Effects of Electrospun Carbon Nanofibers’ Interlayers on High-Performance Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Results
2.1. Nanofiber Characterization
2.1.1. Characterization of PI and PI+ Melamine
2.1.2. Morphology of the Electrospun N-Rich Carbon Nanofibers
2.1.3. Raman/X-ray Diffraction
2.1.4. X-ray Photoelectron Spectroscopy Analysis
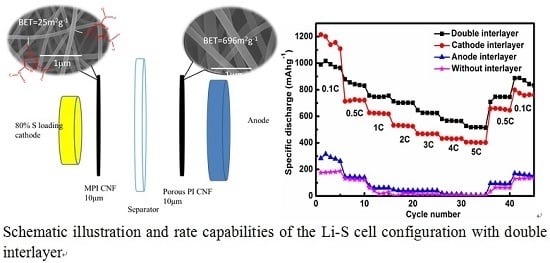
2.2. Electrochemical Performance
3. Discussion
3.1. The Role of Interlayer for Preventing Polysulfide Diffusion
3.2. Cathode Interlayer
3.3. Anode Interlayer
4. Experimental
4.1. Materials
4.2. Fabrication of N-Drop Carbon Nanofibers
4.3. Characterization
5. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulfur composites for high-power lithium–sulfur batteries. Angew. Chem. Int. Ed. Engl. 2011, 123, 6026–6030. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Morcrette, M.; Guéguen, A.; Dedryvère, R.; Tarascon, J.M. Li–S batteries: Simple approaches for superior performance. Energy Environ. Sci. 2013, 6, 176–182. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, F.; Fang, J.; Li, Q.; Jia, M.; Lai, Y.; Zhi, Z.; Li, J. Nickel foam as interlayer to improve the performance of lithium–sulfur battery. J. Solid State Electrochem. 2014, 18, 1025–1029. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Wang, Q.; Shen, C.; Peng, P.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur battery with self-assembly polypyrrole nanotube film as the functional interlayer. J. Power Sources 2015, 273, 511–516. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, B.; Xu, Z.L.; Abouali, S.; Garakani, M.A.; Huang, J.; Kim, J.K. Novel interlayer made from Fe3C/carbon nanofiber webs for high performance lithium–sulfur batteries. J. Power Sources 2015, 285, 43–50. [Google Scholar] [CrossRef]
- Zhang, D.A.; Wang, Q.; Wang, Q.; Sun, J.; Xing, L.L.; Xue, X.Y. High capacity and cyclability of hierarchical MoS2/SnO2 nanocomposites as the cathode of Lithium–Sulfur battery. Electrochim. Acta 2015, 173, 476–482. [Google Scholar] [CrossRef]
- Su, Y.S.; Manthiram, A. Lithium–Sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.; Su, Y.S.; Fu, Y.; Manthiram, A. Improved Lithium–Sulfur cells with a treated carbon paper interlayer. Phys. Chem. Chem. Phys. 2013, 15, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, G.; Lai, Y.; Li, J. A freestanding hollow carbon nanofiber/reduced graphene oxide interlayer for high-performance lithium–sulfur batteries. J. Alloys Compd. 2016, 663, 501–506. [Google Scholar] [CrossRef]
- Wang, J.G.; Xie, K.; Wei, B. Advanced engineering of nanostructured carbons for lithium–sulfur batteries. Nano Energy 2015, 15, 413–444. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Seh, Z.W.; Fu, Z.; Zhang, R.; Cui, Y. Understanding the anchoring effect of two-dimensional layered materials for lithium–sulfur batteries. Nano Lett. 2015, 15, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, J.; Yang, J.; Xie, J.; Huang, B. Seaweed-Like Porous Carbon from The Decomposition of Polypyrrole Nanowires for Application in Lithium Ion Batteries. J. Mater. Chem. A 2013, 1, 5037–5044. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen–doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Wang, X.; Mayes, R.T.; Dai, S. Lithium–Sulfur Batteries Based on Nitrogen–Doped Carbon and an Ionic–Liquid Electrolyte. ChemSusChem 2012, 5, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, J.; Chen, H.; Li, W.; Qiao, W.; Long, D.; Ling, L. High efficiency immobilization of sulfur on nitrogen-enriched mesoporous carbons for Li-S batteries. ACS Appl. Mater. Interfaces 2013, 5, 5630–5638. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2011, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen–Doped Mesoporous Carbon Promoted Chemical Adsorption of Sulfur and Fabrication of High–Areal–Capacity Sulfur Cathode with Exceptional Cycling Stability for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Song, J.; Gordin, M.L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Duan, Y.; Ren, Y.; Wang, D. Strong Lithium Polysulfide Chemisorption on Electroactive Sites of Nitrogen–Doped Carbon Composites for High–Performance Lithium–Sulfur Battery Cathodes. Angew. Chem. Int. Ed. 2015, 54, 4325–4329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, G.; Lai, Y.; Li, J.; Zhang, Z.; Chen, W. Nitrogen-doped porous hollow carbon sphere-decorated separators for advanced lithium–sulfur batteries. J. Power Sources 2015, 300, 157–163. [Google Scholar] [CrossRef]
- Wang, G.; Lai, Y.; Zhang, Z.; Li, J.; Zhang, Z. Enhanced rate capability and cycle stability of lithium-sulfur batteries with a bifunctional MCNT@PEG-modified separator. J. Mater. Chem. A 2015, 3, 7139–7144. [Google Scholar] [CrossRef]
- Xing, L.B.; Xi, K.; Li, Q.; Su, Z.; Lai, C.; Zhao, X.; Kumar, R.V. Nitrogen, sulfur-codoped graphene sponge as electroactive carbon interlayer for high-energy and-power lithium–sulfur batteries. J. Power Sources 2016, 303, 22–28. [Google Scholar] [CrossRef]
- McCloskey, B.D. Attainable Gravimetric and Volumetric Energy Density of Li–S and Li Ion Battery Cells with Solid Separator-Protected Li Metal Anodes. J. Phys. Chem. Lett. 2015, 6, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Hagen, M.; Dörfler, S.; Fanz, P.; Berger, T.; Speck, R.; Tübke, J.; Althuesb, H.; Hoffmannc, M.J.; Scherrc, C.; Kaskel, S. Development and costs calculation of lithium–sulfur cells with high sulfur load and binder free electrodes. J. Power Sources 2013, 224, 260–268. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, G.; Liu, C.; Liu, N.; Li, W.; Yan, K.; Yao, H.; Chu, S.; Cui, Y. Polymer nanofiber-guided uniform lithium deposition for battery electrodes. Nano Lett. 2015, 15, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Angell, C.A. A Novel Electrolyte Solvent for Rechargeable Lithium and Lithium–Ion Batteries. J. Electrochem. Soc. 1996, 143, 4047–4053. [Google Scholar] [CrossRef]
- Matsuda, Y.; Morita, M.; Yamada, K.; Hirai, K. Characteristics of sulfolane–based electrolytes for rechargeable lithium batteries. J. Electrochem. Soc. 1985, 132, 2538–2543. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.K.; Kong, L.L.; Liu, S.; Li, G.R.; Gao, X.P. Protected lithium anode with porous Al2O3 layer for lithium–sulfur battery. J. Mater. Chem. A 2015, 3, 12213–12219. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Wu, M.; Wu, X.; Zhang, J. Enhanced cycle performance of Li–S battery with a polypyrrole functional interlayer. J. Power Sources 2014, 267, 542–546. [Google Scholar] [CrossRef]
- Fu, K.; Xue, L.; Yildiz, O.; Li, S.; Lee, H.; Li, Y.; Xu, G.; Zhou, L.; Bradford, P.; Zhang, X. Effect of CVD carbon coatings on Si@CNF composite as anode for lithium-ion batteries. Nano Energy 2013, 2, 976–986. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Long-term cycling studies on electrospun carbon nanofibers as anode material for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 12175–12184. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Zhang, J.; Shi, C.; Zhou, A.; Bian, C.; Li, W. Thin film composite nanofiltration membrane prepared by the interfacial polymerization of 1,2,4,5-benzene tetracarbonyl chloride on the mixed amines cross-linked poly (ether imide) support. J. Membr. Sci. 2016, 520, 19–28. [Google Scholar] [CrossRef]
- Uysal, Ş.; Uçan, H.İ. The synthesis and characterization of melamine based and [Fe/CrSalen] or [Fe/CrSalophen] capped polimer Schiff base complexes. J. Inorg. Organomet. Polym. Mater. 2010, 20, 720–726. [Google Scholar] [CrossRef]
