Mechanics of Pickering Drops Probed by Electric Field–Induced Stress
Abstract
:1. Introduction
2. Results
2.1. Electric Field–Induced Deformation of Pickering Drops
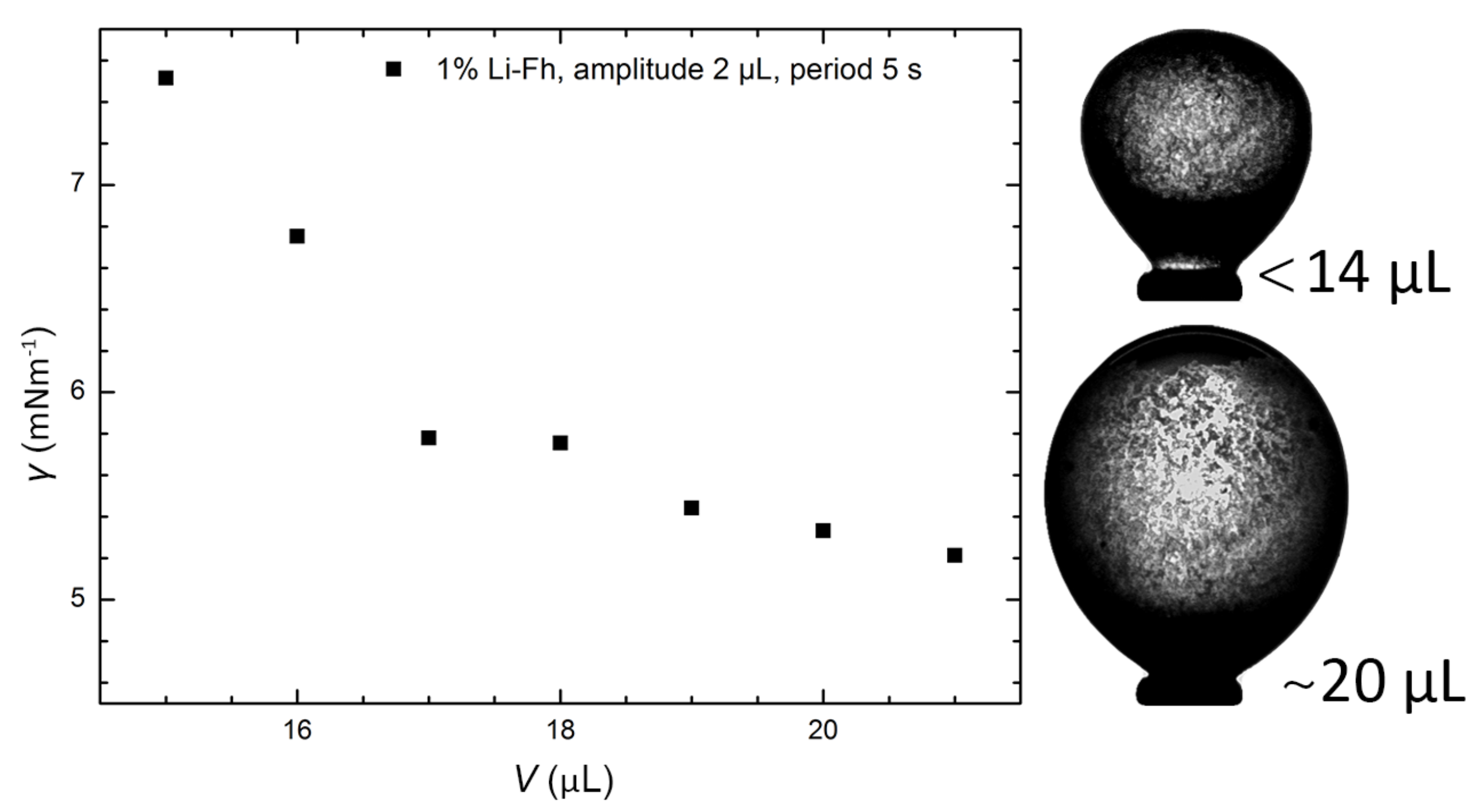
2.2. Surface Tension of Pickering Drops in the Absence of Electric Fields
3. Discussion
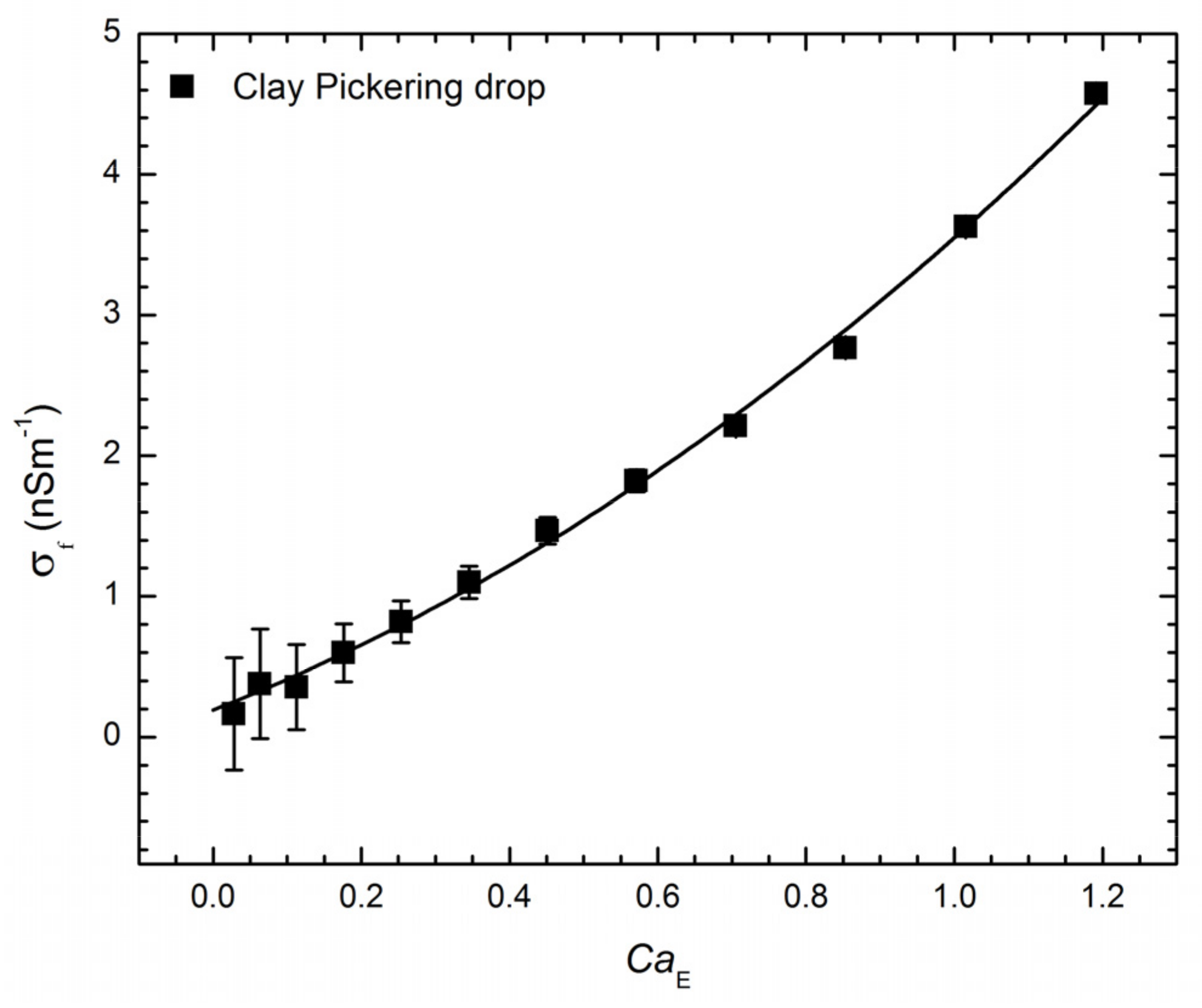
3.1. Pickering Drop Deformation in an Electric Field
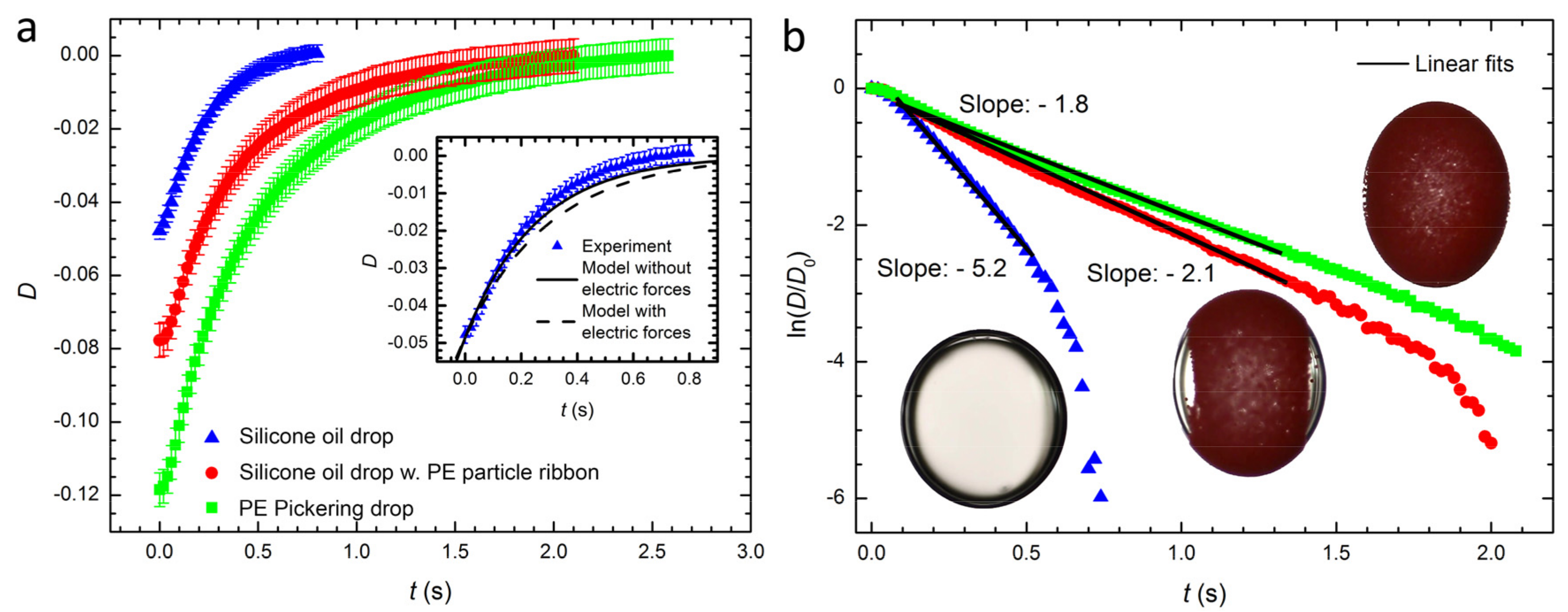
3.2. Relaxation of Drop or Pickering Drop Deformation after Turning off the Electric Field
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Estimation of Particle Coverage
5.3. Electrical Measurements
5.4. Oscillation Drop Tensiometry
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Binks, B.P.; Whitby, C.P. Silica particle-stabilized emulsions of silicone oil and water: Aspects of emulsification. Langmuir 2004, 20, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid interface sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Gholamipour-Shirazi, A.; Carvalho, M.; Fossum, J. Controlled microfluidic emulsification of oil in a clay nanofluid: Role of salt for Pickering stabilization. Eur. Phys. J. Spec. Top. 2016, 225, 757–765. [Google Scholar] [CrossRef]
- Subramaniam, A.B.; Abkarian, M.; Stone, H.A. Controlled assembly of jammed colloidal shells on fluid droplets. Nat. Mater. 2005, 4, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Józefczak, A. Patchy colloidosomes—An emerging class of structures. Eur. Phys. J. Spec. Top. 2016, 225, 741–756. [Google Scholar] [CrossRef]
- Nudurupati, S.; Janjua, M.; Aubry, N.; Singh, P. Concentrating particles on drop surfaces using external electric fields. Electrophoresis 2008, 29, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Nudurupati, S.; Janjua, M.; Singh, P.; Aubry, N. Effect of parameters on redistribution and removal of particles from drop surfaces. Soft Matter 2010, 6, 1157–1169. [Google Scholar] [CrossRef]
