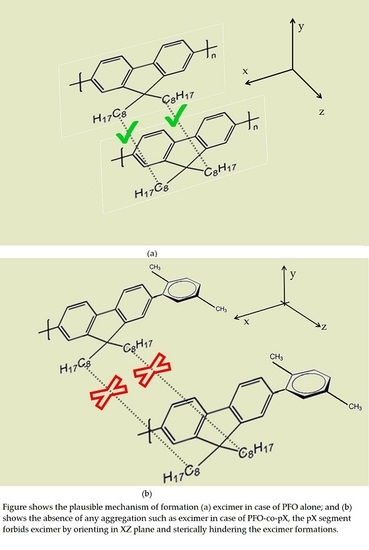
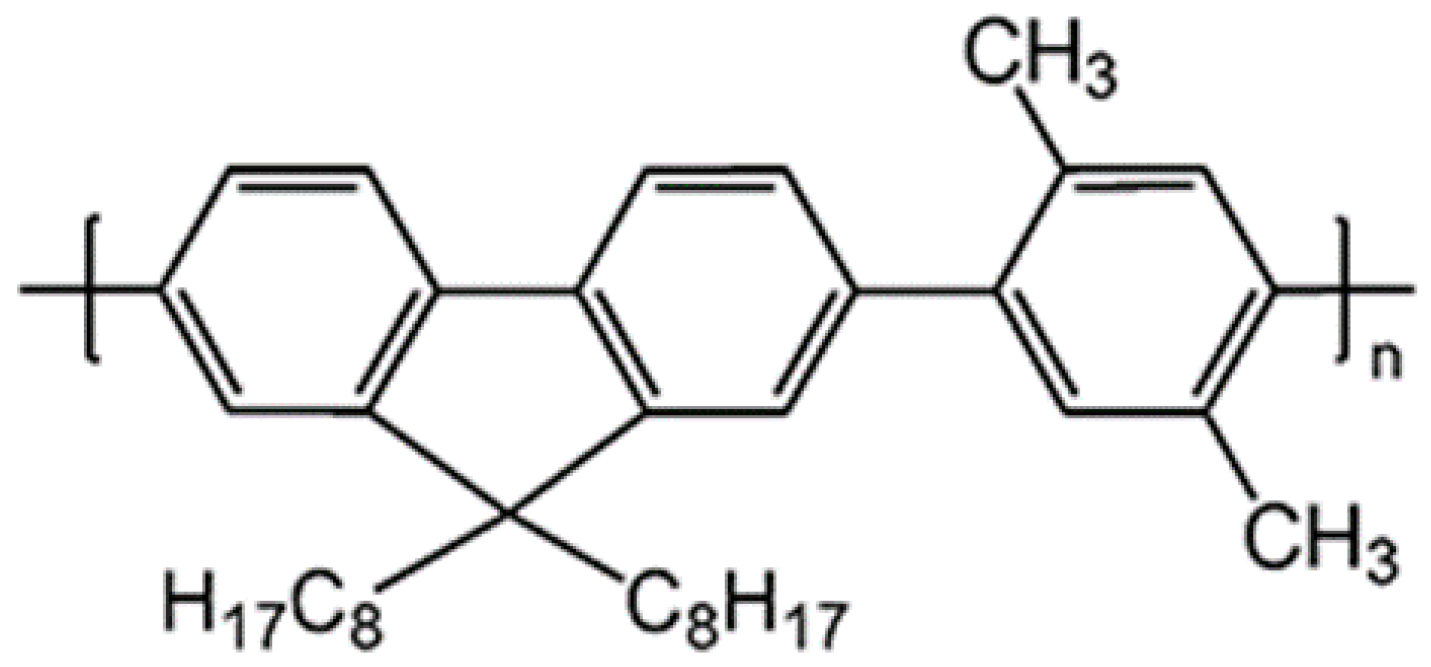
An Efficient Violet Amplified Spontaneous Emission (ASE) from a Conjugated Polymer (PFO-co-pX) in Solution
Abstract
:1. Introduction
2. Experimental Section
3. Results
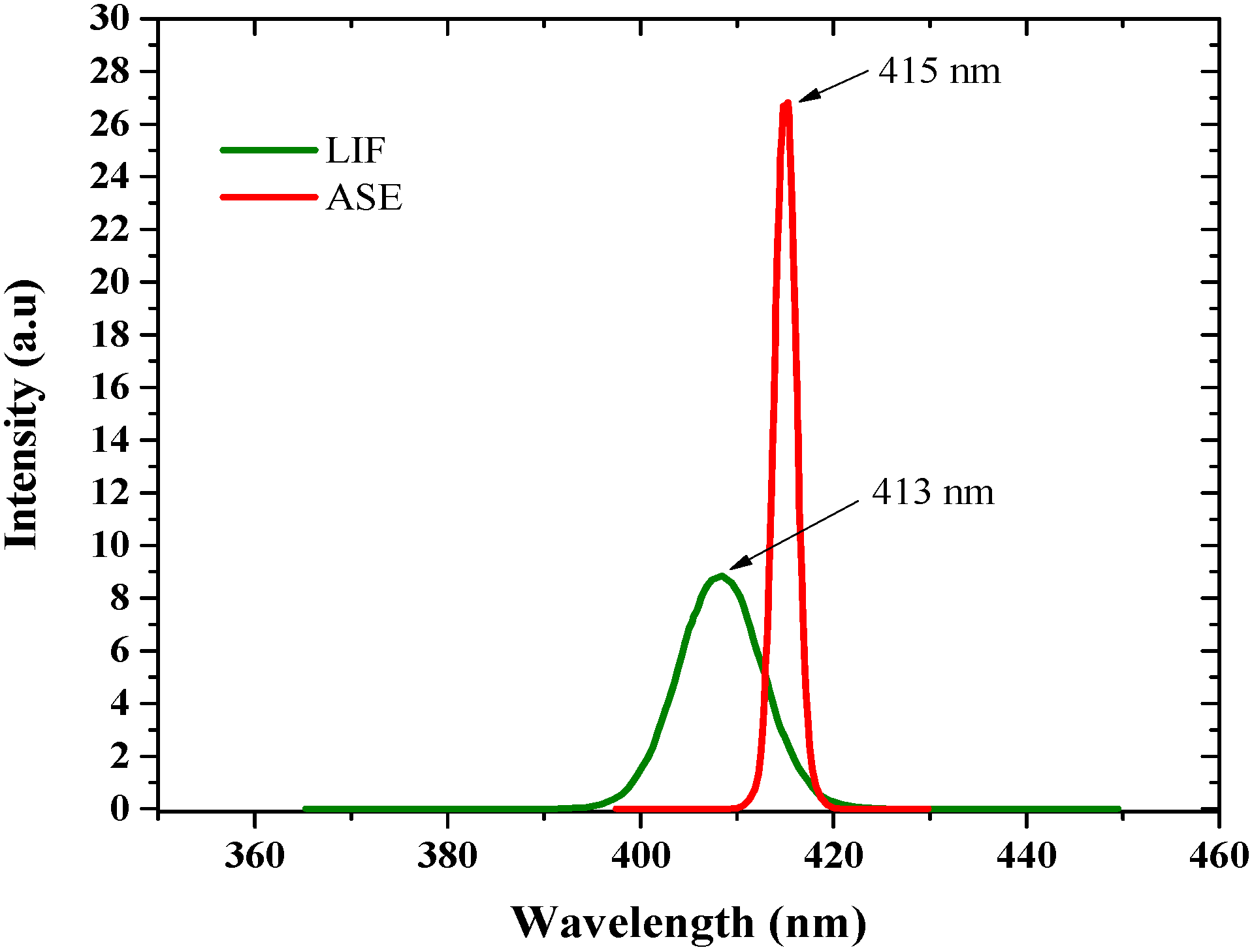
3.1. Spectral Properties
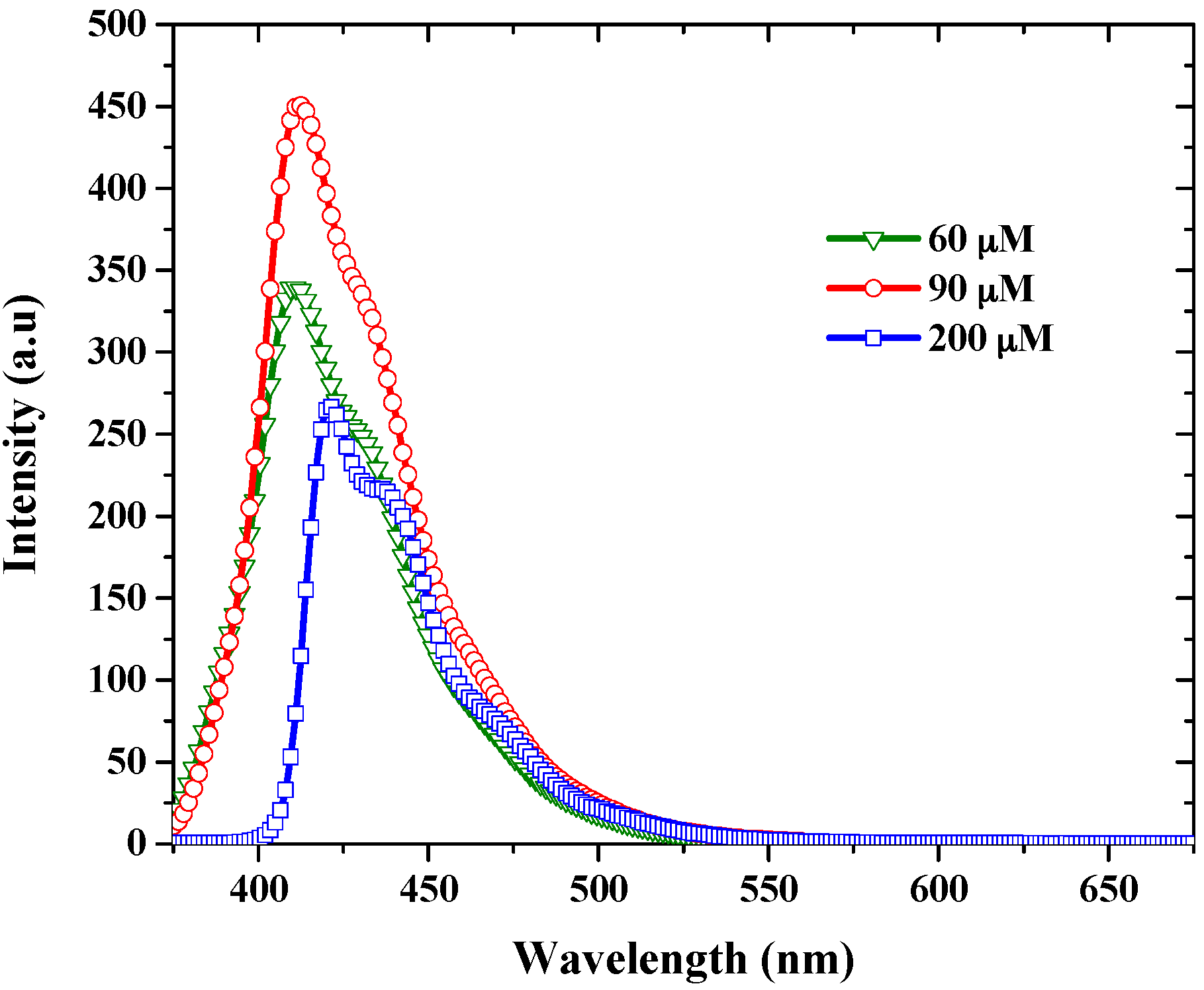
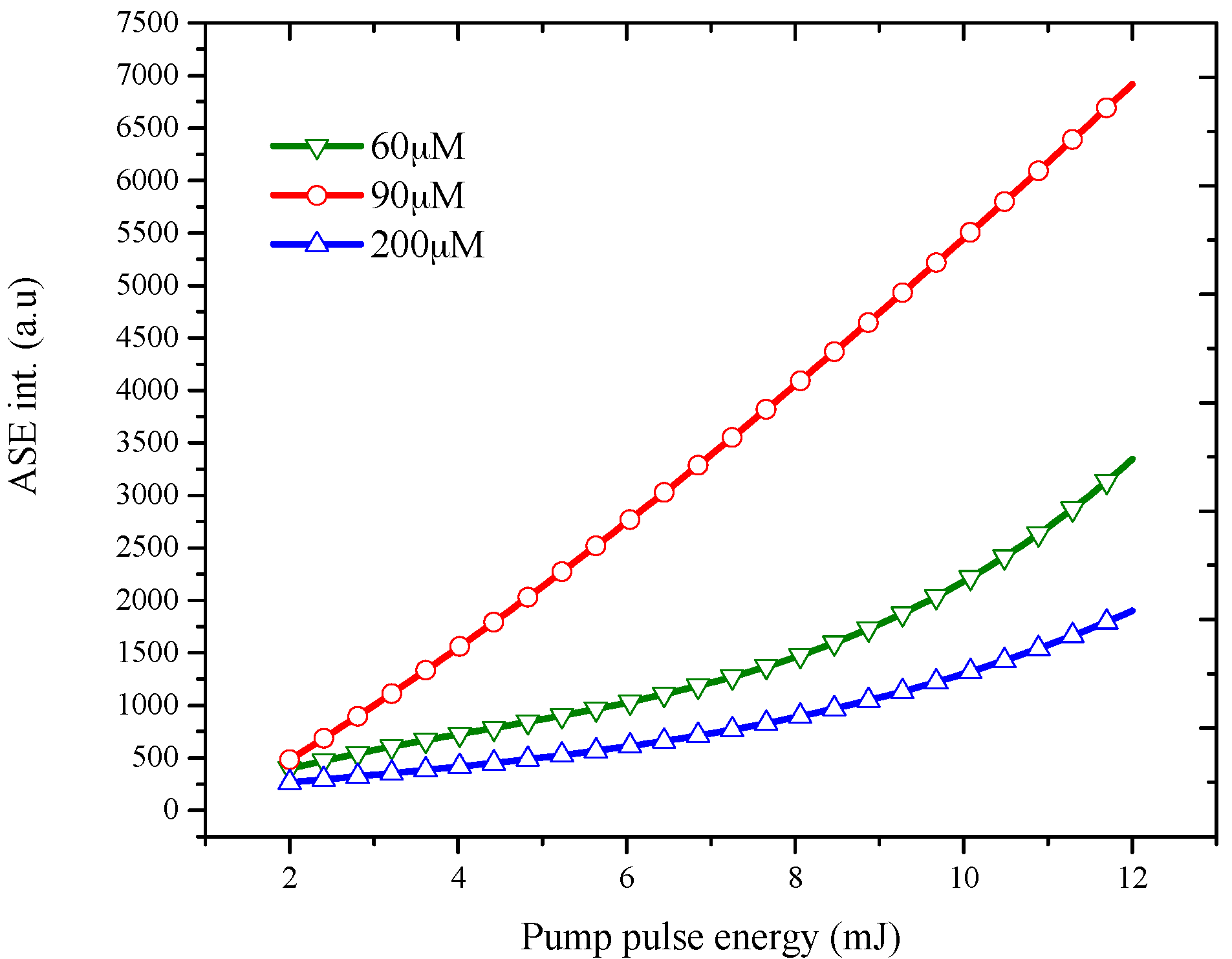
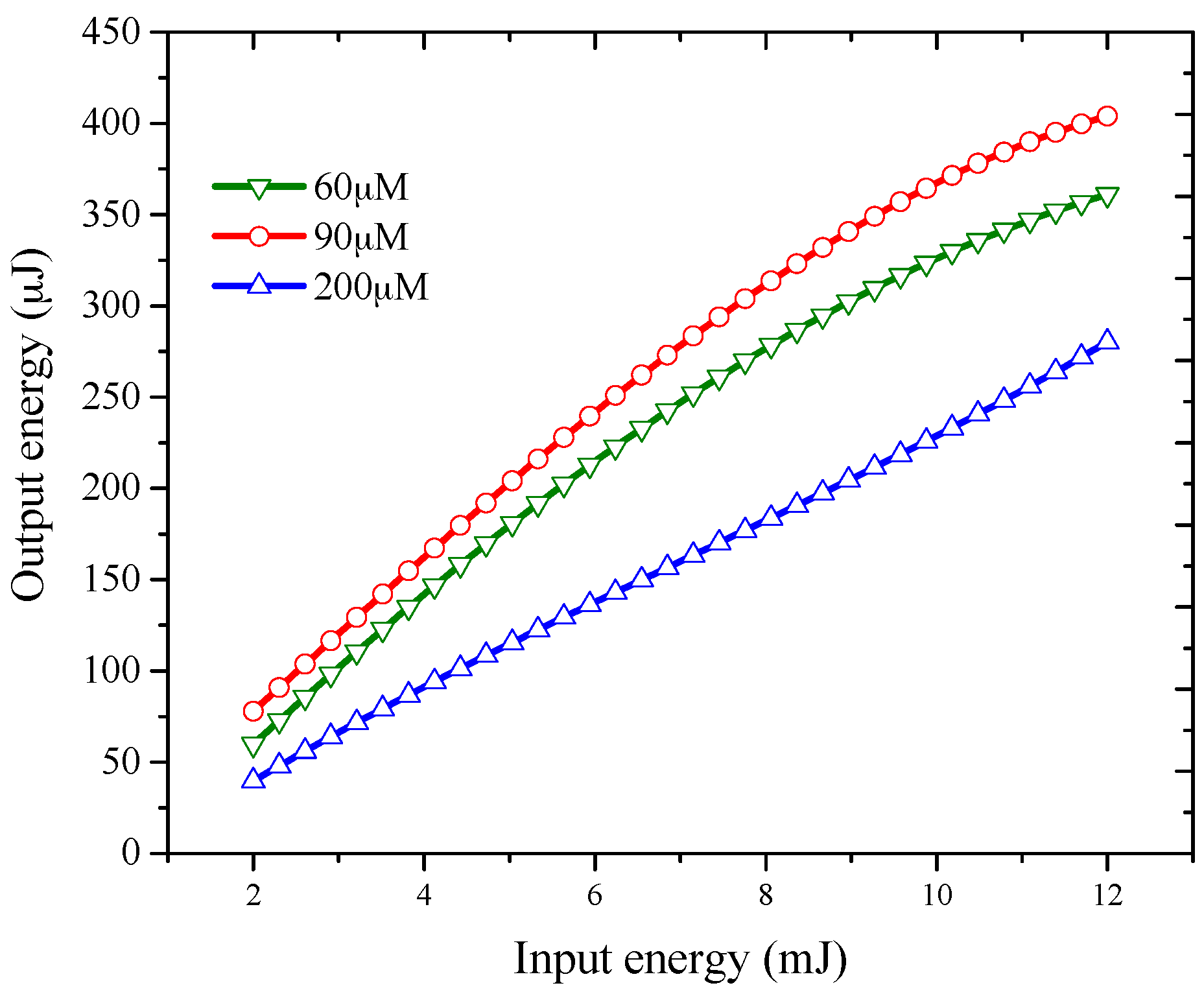
3.2. Amplified Spontaneous Emission Properties of PFO-co-pX
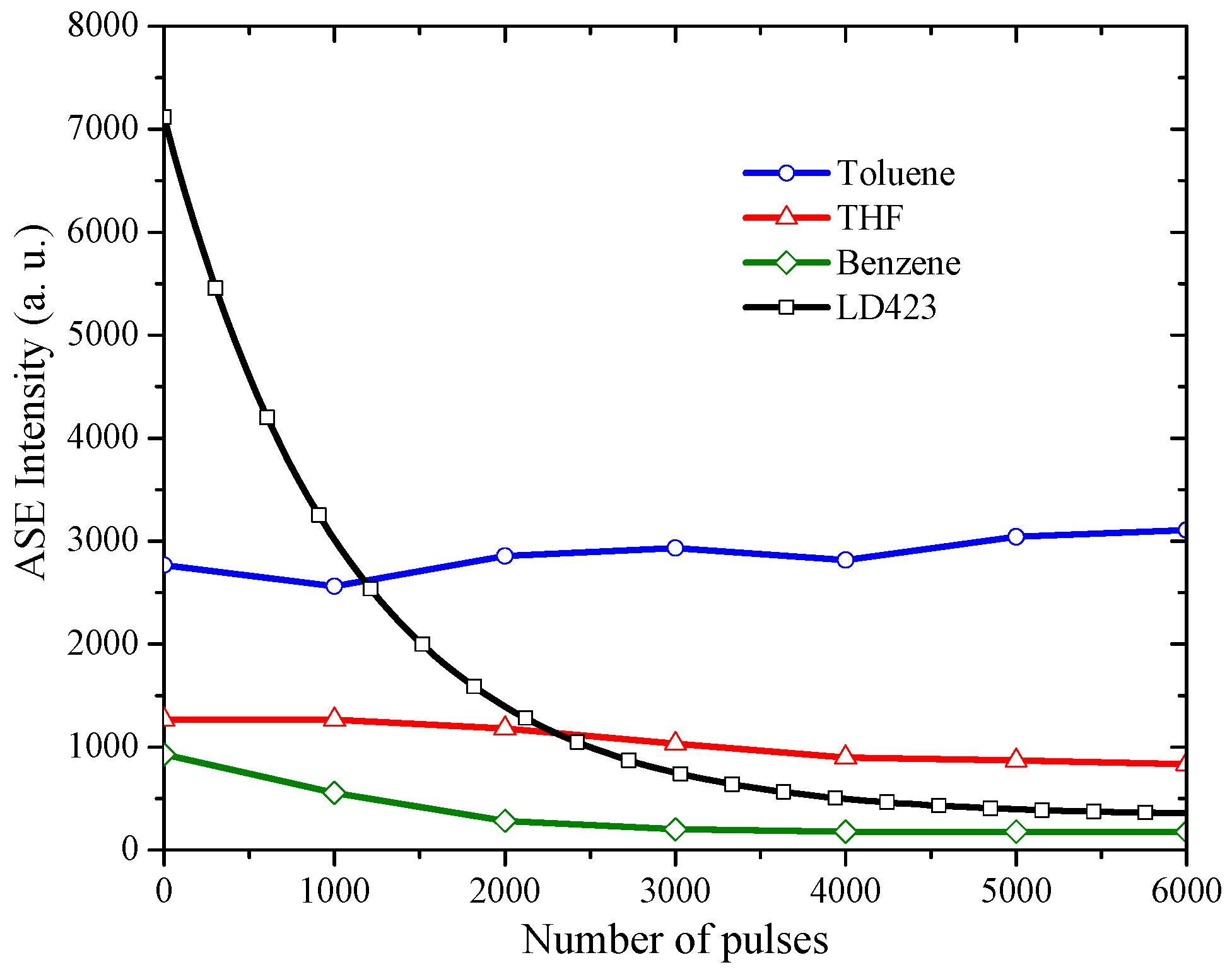
3.3. The Stability of ASE Spectra
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zuhaier, A.-K.I.; Mohammad, K.; Devanesan, S.; AlSalhi, M.S.; Prasad, S.; Masilamani, V. Shelf-life enhancement of donor blood by He–Ne laser biostimulation. Curr. Sci. 2015, 109, 1151. [Google Scholar]
- Wolbarsht, M.L. Laser Applications in Medicine and Biology, 1st ed.; Springer US: New York, NY, USA, 1971; Volume 1. [Google Scholar]
- Masilamani, V.; Das, B.; Secor, J.; AlSalhi, M.; Devanesan, S.; Prasad, S.; Rabah, D.; Alfano, R. Optical biopsy of benign and malignant tissue by time resolved spectroscopy. Technol. Cancer Res. Treat. 2013, 12, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Paschotta, R. Encyclopedia of Laser Physics and Technology; RP Photonics Consulting GmbH: Weinheim, Germany, 2008. [Google Scholar]