- Pandey, J.; Prajapati, P.; Shimpi, M.R.; Tandon, P. Studies of molecular structure, hydrogen bonding and chemical activity of a nitrofurantoin-l-proline cocrystal: A combined spectroscopic and quantum chemical approach. RSC Adv. 2016, 6, 74135–74154. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, Z.; Li, Q.; Guan, Z. Experimental investigation of the governing parameters in the electrospinning of polyethylene oxide solution. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 580–585. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Preparation of non-covalently functionalized graphene using 9-anthracene carboxylic acid. Nanotechnology 2011, 22, 405603. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Yang, Y.; Yu, L.; Gao, T.J.; Huang, Z.H.; Kang, F.Y. Polyimide–based porous hollow carbon nanofibers for supercapacitor electrode. J. Appl. Polym. Sci. 2016, 133, 19. [Google Scholar] [CrossRef]
- Yin, L.C.; Liang, J.; Zhou, G.M.; Li, F.; Saito, R.; Cheng, H.M. Understanding the interactions between lithium polysulfides and N-doped graphene using density functional theory calculations. Nano Energy 2016, 25, 203–210. [Google Scholar] [CrossRef]
- Hou, T.Z.; Peng, H.J.; Huang, J.Q.; Zhang, Q.; Li, B. The formation of strong-couple interactions between nitrogen-doped graphene and sulfur/lithium (poly) sulfides in lithium-sulfur batteries. 2D Mater. 2015, 2, 014011. [Google Scholar] [CrossRef]
- Hou, T.Z.; Peng, H.J.; Huang, J.Q.; Zhang, Q.; Li, B. Nitrogen-doped graphene/sulfur composite as cathode material for high capacity lithium–sulfur batteries. J. Power Sources 2014, 256, 361–368. [Google Scholar]
- Han, K.; Shen, J.; Hao, S.; Ye, H.; Wolverton, C.; Kung, M.C.; Kung, H.H. Free–Standing Nitrogen–doped Graphene Paper as Electrodes for High–Performance Lithium/Dissolved Polysulfide Batteries. ChemSusChem 2014, 7, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, W.; Zhao, W.; Li, G.; Hou, Y.; Liu, M.; Zhou, L.; Ye, F.; Li, H.; Wei, Z.; et al. High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Su, K.; Wan, W.; Guo, H.; Zhou, H.; Chen, J.; Zhang, X.; Huang, Y. High sulfur loading composite wrapped by 3D nitrogen-doped graphene as a cathode material for lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 5018–5023. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hou, H.; Zhao, Y.; Zhu, Z.; Fong, H. Electrospun polyimide nanofibers and their applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun Nanomaterials for Supercapacitor Electrodes: Designed Architectures and Electrochemical Performance. Adv. Energy Mater. 2016, 7, 1601301. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical impedance spectroscopy study of a lithium/sulfur battery: Modeling and analysis of capacity fading. J. Electrochem. Soc. 2013, 160, A553–A558. [Google Scholar] [CrossRef]
- Reddy, M.V.; Wei Wen, B.L.; Loh, K.P.; Chowdari, B.V.R. Energy storage studies on InVO4 as high performance anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Preparation and characterization of LiNi0.5Co0.5O2 and LiNi0.5Co0.4Al0.1O2 by molten salt synthesis for Li ion batteries. J. Phys. Chem. C 2007, 111, 11712–11720. [Google Scholar] [CrossRef]
- Kolosnitsyn, V.S.; Kuzmina, E.V.; Karaseva, E.V.; Mochalov, S.E. A study of the electrochemical processes in lithium–sulphur cells by impedance spectroscopy. J. Power Sources 2011, 196, 1478–1482. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Long-term cycling studies on 4V-cathode, lithium vanadium fluorophosphate. J. Power Sources 2010, 195, 5768–5774. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Nano-(V1/2Sb1/2Sn)O4: A high capacity, high rate anode material for Li-ion batteries. J. Mater. Chem. 2011, 21, 10003–10011. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, Q.; Peng, H.J.; Liu, X.Y.; Qian, W.Z.; Wei, F. Ionic shield for polysulfides towards highly-stable lithium–sulfur batteries. Energy Environ. Sci. 2014, 7, 347–353. [Google Scholar] [CrossRef]