- Dommersnes, P.; Rozynek, Z.; Mikkelsen, A.; Castberg, R.; Kjerstad, K.; Hersvik, K.; Fossum, J.O. Active structuring of colloidal armour on liquid drops. Nat. Commun. 2013, 4, 2066. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Mikkelsen, A.; Dommersnes, P.; Fossum, J.O. Electroformation of Janus and patchy capsules. Nat. Commun. 2014, 5, 3945. [Google Scholar] [CrossRef] [PubMed]
- Dommersnes, P.; Fossum, J.O. Surface structuring of particle laden drops using electric fields. Eur. Phys. J. Spec. Top. 2016, 225, 715–728. [Google Scholar] [CrossRef]
- Dinsmore, A.D.; Hsu, M.F.; Nikolaides, M.G.; Marquez, M.; Bausch, A.R.; Weitz, D.A. Colloidosomes: Selectively permeable capsules composed of colloidal particles. Science 2002, 298, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.S.; Studart, A.R. Monodisperse functional colloidosomes with tailored nanoparticle shells. Langmuir 2011, 27, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Cayre, O.J.; Hitchcock, J.; Manga, M.S.; Fincham, S.; Simoes, A.; Williams, R.A.; Biggs, S. pH-responsive colloidosomes and their use for controlling release. Soft Matter 2012, 8, 4717–4724. [Google Scholar] [CrossRef]
- Fery, A.; Weinkamer, R. Mechanical properties of micro-and nanocapsules: Single-capsule measurements. Polymer 2007, 48, 7221–7235. [Google Scholar] [CrossRef]
- Knoche, S.; Vella, D.; Aumaitre, E.; Degen, P.; Rehage, H.; Cicuta, P.; Kierfeld, J. Elastometry of deflated capsules: Elastic moduli from shape and wrinkle analysis. Langmuir 2013, 29, 12463–12471. [Google Scholar] [CrossRef] [PubMed]
- Monteux, C.; Kirkwood, J.; Xu, H.; Jung, E.; Fuller, G.G. Determining the mechanical response of particle-laden fluid interfaces using surface pressure isotherms and bulk pressure measurements of droplets. Phys. Chem. Chem. Phys. 2007, 9, 6344–6350. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.S.; Shum, H.C.; Weitz, D.A. Controlled buckling and crumpling of nanoparticle-coated droplets. Langmuir 2010, 26, 18612–18616. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.K.; Carl, P.; Gorevski, N.; Russell, T.P.; Wang, Q.; Boker, A.; Fery, A. Separating membrane and surface tension contributions in Pickering droplet deformation. Soft Matter 2008, 4, 2259–2266. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Tabor, R.F.; Ong, L.; Stevens, G.W.; Dagastine, R.R. Nano-mechanical properties of clay-armoured emulsion droplets. Soft Matter 2012, 8, 3112–3121. [Google Scholar] [CrossRef]