- Ibnaouf, K.; Prasad, S.; Aldwayyan, A.; AlSalhi, M.S.; Masilamani, V. Amplified spontaneous emission spectra from the superexciplex of coumarin 138. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Devaraj, D.; Masilamani, V. High power amplified spontaneous emission from an oligomer in solution. J. Lumin. 2015, 168, 109–113. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Prasad, S.; Hamdan, A.; AlSalhi, M.; Aldwayyan, A.; Zaman, M.; Masilamani, V. Photoluminescence spectra of cdse/zns quantum dots in solution. Spectrochi. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Alameh, K.; Devaraj, D.; Hamdan, A.; Karim, M.; Zaman, M.; Masilamani, V. Laser from optically pumped quantum dot cdse/zns in a colloidal liquid. J. Nanosci. Nanotechnol. 2015, 15, 6710–6713. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; AlHesseny, H.S.; AlSalhi, M.S.; Devaraj, D.; Masilamai, V. A high power, frequency tunable colloidal quantum dot (cdse/zns) laser. Nanomaterials 2017, 7, 29. [Google Scholar] [CrossRef]
- Tessler, N. Lasers based on semiconducting organic materials. Adv. Mater. 1999, 11, 363–370. [Google Scholar] [CrossRef]
- McGehee, M.D.; Heeger, A.J. Semiconducting (conjugated) polymers as materials for solid-state lasers. Adv. Mater. 2000, 12, 1655–1668. [Google Scholar] [CrossRef]
- Kallinger, C.; Hilmer, M.; Haugeneder, A.; Perner, M.; Spirkl, W.; Lemmer, U.; Feldmann, J.; Scherf, U.; Müllen, K.; Gombert, A. A flexible conjugated polymer laser. Adv. Mater. 1998, 10, 920–923. [Google Scholar] [CrossRef]
- Turnbull, G.; Andrew, P.; Jory, M.; Barnes, W.L.; Samuel, I. Relationship between photonic band structure and emission characteristics of a polymer distributed feedback laser. Phys. Rev. B 2001, 64, 125122. [Google Scholar] [CrossRef] [Green Version]
- Bauer, C.; Giessen, H.; Schnabel, B.; Kley, E.B.; Schmitt, C.; Scherf, U.; Mahrt, R. A surface-emitting circular grating polymer laser. Adv. Mater. 2001, 13, 1161–1164. [Google Scholar] [CrossRef]
- Holzer, W.; Penzkofer, A.; Pertsch, T.; Danz, N.; Bräuer, A.; Kley, E.; Tillmann, H.; Bader, C.; Hörhold, H.-H. Corrugated neat thin-film conjugated polymer distributed-feedback lasers. Appl. Phys. B Lasers Opt. 2002, 74, 333–342. [Google Scholar] [CrossRef]
- Jenekhe, S.A. Excited-state complexes of conjugated polymers. Adv. Mater. 1995, 7, 309–311. [Google Scholar] [CrossRef]
- Moses, D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. [Google Scholar] [CrossRef]
