Surface Modification of Multi-Walled Carbon Nanotubes via Hemoglobin-Derived Iron and Nitrogen-Rich Carbon Nanolayers for the Electrocatalysis of Oxygen Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of CNT-Based Catalysts
2.3. Characterizations and Electrochemical Tests of CNT-Based Catalysts
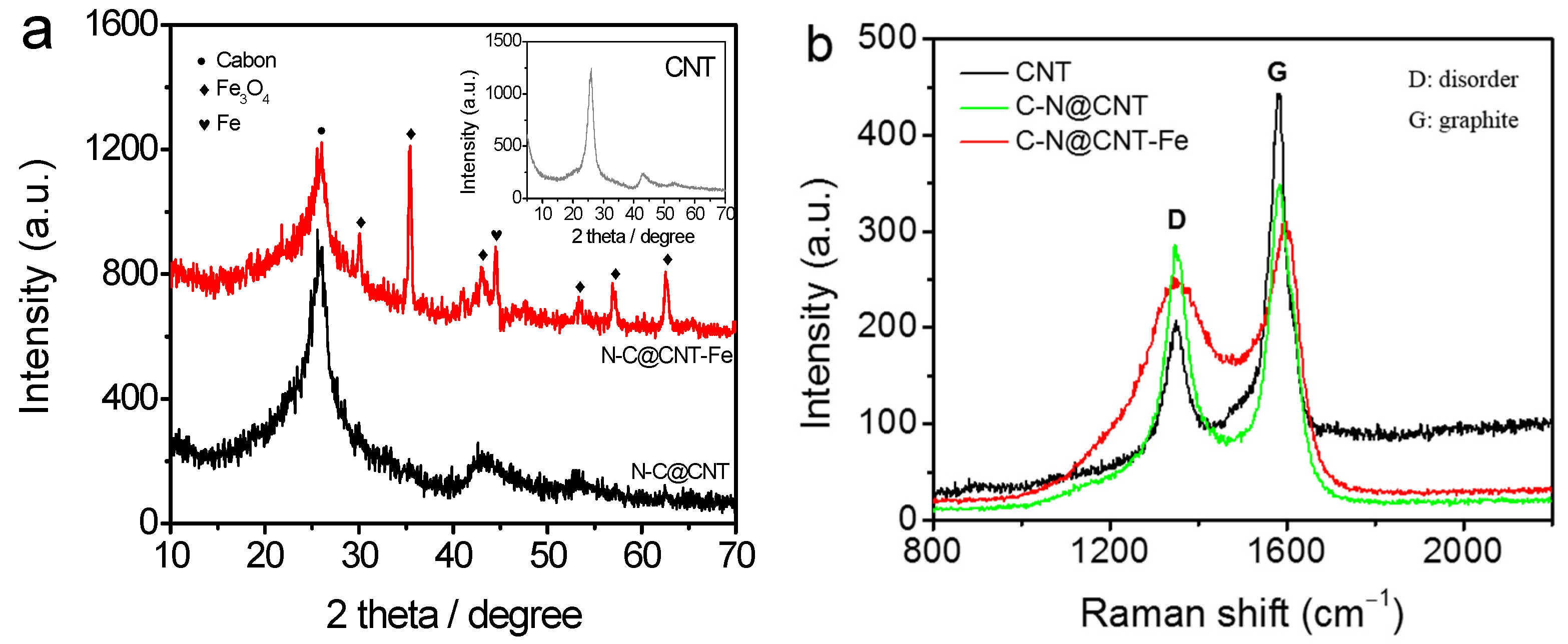
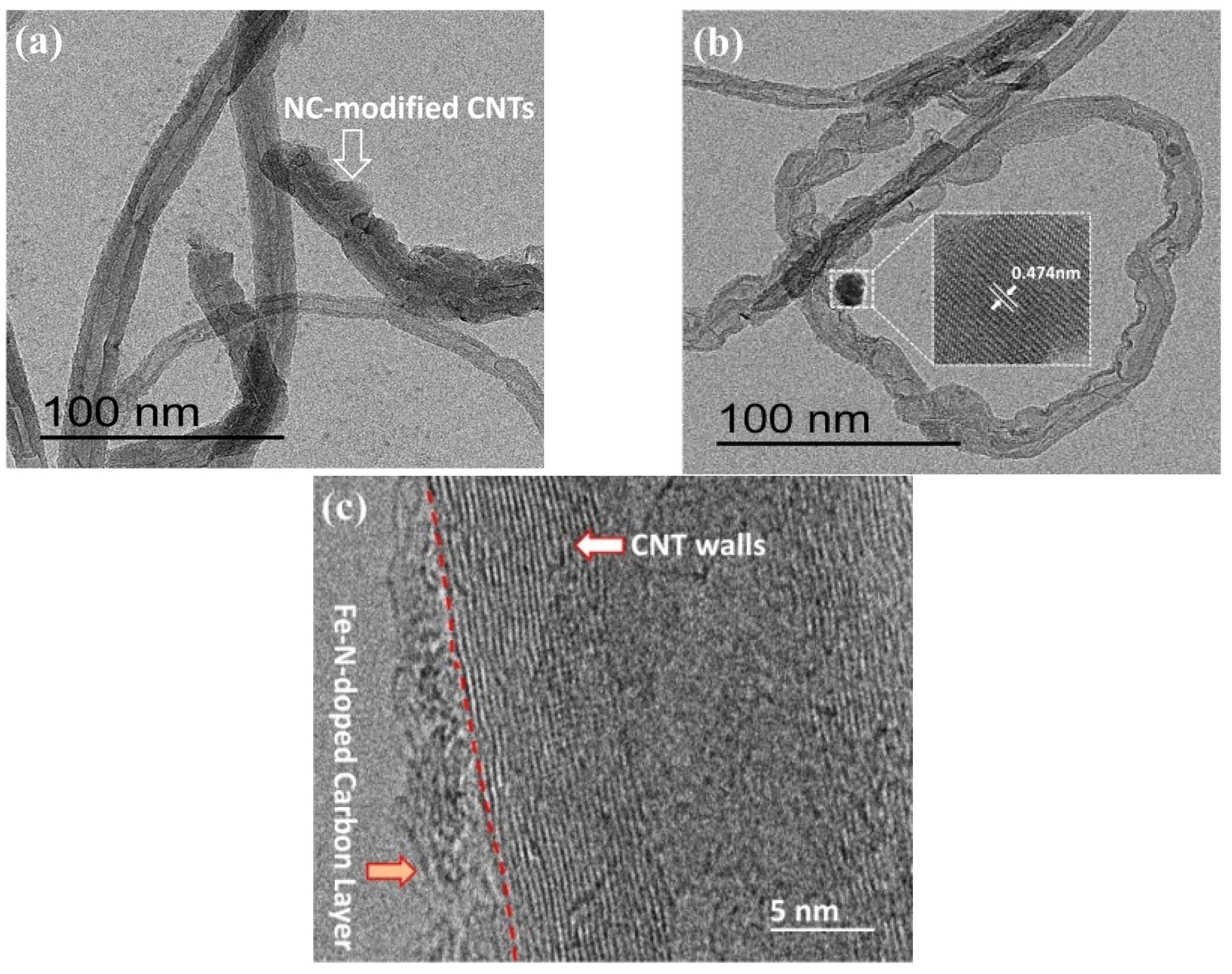
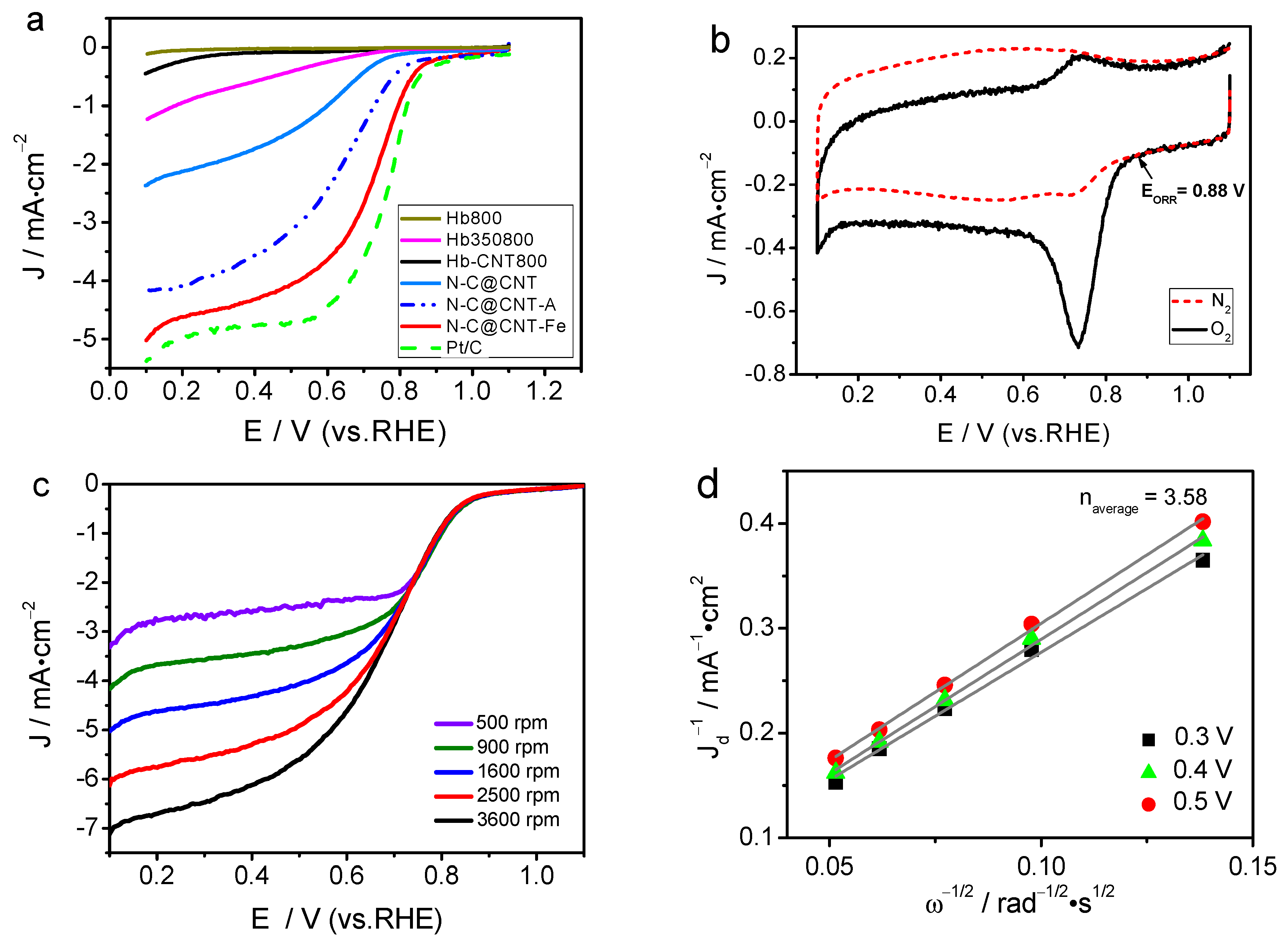
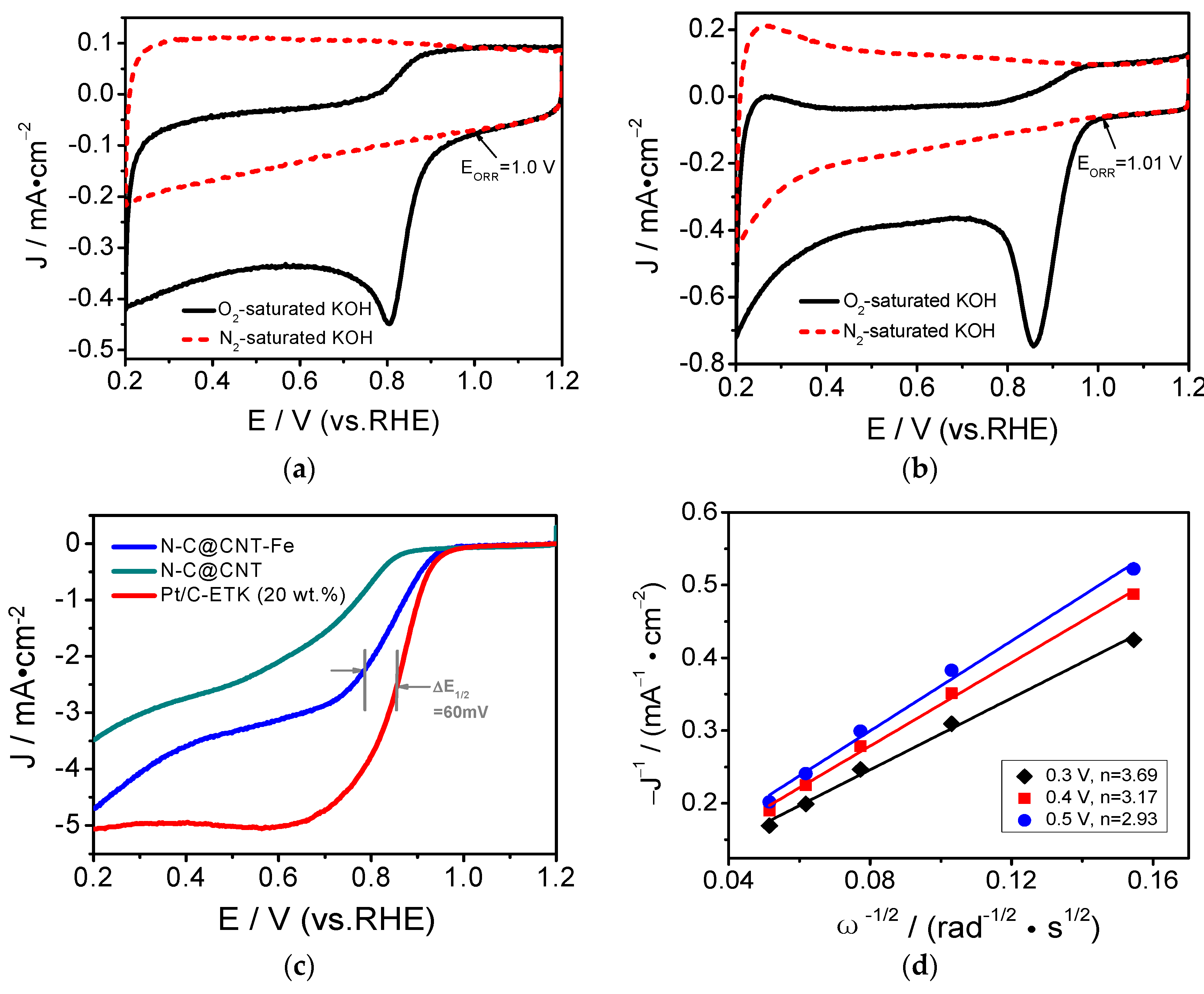
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Levy, N.; Mahammed, A.; Kosa, M.; Major, D.T.; Gross, Z.; Elbaz, L. Metallocorroles as nonprecious-metal catalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 14080–14084. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Z.; Liao, W.L.; Sun, L.T.; Chen, C.G. Synthesis of non-noble nitrogen-containing catalysts for cathodic oxygen reduction reaction: A critical review. Int. J. Electrochem. Sci. 2015, 10, 2467–2477. [Google Scholar]
- Wang, Q.; Zhou, Z.-Y.; Lai, Y.-J.; You, Y.; Liu, J.-G.; Wu, X.-L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M.; et al. Phenylenediamine-based FeNx/C catalyst with high activity for oxygen reduction in acid medium and its active-site probing. J. Am. Chem. Soc. 2014, 136, 10882–10885. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Hu, R.; Liao, W.; Li, Z.; Sun, L.; Shi, D.; Li, Y.; Chen, C. Protein-enriched fish “biowaste” converted to three-dimensional porous carbon nano-network for advanced oxygen reduction electrocatalysis. Electrochim. Acta 2017, 236, 228–238. [Google Scholar] [CrossRef]