- Reddy, M.V.; Jose, R.; Teng, T.H.; Chowdari, B.V.R.; Ramakrishna, S. Preparation and electrochemical studies of electrospun TiO2 nanofibers and molten salt method nanoparticles. Electrochim. Acta 2010, 55, 3109–3117. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Y.; Reddy, M.V.; Nair, A.S.; Chowdari, B.V.R.; Ramakrishna, S. Long term cycling studies of electrospun TiO2 nanostructures and their composites with MWCNTs for rechargeable Li-ion batteries. RSC Adv. 2012, 2, 531–537. [Google Scholar] [CrossRef]
- Viet, A.L.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R.; Ramakrishna, S. Nanostructured Nb2O5 polymorphs by electrospinning for rechargeable lithium batteries. J. Phys. Chem. C 2009, 114, 664–671. [Google Scholar] [CrossRef]
- Viet, A.L.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R.; Ramakrishna, S. Electrospun α-Fe2O3 nanostructures for supercapacitor applications. J. Mater. Chem. A 2013, 1, 11698–11704. [Google Scholar]
- Wu, Y.; Zhu, P.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Maghemite nanoparticles on electrospun CNFs template as prospective lithium-ion battery anode. ACS Appl. Mater. Interfaces 2014, 6, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, P.; Zhao, X.; Reddy, M.V.; Peng, S.; Chowdari, B.V.R.; Ramakrishna, S. Highly improved rechargeable stability for lithium/silver vanadium oxide battery induced via electrospinning technique. J. Mater. Chem. A 2013, 1, 852–859. [Google Scholar] [CrossRef]
- Le Viet, A.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R.; Ramakrishna, S. Electrochemical properties of bare and Ta-substituted Nb2O5 nanostructures. Electrochim. Acta 2011, 56, 1518–1528. [Google Scholar] [CrossRef]
- Reddy, M.V.; Jose, R.; Le Viet, A.; Ozoemena, K.I.; Chowdari, B.V.R.; Ramakrishna, S. Studies on the lithium ion diffusion coefficients of electrospun Nb2O5 nanostructures using galvanostatic intermittent titration and electrochemical impedance spectroscopy. Electrochim. Acta 2014, 128, 198–202. [Google Scholar] [CrossRef]
- Le Viet, A.; Jose, R.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Nb2O5 photoelectrodes for dye-sensitized solar cells: Choice of the polymorph. J. Phys. Chem. C 2010, 114, 21795–21800. [Google Scholar] [CrossRef]












| CNF | MCNF | |
|---|---|---|
| C1s Atomic % | 90.12% | 90.42% |
| N1s Atomic % | 2.44% | 5.11% |
| O1s Atomic % | 7.45% | 4.58% |
| Pyridinic (N-6) | 0.23% (398.4 eV) | 1.76% (398.4 eV) |
| pyrrolic/pyridine (N-5) | 1.76% (400.8 eV) | 3.25% (400.8 eV) |
| quaternary (N-Q) | 0.41% (405.4 eV) | 0.09% (403.8 eV) |
| Sample | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| CNF | 696 | 0.29 |
| MCNF | 30 | 0.05 |
| Structure | Double Interlayer | Cathode Interlayer | Anode Interlayer | Without Interlayer |
|---|---|---|---|---|
| ∆Ep1 (mV) | 80 | 170 | 190 | 280 |
| ∆Ep2 (mV) | 280 | 440 | 390 | 480 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Le, T.; Yang, Y.; Yu, Z.; Huang, Z.; Kang, F. Effects of Electrospun Carbon Nanofibers’ Interlayers on High-Performance Lithium–Sulfur Batteries. Materials 2017, 10, 376. https://doi.org/10.3390/ma10040376
Gao T, Le T, Yang Y, Yu Z, Huang Z, Kang F. Effects of Electrospun Carbon Nanofibers’ Interlayers on High-Performance Lithium–Sulfur Batteries. Materials. 2017; 10(4):376. https://doi.org/10.3390/ma10040376
Chicago/Turabian StyleGao, Tianji, TrungHieu Le, Ying Yang, Zhihao Yu, Zhenghong Huang, and Feiyu Kang. 2017. "Effects of Electrospun Carbon Nanofibers’ Interlayers on High-Performance Lithium–Sulfur Batteries" Materials 10, no. 4: 376. https://doi.org/10.3390/ma10040376