded as inclusions, sharing the same permittivity: ε
i = ε
0 = 1. In this instance, the volume fraction will be less complicated, which is expressed as follows:
Substituting Equations (9) into (3), yielding:
Multiply the numerator and denominator with
V∙ρ
g, and simplify it, finally we obtain:
Similarly, M, V, ρg, and are constant or measurable, it is obvious that the Equation (11) achieved the goal to make the connection between εeff and vg.
3. Experiment Setup
3.1. Materials and Sample Preparations
The original GAC came from the commercial activated carbon (Analytical pure, KELON Chemical Reagent Factory, Chengdu, China). All of the GAC samples are dried for 2 days at 90 °C with the electric drying oven, except sample B, which was initially soaked in deionized water and then be frozen at −40 °C for 1 day. This process was done to physically enlarge the pore volume. The other samples were named as A, C1, C2 and C3. Generally, sample A was the blank, sample B was treated by freezing procedure, samples C1–C3 were treated by microwave irradiation with a modified domestic microwave oven. The modification involved replacing the magnetron by an industrial one, which is cooled by water and the model named as 2M410A. All of the samples C1–C3 were treated by a microwave in the same anode current 0.4 A, but for different durations (min). In addition, to prevent the GAC from over treatment and potentially burning down, the maximum microwave irradiation duration should be less than 3 min. Thus, in order to observe the effect of different microwave irradiation duration on the properties of GAC samples, the microwave irradiation duration for samples C1–C3 was set to be 1, 2, and 3 min, respectively.
3.2. Measurement and Characterization
(a) Measurement of the effective complex permittivity: resonant cavity method.
Specific measurement apparatus and particular principles were referenced in [
17]. In general, the measurement system was composed of two parts:
A metal can cavity and the accompanying open-end coaxial probe were well-designed. The can was finely manufactured with a fixed size. Thus, when the can was filled with the GAC samples, the heap volume V was the same as the volume of the can.
A vector network analyzer (VNA) (N5230A, Agilent, Santa Rosa, United States) was used to measure the magnitude and phase of scattering parameter S11. Based on a well-trained back propagation (BP) neural network as the core algorithm, it was quite convenient to infer the effective complex permittivity of the sample.
(b) Measurement of the fundamental physical coefficient: the heap mass M, and the granule density ρg.
A total of 20–30 grains of GAC were picked arbitrarily. The mass and geometric dimensions of each granular was determined by electronic balance and vernier caliper, respectively. The data was used to calculate the arithmetic mean of the mass and volume for each grain. Finally, the mass of the granular was divided by the volume to obtain the density ρg.
(c) Characterization of the pore volume.
Pore volume was determined using the automatic surface area and pore analyzer (ASAP2020, Micromeritcs, Norcross, GA, USA). Nitrogen isotherm adsorption (at relative pressure of 0.99 atm) was used to calculate the pore volume by the static volumetric method.
4. Results and Discussion
The detailed experimental results are collected in
Table 1.