- Vij, V.; Tiwari, J.N.; Lee, W.-G.; Yoon, T.; Kim, K.S. Hemoglobin-carbon nanotube derived noble-metal-free Fe5C2-based catalyst for highly efficient oxygen reduction reaction. Sci. Rep. 2016, 6, 20132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bai, Z.; Wu, M.; Qiao, J.; Chen, Z. 3-Dimensional porous N-doped graphene foam as a non-precious catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 3343–3350. [Google Scholar] [CrossRef]
- Nie, Y.; Xie, X.; Chen, S.; Ding, W.; Qi, X.; Wang, Y.; Wang, J.; Li, W.; Wei, Z.; Shao, M. Towards effective utilization of nitrogen-containing active sites: Nitrogen-doped carbon layers wrapped CNTs electrocatalysts for superior oxygen reduction. Electrochim. Acta 2016, 187, 153–160. [Google Scholar] [CrossRef]
- Guo, C.; Chen, C.; Luo, Z. The structural changes of blood pyropolymers and their beneficial electrocatalytic activity toward oxygen reduction. Chin. Sci. Bull. 2013, 58, 3698–3703. [Google Scholar] [CrossRef]
- Guo, C.; Liao, W.; Chen, C. Fe/N/C catalysts derived from blood protein and their electrocatalytic activity towards the oxygen reduction reaction in acidic solution. Chin. Sci. Bull. 2014, 59, 3424–3429. [Google Scholar] [CrossRef]