- Subramaniam, A.B.; Abkarian, M.; Mahadevan, L.; Stone, H.A. Mechanics of interfacial composite materials. Langmuir 2006, 22, 10204–10208. [Google Scholar] [CrossRef] [PubMed]
- Bécu, L.; Benyahia, L. Strain-induced droplet retraction memory in a Pickering emulsion. Langmuir 2009, 25, 6678–6682. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.; Gräf, V.; Walz, E.; Schuchmann, H.P. Production of particle-stabilized nonspherical emulsion drops in simple shear flow. Chem. Eng. Technol. 2015, 38, 1490–1493. [Google Scholar] [CrossRef]
- Mulligan, M.K.; Rothstein, J.P. Deformation and breakup of micro- and nanoparticle stabilized droplets in microfluidic extensional flows. Langmuir 2011, 27, 9760–9768. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, A.; Wojciechowski, J.; Rajňák, M.; Kurimský, J.; Khobaib, K.; Kertmen, A.; Rozynek, Z. Electric field-driven assembly of sulfonated polystyrene microspheres. Materials 2017, 10, 329. [Google Scholar] [CrossRef]
- Collins, R.T.; Sambath, K.; Harris, M.T.; Basaran, O.A. Universal scaling laws for the disintegration of electrified drops. Proc. Natl. Acad. Sci. USA 2013, 110, 4905–4910. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.T.; Jones, J.J.; Harris, M.T.; Basaran, O.A. Electrohydrodynamic tip streaming and emission of charged drops from liquid cones. Nat. Phys. 2008, 4, 149–154. [Google Scholar] [CrossRef]
- Rozynek, Z.; Dommersnes, P.; Mikkelsen, A.; Michels, L.; Fossum, J.O. Electrohydrodynamic controlled assembly and fracturing of thin colloidal particle films confined at drop interfaces. Eur. Phys. J. Spec. Top. 2014, 223, 1859–1867. [Google Scholar] [CrossRef]
- Ouriemi, M.; Vlahovska, P.M. Electrohydrodynamic deformation and rotation of a particle-coated drop. Langmuir 2015, 31, 6298–6305. [Google Scholar] [CrossRef] [PubMed]