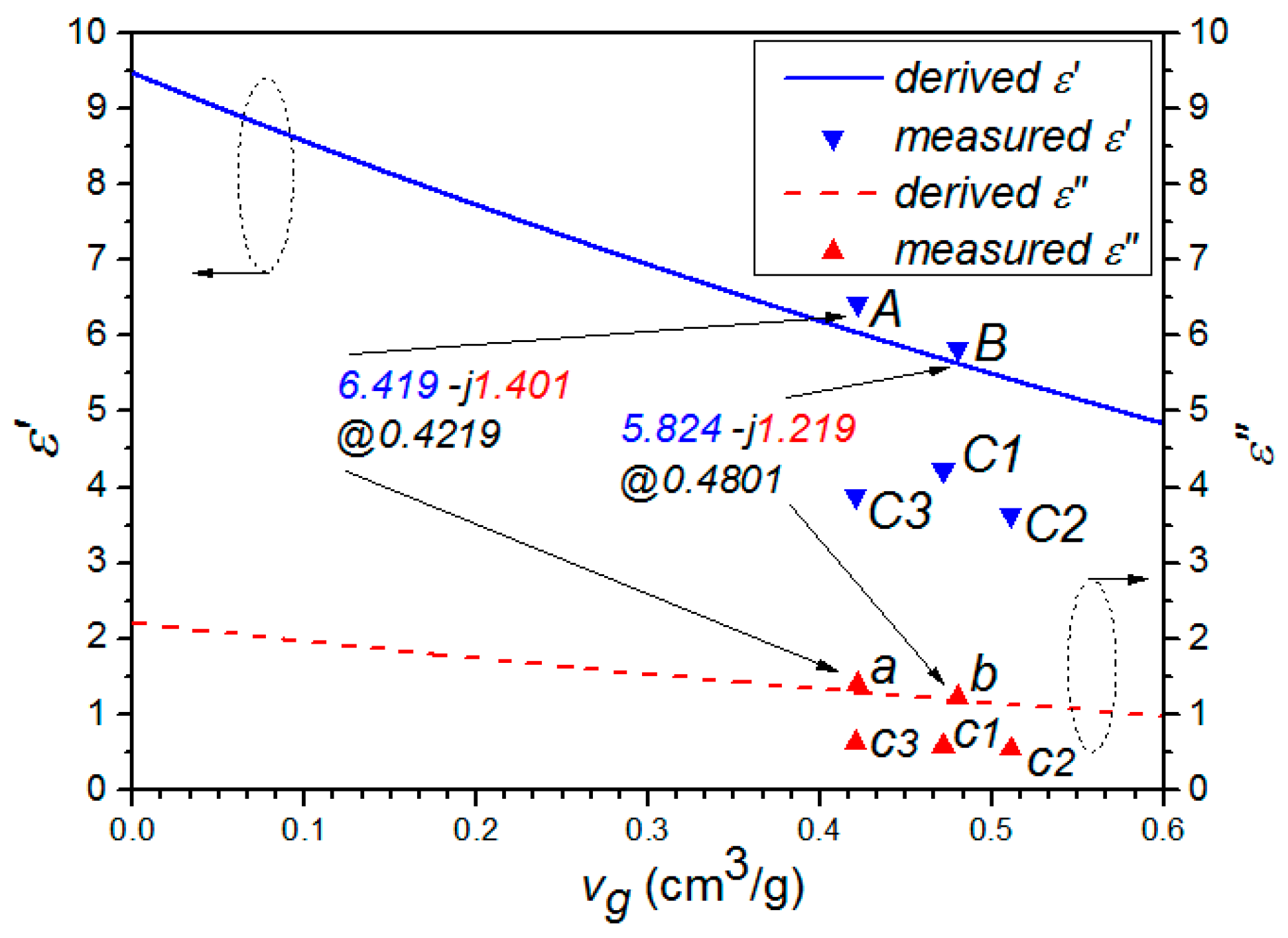
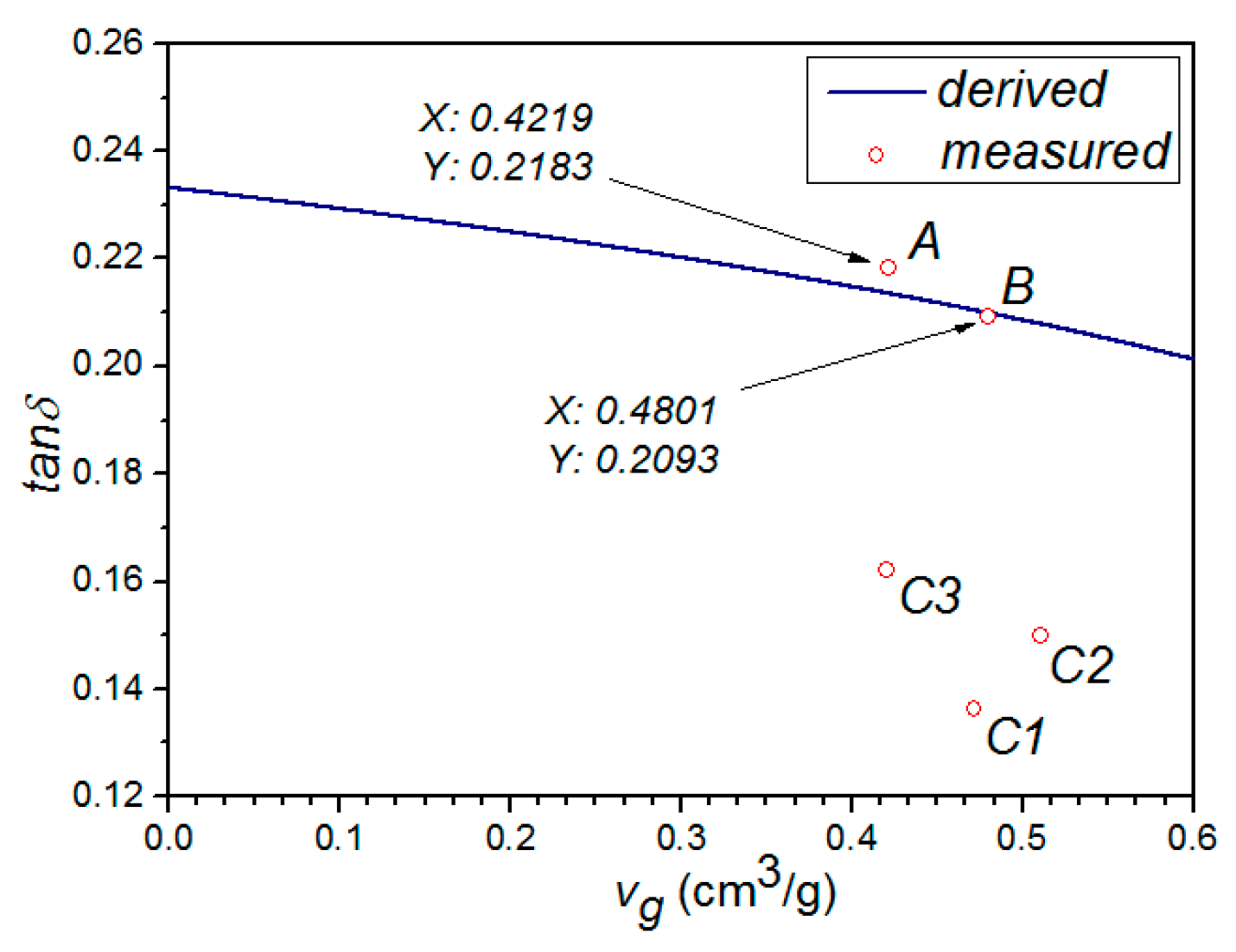
In
Table 1, compared the properties of sample A to sample B, it showed that the frozen process can enlarge the pore volume by about 13.8% or (0.4801 − 0.4219)/0.4219 and reduce the real and imaginary part of the complex permittivity by about 9.3% or (6.419 − 5.824)/6.419 and 13.0% or (1.401 − 1.219)/1.401, respectively. Similarly, the pore volume of samples C1–C3 was enlarged by the microwave irradiation, while the permittivity decreased accordingly. However, we can also noticed that when the microwave radiation duration reaches a certain extent, the pore volume of the sample will be decreased, as shown for sample C3 in
Table 1. Therefore, in order to prevent the sample from over treatment and potentially burning down, a reasonable microwave radiation duration was required.
In order to further study the relationship between the pore volume and the permittivity of the GAC samples, we take the average density (
= 0.7673 g/cm
3) and heap mass (
= 409.483 g) to represent the GAC’s granule density and heap mass, respectively. Substituting
,
, the heap volume (
V = 785.398 cm
3) and the measured pore volume (
vg) of each GAC in
Table 1 into Equations (5), (8), and (11), we can obtain the
in both Model A and Model B. The results are presented in
Table 2 and
Table 3.