- Guo, C.-Z.; Chen, C.-G.; Luo, Z.-L. A novel nitrogen-containing electrocatalyst for oxygen reduction reaction from blood protein pyrolysis. J. Power Sources 2014, 245, 841–845. [Google Scholar] [CrossRef]
- Guo, C.; Liao, W.; Li, Z.; Chen, C. Exploration of the catalytically active site structures of animal biomass-modified on cheap carbon nanospheres for oxygen reduction reaction with high activity, stability and methanol-tolerant performance in alkaline medium. Carbon 2015, 85, 279–288. [Google Scholar] [CrossRef]
- Maruyama, J.; Abe, I. Carbonized hemoglobin functioning as a cathode catalyst for polymer electrolyte fuel cells. Chem. Mater. 2006, 18, 1303–1311. [Google Scholar] [CrossRef]
- Ding, Y.; Jia, W.; Zhang, H.; Li, B.; Gu, Z.; Le, Y. Carbonized hemoglobin nanofibers for enhanced H2O2 detection. Electroanalysis 2010, 22, 1911–1917. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L.; Chang, D.W.; Baek, J.-B. Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction. ACS Nano 2011, 5, 6202–6209. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liao, W.; Li, Z.; Sun, L.; Chen, C. Easy conversion of protein-rich enoki mushroom biomass to a nitrogen-doped carbon nanomaterial as a promising metal-free catalyst for oxygen reduction reaction. Nanoscale 2015, 7, 15990–15998. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, D.; Feng, X.; Muellen, K. Potentiometric sensing of neutral species based on a uniform-sized molecularly imprinted polymer as a receptor. Angew. Chem. Int. Ed. 2010, 49, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-Z.; Liao, W.