- O’Carroll, D.; Iacopino, D.; O’Riordan, A.; Lovera, P.; O’Connor, É.; O’Brien, G.A.; Redmond, G. Poly(9,9-dioctylfluorene) nanowires with pronounced β-phase morphology: Synthesis, characterization, and optical properties. Adv. Mater. 2008, 20, 42–48. [Google Scholar] [CrossRef]
- Xia, R.; Heliotis, G.; Hou, Y.; Bradley, D.D. Fluorene-based conjugated polymer optical gain media. Org. Electron. 2003, 4, 165–177. [Google Scholar] [CrossRef]
- Mustapha, N.; Ibnaouf, K.; Fekkai, Z.; Hennache, A.; Prasad, S.; Alyamani, A. Improved efficiency of solar cells based on behp-co-meh-ppv doped with zno nanoparticles. Opt. Int. J. Light Electron Opt. 2013, 124, 5524–5527. [Google Scholar] [CrossRef]
- Knaapila, M.; Bright, D.W.; Nehls, B.S.; Garamus, V.M.; Almásy, L.; Schweins, R.; Scherf, U.; Monkman, A.P. Development of intermolecular structure and beta-phase of random poly[9,9-bis(2-ethylhexyl) fluorene]-co-(9,9-dioctylfluorene) in methylcyclohexane. Macromolecules 2011, 44, 6453–6460. [Google Scholar] [CrossRef]
- Brown, A.; Bradley, D.; Burroughes, J.; Friend, R.; Greenham, N.; Burn, P.; Holmes, A.; Kraft, A. Poly(p-phenylenevinylene) light-emitting diodes: Enhanced electroluminescent efficiency through charge carrier confinement. Appl. Phys. Lett. 1992, 61, 2793–2795. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Hou, Q.; Peng, J.; Yang, W.; Cao, Y. High-efficiency white-light emission from a single copolymer: Fluorescent blue, green, and red chromophores on a conjugated polymer backbone. Adv. Mater. 2007, 19, 1113–1117. [Google Scholar] [CrossRef]
- Schön, J.; Kloc, C.; Dodabalapur, A.; Batlogg, B. An organic solid state injection laser. Science 2000, 289, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Riechel, S.; Kallinger, C.; Lemmer, U.; Feldmann, J.; Gombert, A.; Wittwer, V.; Scherf, U. A nearly diffraction limited surface emitting conjugated polymer laser utilizing a two-dimensional photonic band structure. Appl. Phys. Lett. 2000, 77, 2310–2312. [Google Scholar] [CrossRef]
- McGehee, M.D.; Gupta, R.; Veenstra, S.; Miller, E.K.; Díaz-García, M.A.; Heeger, A.J. Amplified spontaneous emission from photopumped films of a conjugated polymer. Phys. Rev. B 1998, 58, 7035–7039. [Google Scholar] [CrossRef]
- Qiu, Z.; Han, T.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Polyarylcyanation of diyne: A one-pot three-component convenient route for in situ generation of polymers with AIE characteristics. Macromolecules 2016, 49, 8888–8898. [Google Scholar] [CrossRef]