- Ouriemi, M.; Vlahovska, P.M. Electrohydrodynamics of particle-covered drops. J. Fluid Mech. 2014, 751, 106–120. [Google Scholar] [CrossRef]
- Rozynek, Z.; Kaczmarek-Klinowska, M.; Magdziarz, A. Assembly and rearrangement of particles confined at a surface of a droplet, and intruder motion in electro-shaken particle films. Materials 2016, 9, 679. [Google Scholar] [CrossRef]
- Chen, G.; Tan, P.; Chen, S.; Huang, J.; Wen, W.; Xu, L. Coalescence of Pickering emulsion droplets induced by an electric field. Phys. Rev. Lett. 2013, 110, 064502. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Singh, P.; Aubry, N. Destabilization of Pickering emulsions using external electric fields. Electrophoresis 2010, 31, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.B.; Caggioni, M.; Ergun, R.; Hartel, R.W.; Spicer, P.T. Arrested coalescence in Pickering emulsions. Soft Matter 2011, 7, 7710–7716. [Google Scholar] [CrossRef]
- Studart, A.R.; Shum, H.C.; Weitz, D.A. Arrested coalescence of particle-coated droplets into nonspherical supracolloidal structures. J. Phys. Chem. B 2009, 113, 3914–3919. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, A.; Dommersnes, P.; Fossum, J.O. Electric stress-induced slip lines in jammed particle monolayers. Rev. Cuba. Fis. 2016, 33, 47–49. [Google Scholar]
- Saville, D.A. Electrohydrodynamics: The Taylor-Melcher leaky dielectric model. Annu. Rev. Fluid Mech. 1997, 29, 27–64. [Google Scholar] [CrossRef]
- Taylor, G. Studies in electrohydrodynamics. I. The circulation produced in a drop by electrical field. Proc. R. Soc. Math. Phys. Eng. Sci. 1966, 291, 159–166. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Nagayama, K. Capillary interactions between particles bound to interfaces, liquid films and biomembranes. Adv. Colloid Interface Sci. 2000, 85, 145–192. [Google Scholar] [CrossRef]
- Lanauze, J.A.; Walker, L.M.; Khair, A.S. Nonlinear electrohydrodynamics of slightly deformed oblate drops. J. Fluid Mech. 2015, 774, 245–266. [Google Scholar] [CrossRef]