-L.; Chen, C.-G. Design of a non-precious metal electrocatalyst for alkaline electrolyte oxygen reduction by using soybean biomass as the nitrogen source of electrocatalytically active center structures. J. Power Sources 2014, 269, 841–847. [Google Scholar] [CrossRef]
- Shui, J.; Wang, M.; Du, F.; Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 2015, 1–7, e1400129. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wei, Z.; Chen, S.; Qi, X.; Yang, T.; Hu, J.; Wang, D.; Wan, L.-J.; Alvi, S.F.; Li, L. Space-confinement-induced synthesis of pyridinic- and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction. Angew. Chem. Int. Ed. 2013, 52, 11755–11759. [Google Scholar] [CrossRef] [PubMed]
- Artyushkova, K.; Walker, C.; Patterson, W.; Atanassov, P. Hierarchically structured non-PGM oxygen reduction electrocatalyst based on microemulsion-templated silica and pyrolyzed iron and cyanamide precursors. Electrocatalysis 2014, 5, 241–247. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Y.; Ding, W.; Chen, S.G.; Xiong, K.; Qi, X.Q.; Zhang, Y.; Wang, J.; Wei, Z.D. Unification of catalytic oxygen reduction and hydrogen evolution reactions: Highly dispersive Co nanoparticles encapsulated inside Co and nitrogen co-doped carbon. Chem. Commun. 2015, 51, 8942–8945. [Google Scholar] [CrossRef] [PubMed]