- Deshapande, N.; Pujar, G.; Sunagar, M.G.; Gaonkar, S.; Belavagi, N.S.; Inamdar, S.; Bathula, C.; Khazi, I.A.M. Synthesis and optoelectronic exploration of highly conjugated 1,3,4-oxadiazole containing donor-π-acceptor chromophores. ChemistrySelect 2017, 2, 1793–1801. [Google Scholar] [CrossRef]
- Setayesh, S.; Grimsdale, A.C.; Weil, T.; Enkelmann, V.; Müllen, K.; Meghdadi, F.; List, E.J.; Leising, G. Polyfluorenes with polyphenylene dendron side chains: Toward non-aggregating, light-emitting polymers. J. Am. Chem. Soc. 2001, 123, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Hou, L.; Wu, H.; Wang, X.; Shen, H.; Cao, W.; Yang, W.; Cao, Y. High-efficiency, environment-friendly electroluminescent polymers with stable high work function metal as a cathode: Green-and yellow-emitting conjugated polyfluorene polyelectrolytes and their neutral precursors. J. Am. Chem. Soc. 2004, 126, 9845–9853. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Zhang, J.; Grimsdale, A.C.; Müllen, K.; Gaal, M.; List, E.J. Poly(tetraarylindenofluorene)s: New stable blue-emitting polymers. Macromolecules 2003, 36, 8240–8245. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Prasad, S.; Masilamani, V.; AlSalhi, M. Evidence for amplified spontaneous emission from double excimer of conjugated polymer (pdhf) in a liquid solution. Polymer 2013, 54, 2401–2405. [Google Scholar] [CrossRef]