From
Table 2 and
Table 3, we can clearly see that the results obtained by Model A are slightly better than those obtained by Model B. The maximum difference of the real and imaginary part of complex permittivity and the loss tangent
obtained by model A and model B is 4.930% or (17.750 − 16.875)/17.750, 7.100% or (2.000 − 1.856)/2.000, and 2.496% or (0.1963 − 0.1914)/0.1963, respectively. Nevertheless, under the condition that there are individual differences in the samples,
Table 2 and
Table 3 verified that model A and B could provide the results with little difference. Hence, in the following discussion, we just take the data of Model A into account. Considering that the loss tangent plays a crucial role in microwave heating, the results can be clearly grouped according to whether it was processed with microwave. To illustrate this more specifically, we take the average of the non-microwave treatment samples A and B:
Substituting
,
,
V, and Equations (12) into (8), we can derive the relationship between ε
eff and
vg, as shown in
Figure 2. Similarly, the relationship between tan δ(ε
eff) and
vg can also be obtained, as shown in
Figure 3.
In
Figure 2 and
Figure 3, it can be clearly seen that the properties of samples A and B fit the derived curve well, while those of samples C1–C3 have a considerable shift from the curve. It indicated that microwave treatment have a significant effect on the structure of GAC, making the classical model no longer suitable for describing its permittivity. This is reasonable, because the derivation in both Model A and Model B did not take the microwave factor into account. Meanwhile, we can also notice that both ε
eff and tan δ(ε
eff) are decreased with the increment of
vg. This may be due to the fact that the air (low permittivity) composition in mixture increases with the increment of
vg.
5. Conclusions
This paper studied the relationship between the effective complex permittivity and pore volume of GAC. The relationship was derived based on MGA theory. Two quantificational expressions were established in different equivalent models, respectively. A well-designed resonant cavity and the accompanying open-end coaxial probe were introduced to measure the effective complex permittivity of the GAC. The automatic surface area and pore analyzer was also applied to carry out the BET testing to acquire the pore volume of the GAC. Results obtained by the two expressions were extremely similar. Meanwhile, the theoretical results are in a good agreement with those from the experiment. In addition, the results indicated that microwave treatment had a great impact on the structure of GAC, making the classical model no longer suitable for describing its permittivity.
This study provided a bridge to link the effective complex permittivity and pore volume of GAC. In addition, it provided a potential and convenient method for the rapid assisted characterization of GAC. In the future study, we will try to take the microwave factor into account during derivation, and study the relationship between the permittivity and pore size distribution or different absorbability (porosity).
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 61501311; 61601312).
Author Contributions
Yang Yang conceived and designed the experiments; Chao Shi and Yi Zhang performed the experiments; Yang Yang developed the model, analyzed the data and wrote the initial draft of the manuscript; Jinghua Ye reviewed and contributed to the final manuscript; Huangcheng Zhu and Kama Huang contributed the location and equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohan, D.; Singh, K.P. Granular Activated Carbon. In Water Encyclopedia, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 92–107. [Google Scholar]
- Walker, G.M.; Weatherley, L.R. Adsorption of acid dyes on to granular activated carbon in fixed beds. Water Res. 1997, 31, 2093–2101. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Wan, M.A. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.K.; Chandra, P.; Chatterjee, P.K. A review on the fuel gas cleaning technologies in gasification process. J. Environ. Chem. Eng. 2015, 3, 689–702. [Google Scholar]