- Rozynek, Z.; Knudsen, K.D.; Fossum, J.O.; Meheust, Y.; Wang, B.; Zhou, M. Electric field induced structuring in clay-oil suspensions: New insights from WAXS, SEM, leak current, dielectric permittivity, and rheometry. J. Phys. Condens. Matter 2010, 22, 324104. [Google Scholar] [CrossRef] [PubMed]
- Esmaeeli, A.; Sharifi, P. Transient electrohydrodynamics of a liquid drop. Phys. Rev. E 2011, 84, 036308. [Google Scholar] [CrossRef] [PubMed]
- McConnell, L.C.; Vlahovska, P.M.; Miksis, M.J. Vesicle dynamics in uniform electric fields: Squaring and breathing. Soft Matter 2015, 11, 4840–4846. [Google Scholar] [CrossRef] [PubMed]
- Salipante, P.F.; Vlahovska, P.M. Vesicle deformation in DC electric pulses. Soft Matter 2014, 10, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Karyappa, R.B.; Deshmukh, S.D.; Thaokar, R.M. Deformation of an elastic capsule in a uniform electric field. Phys. Fluids 2014, 26, 122108. [Google Scholar] [CrossRef]
- Ha, J.W.; Yang, S.M. Electrohydrodynamic effects on the deformation and orientation of a liquid capsule in a linear flow. Phys. Fluids 2000, 12, 1671–1684. [Google Scholar] [CrossRef]
- Winterhalter, M.; Helfrich, W. Deformation of spherical vesicles by electric-fields. J. Colloid Interface Sci. 1988, 122, 583–586. [Google Scholar] [CrossRef]
- Rallison, J. The deformation of small viscous drops and bubbles in shear flows. Annu. Rev. Fluid Mech. 1984, 16, 45–66. [Google Scholar] [CrossRef]
- Luciani, A.; Champagne, M.; Utracki, L. Interfacial tension coefficient from the retraction of ellipsoidal drops. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 1393–1403. [Google Scholar] [CrossRef]
- Lanauze, J.A.; Walker, L.M.; Khair, A.S. Relaxation or breakup of a low-conductivity drop upon removal of a uniform dc electric field. Phys. Rev. Fluids 2016, 1, 033902. [Google Scholar] [CrossRef]