- Tylus, U.; Jia, Q.; Strickland, K.; Ramaswamy, N.; Serov, A.; Atanassov, P.; Mukerjee, S. Elucidating oxygen reduction active sites in pyrolyzed metal–nitrogen coordinated non-precious-metal electrocatalyst systems. J. Phys. Chem. C 2014, 118, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Charreteur, F.; Ruggeri, S.; Jaouen, F.; Dodelet, J.P. Increasing the activity of Fe/N/C catalysts in PEM fuel cell cathodes using carbon blacks with a high-disordered carbon content. Electrochim. Acta 2008, 53, 6881–6889. [Google Scholar] [CrossRef]
- Schilling, T.; Bron, M. Oxygen reduction at Fe–N-modified multi-walled carbon nanotubes in acidic electrolyte. Electrochim. Acta 2008, 53, 5379–5385. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Chen, H.M.; Liu, R.-S.; Asakura, K.; Zhang, L.; Zhang, J.; Lo, M.-Y.; Peng, Y.-M. Carbon incorporated FeN/C electrocatalyst for oxygen reduction enhancement in direct methanol fuel cells: X-ray absorption approach to local structures. Electrochim. Acta 2011, 56, 8734–8738. [Google Scholar] [CrossRef]
- Liu, G.C.-K.; Dahn, J.R. Fe–N–C oxygen reduction catalysts supported on vertically aligned carbon nanotubes. Appl. Catal. A 2008, 347, 43–49. [Google Scholar] [CrossRef]
- Onodera, T.; Suzuki, S.; Mizukami, T.; Kanzaki, H. Enhancement of oxygen reduction activity with addition of carbon support for non-precious metal nitrogen doped carbon catalyst. J. Power Sources 2011, 196, 7994–7999. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Cao, X.; Jin, J.; Liu, Q.; Zhang, J. FeCo–Nx embedded graphene as high performance catalysts for oxygen reduction reaction. J. Appl. Catal. B 2013, 130–131, 143–151. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Sun, L.; Hu, R.; Liao, W.; Li, Z.; Li, Y.; Guo, C. Surface Modification of Multi-Walled Carbon Nanotubes via Hemoglobin-Derived Iron and Nitrogen-Rich Carbon Nanolayers for the Electrocatalysis of Oxygen Reduction. Materials 2017, 10, 564. https://doi.org/10.3390/ma10050564
Li W, Sun L, Hu R, Liao W, Li Z, Li Y, Guo C. Surface Modification of Multi-Walled Carbon Nanotubes via Hemoglobin-Derived Iron and Nitrogen-Rich Carbon Nanolayers for the Electrocatalysis of Oxygen Reduction. Materials. 2017; 10(5):564. https://doi.org/10.3390/ma10050564
Chicago/Turabian StyleLi, Wensheng, Lingtao Sun, Rong Hu, Wenli Liao, Zhongbin Li, Yanrong Li, and Chaozhong Guo. 2017. "Surface Modification of Multi-Walled Carbon Nanotubes via Hemoglobin-Derived Iron and Nitrogen-Rich Carbon Nanolayers for the Electrocatalysis of Oxygen Reduction" Materials 10, no. 5: 564. https://doi.org/10.3390/ma10050564
APA StyleLi, W., Sun, L., Hu, R., Liao, W., Li, Z., Li, Y., & Guo, C. (2017). Surface Modification of Multi-Walled Carbon Nanotubes via Hemoglobin-Derived Iron and Nitrogen-Rich Carbon Nanolayers for the Electrocatalysis of Oxygen Reduction. Materials, 10(5), 564. https://doi.org/10.3390/ma10050564