- Alsalhi, M.; Ibnaouf, K.; Masilamani, V.; Yassin, O. Excimer state of a conjugate polymer (meh-ppv) in liquid solutions. Laser Phys. 2007, 17, 1361–1366. [Google Scholar] [CrossRef]
- Ibnaouf, K. Amplified spontaneous emission spectra of poly (9,9-dioctylfluorenyl-2,7-diyl) under pulsed laser excitation. Synth. Met. 2015, 209, 534–543. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Masilamani, V. Laser from the dimer state of a conjugated polymer (pfo) in solution. Polymer 2014, 55, 727–732. [Google Scholar] [CrossRef]
- Schwartz, B.J. Conjugated polymers as molecular materials: How chain conformation and film morphology influence energy transfer and interchain interactions. Annu. Rev. Phys. Chem. 2003, 54, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Ibnaouf, K.; Prasad, S.; Masilamani, V.; AlSalhi, M.; Mustapha, N.; Alyamani, A. Triple amplified spontaneous emissions from a conjugated copolymer behp-co-meh-ppv in solution. Phys. E Low Dimens. Syst. Nanostruct. 2013, 53, 66–71. [Google Scholar] [CrossRef]
- Meyer, Y.; d’Azy, O.B.; Martin, M.; Breheret, E. Spectral evolution and relaxation oscillations in dye lasers. Opt. Commun. 1986, 60, 64–68. [Google Scholar] [CrossRef]