- Assighaou, S.; Benyahia, L. Universal retraction process of a droplet shape after a large strain jump. Phys. Rev. E 2008, 77, 5. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.L.; Hemmen, H.; Fonseca, D.M.; Coutant, C.; Knudsen, K.D.; Plivelic, T.S.; Bonn, D.; Fossum, J.O. Swelling transition of a clay induced by heating. Sci. Rep. 2012, 2, 618. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Freer, E.; Wong, H.; Radke, C. Oscillating drop/bubble tensiometry: Effect of viscous forces on the measurement of interfacial tension. J. Colloid Interface Sci. 2005, 282, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Basaran, O.A.; Franses, E.I. Effects of dynamic surface tension and fluid flow on the oscillations of a supported bubble. Colloids Surf. Physicochem. Eng. Asp. 2006, 282, 183–202. [Google Scholar] [CrossRef]
- Liao, Y.C.; Basaran, O.A.; Franses, E.I. Hydrodynamic effects on the oscillations of supported bubbles: Implications for accurate measurements of surface properties. Colloids Surf. Physicochem. Eng. Asp. 2004, 250, 367–384. [Google Scholar] [CrossRef]


| Fluid | (Sm−1) | (Pa s) | (kgm−3) | (mm) | |
|---|---|---|---|---|---|
| Drop (silicone oil) | 2.8 | 0.05 | 961 | 1.0 | |
| Medium (castor oil) | 4.7 | 0.75 | 960 | - | |
| (mNm−1) | |||||
| 0.4 | 1.3 | 1.7 | 0.07 | 4.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikkelsen, A.; Dommersnes, P.; Rozynek, Z.; Gholamipour-Shirazi, A.; Carvalho, M.d.S.; Fossum, J.O. Mechanics of Pickering Drops Probed by Electric Field–Induced Stress. Materials 2017, 10, 436. https://doi.org/10.3390/ma10040436
Mikkelsen A, Dommersnes P, Rozynek Z, Gholamipour-Shirazi A, Carvalho MdS, Fossum JO. Mechanics of Pickering Drops Probed by Electric Field–Induced Stress. Materials. 2017; 10(4):436. https://doi.org/10.3390/ma10040436
Chicago/Turabian StyleMikkelsen, Alexander, Paul Dommersnes, Zbigniew Rozynek, Azarmidokht Gholamipour-Shirazi, Marcio da Silveira Carvalho, and Jon Otto Fossum. 2017. "Mechanics of Pickering Drops Probed by Electric Field–Induced Stress" Materials 10, no. 4: 436. https://doi.org/10.3390/ma10040436
APA StyleMikkelsen, A., Dommersnes, P., Rozynek, Z., Gholamipour-Shirazi, A., Carvalho, M. d. S., & Fossum, J. O. (2017). Mechanics of Pickering Drops Probed by Electric Field–Induced Stress. Materials, 10(4), 436. https://doi.org/10.3390/ma10040436