- Liu, X.; Quan, X.; Bo, L.; Chen, S.; Zhao, Y. Simultaneous pentachlorophenol decomposition and granular activated carbon regeneration assisted by microwave irradiation. Carbon 2004, 42, 415–422. [Google Scholar] [CrossRef]
- Liu, X.; Yu, G.; Han, W. Granular activated carbon adsorption and microwave regeneration for the treatment of 2,4,5-trichlorobiphenyl in simulated soil-washing solution. J. Hazard. Mater. 2007, 147, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Wang, P.; Zhao, S.S.; Zhang, W. Treatment of an industrial chemical waste-water using a granular activated carbon adsorption-microwave regeneration process. J. Chem. Technol. Biotechnol. 2012, 87, 1004–1009. [Google Scholar] [CrossRef]
- Ania, C.O.; Parra, J.B.; Menéndez, J.A.; Pis, J.J. Effect of microwave and conventional regeneration on the microporous and mesoporous network and on the adsorptive capacity of activated carbons. Microporous Mesoporous Mater. 2005, 85, 7–15. [Google Scholar] [CrossRef]
- Shi, C.; Wang, T.; Zhang, F.H.; Hua, J.; Huang, K.M.; Yang, Y. Study on regeneration of granular activated carbon by microwave thermal treatment. J. Sichuan Univ. Sci. Ed. 2015, 52, 101–106. [Google Scholar]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption between atrazine and methylene blue on activated carbon: The importance of pore size distribution. Carbon 2000, 38, 1423–1436. [Google Scholar] [CrossRef]
- Li, L.; Quinlivan, P.A.; Knappe, D.R.U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Lu, Q.; Sorial, G.A. The role of adsorbent pore size distribution in multicomponent adsorption on activated carbon. Carbon 2004, 42, 3133–3142. [Google Scholar] [CrossRef]
- Lu, Q.; Sorial, G.A. Adsorption of phenolics on activated carbon—Impact of pore size and molecular oxygen. Chemosphere 2004, 55, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Y.; Huang, K.M.; Chen, C.; Wang, K.Y. A permittivity measurement method based on cavity perturbation technique. Appl. Mech. Mater. 2014, 590, 629–633. [Google Scholar] [CrossRef]
- Chen, F.; Mao, S.; Wang, X.; Semouchkina, E.; Lanagan, M. Effects of cavity dimensions in split-post dielectric resonator technique for complex permittivity measurements. In Proceedings of the 2012 IEEE Antennas and Propagation Society International Symposium (APSURSI), Chicago, IL, USA, 8–14 July 2012; pp. 1–2.
- Chen, Q.; Huang, K.M.; Yang, X.; Luo, M.; Zhu, H. An artificial nerve network realization in the measurement of material permittivity. Prog. Electromagn. Res. 2011, 116, 347–361. [Google Scholar] [CrossRef]
- Eichelbaum, M.; Stößer, R.; Karpov, A.; Dobner, C.K.; Rosowski, F.; Trunschke, A.; Schlögl, R. The microwave cavity perturbation technique for contact-free and in situ electrical conductivity measurements in catalysis and materials science. Phys. Chem. Chem. Phys. 2012, 14, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ong, C.K.; Tan, B.T.G. Amendment of cavity perturbation method for permittivity measurement of extremely low-loss dielectrics. IEEE Trans. Instrum. Meas. 1999, 48, 1031–1037. [Google Scholar] [CrossRef]
- Roelvink, J.; Trabelsi, S. Measuring the complex permittivity of poultry meat with a planar transmission-line sensor. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference, Minneapolis, MN, USA, 6–9 May 2013; pp. 1689–1693.
- Adous, M.; Quéffélec, P.; Laguerre, L. Coaxial/cylindrical transition line for broadband permittivity measurement of civil engineering materials. Meas. Sci. Technol. 2006, 17, 2241. [Google Scholar] [CrossRef]
- Baker-Jarvis, J.; Vanzura, E.J.; Kissick, W.A. Improved technique for determining complex permittivity with the transmission/reflection method. IEEE Trans. Microw. Theory Tech. 1990, 38, 1096–1103. [Google Scholar] [CrossRef]
- Garnett, J.M. Colours in metal glasses and in metallic films. Philos. Trans. R. Soc. Lond. 1904, 73, 443–445. [Google Scholar] [CrossRef]
- Mallet, P.; Guerin, C.A.; Sentenac, A. Maxwell-Garnett mixing rule in the presence of multiple scattering: Derivation and accuracy. Phys. Rev. B 2005, 72, 014205. [Google Scholar] [CrossRef]
- Li, S.; Chen, R.; Anwar, S.; Lu, W.; Lai, Y.; Chen, H.; Hou, B.; Ren, F.; Gu, B. Applying effective medium theory in characterizing dielectric constant of solids. Prog. Electromagn. Res. Lett. 2012, 35, 145–153. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).