- Cornil, J.; Beljonne, D.; Calbert, J.P.; Brédas, J.L. Interchain interactions in organic π‐conjugated materials: Impact on electronic structure, optical response, and charge transport. Adv. Mater. 2001, 13, 1053–1067. [Google Scholar] [CrossRef]

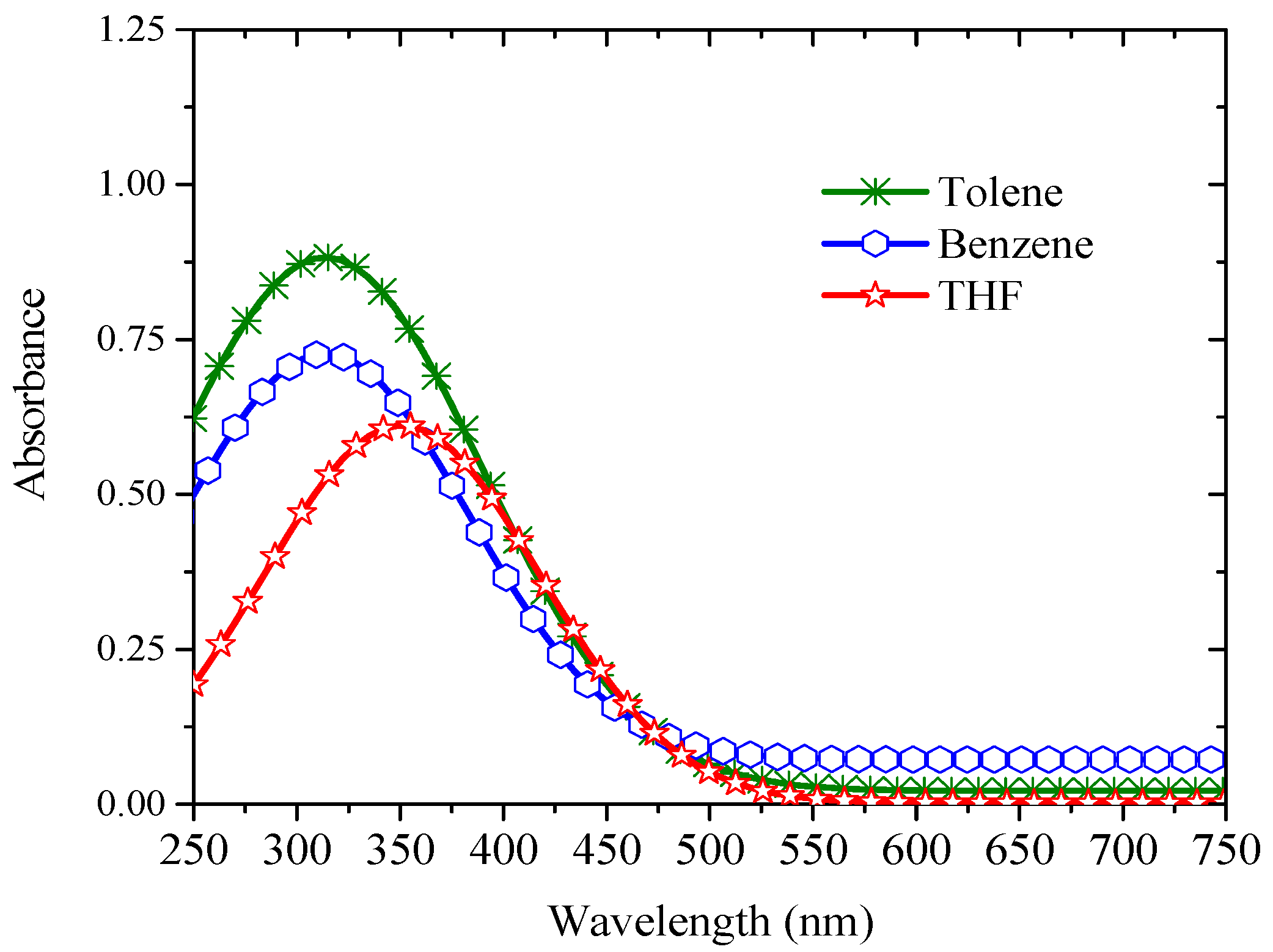


| Solvent | Quantum Yield |
|---|---|
| Toluene | 0.3 |
| THF | 0.29 |
| Benzene | 0.24 |
| Solvent | ASE Intensity (Arbitrary Unit) |
|---|---|
| THF | 1899 |
| Benzene | 3274 |
| Toluene | 9521 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulaziz Alfahd, S.; Prasad Rajendra, S.; Al-Mujammi, W.; Devaraj, D.; Masilamani, V.; AlSalhi, M.S. An Efficient Violet Amplified Spontaneous Emission (ASE) from a Conjugated Polymer (PFO-co-pX) in Solution. Materials 2017, 10, 265. https://doi.org/10.3390/ma10030265
Abdulaziz Alfahd S, Prasad Rajendra S, Al-Mujammi W, Devaraj D, Masilamani V, AlSalhi MS. An Efficient Violet Amplified Spontaneous Emission (ASE) from a Conjugated Polymer (PFO-co-pX) in Solution. Materials. 2017; 10(3):265. https://doi.org/10.3390/ma10030265
Chicago/Turabian StyleAbdulaziz Alfahd, Sara, Saradh Prasad Rajendra, Wafa Al-Mujammi, Durairaj Devaraj, Vadivel Masilamani, and Mohamad Saleh AlSalhi. 2017. "An Efficient Violet Amplified Spontaneous Emission (ASE) from a Conjugated Polymer (PFO-co-pX) in Solution" Materials 10, no. 3: 265. https://doi.org/10.3390/ma10030265