Chemical Treatment of Waste Abaca for Natural Fiber-Reinforced Geopolymer Composite
Abstract
:1. Introduction
2. Materials and Method
2.1. Sources of Raw Materials
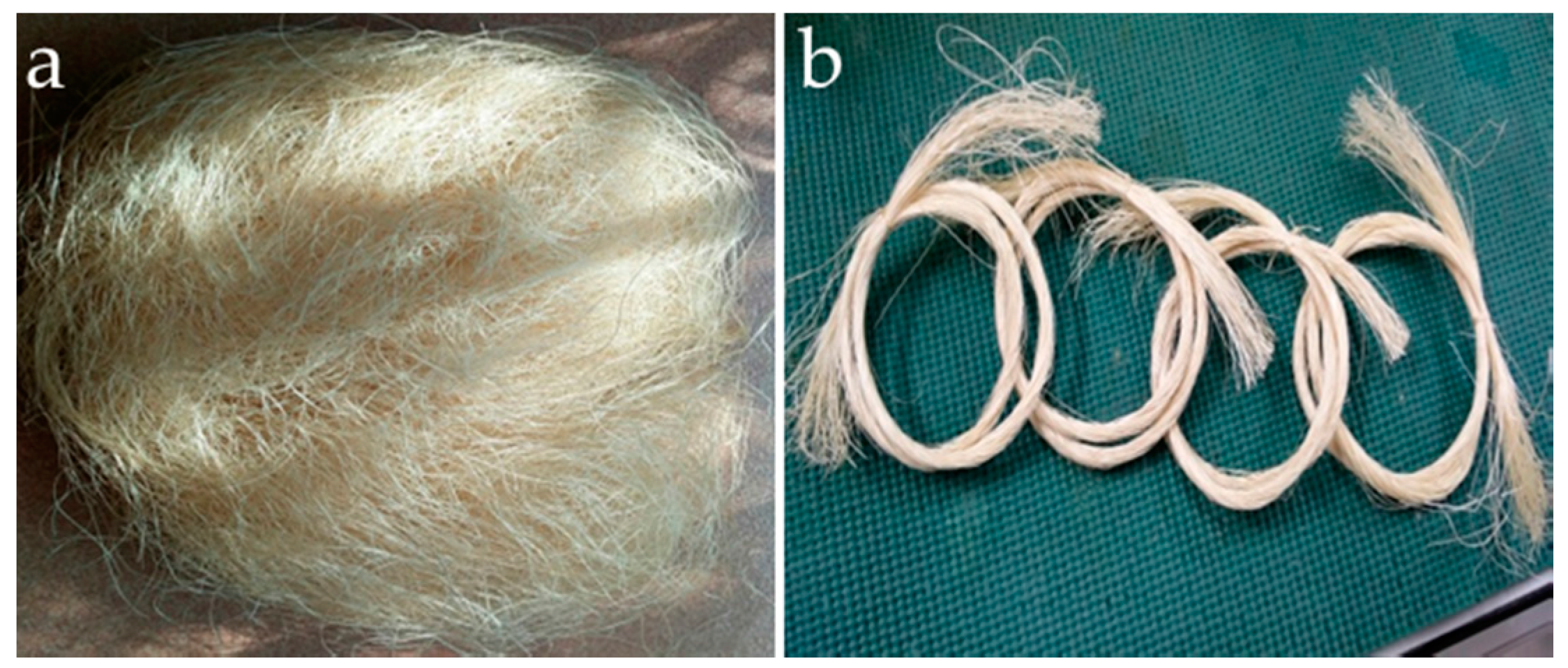
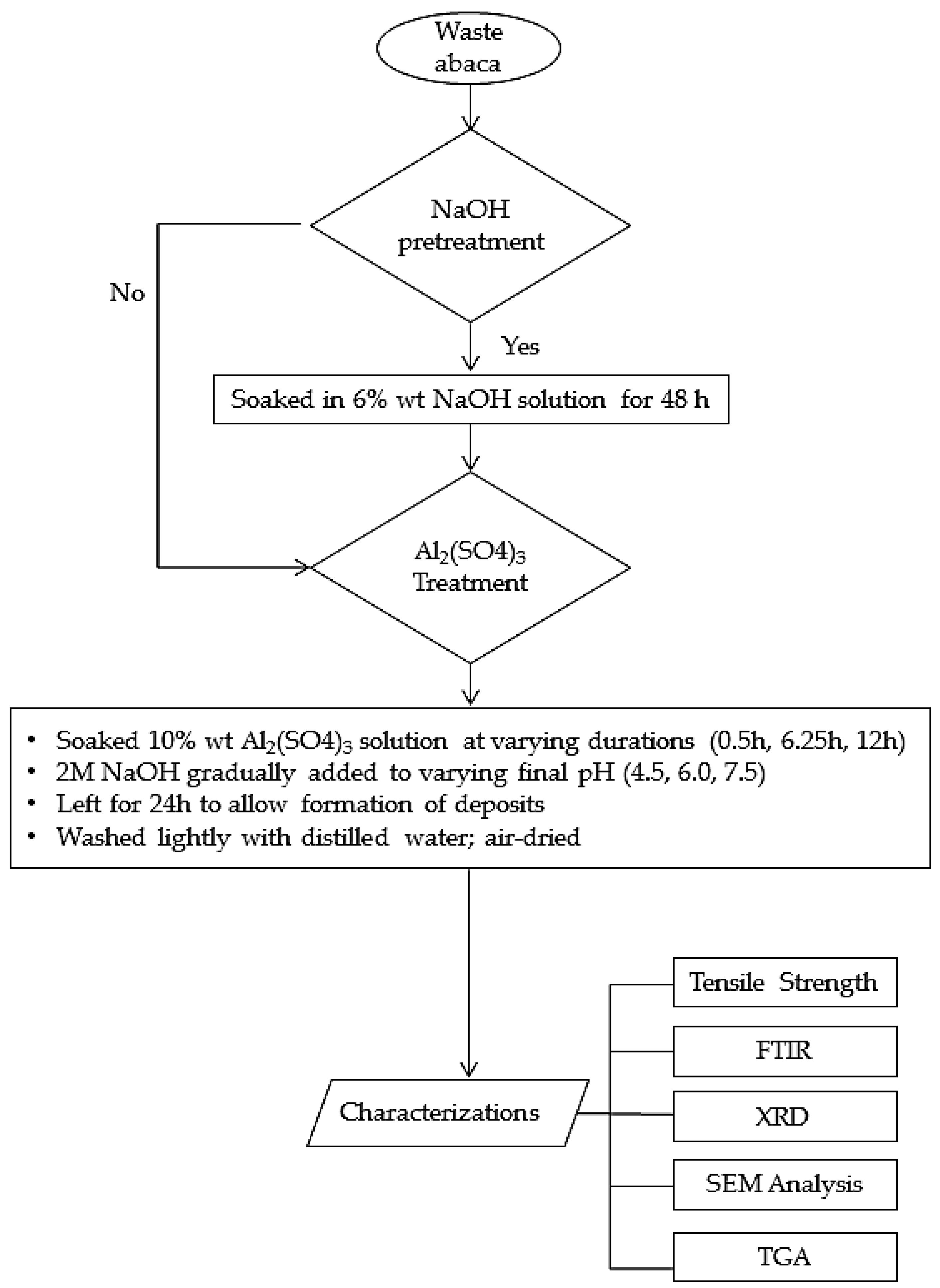
2.2. Chemical Treatment of Waste Abaca Fiber
2.3. Preparation of Abaca Fiber-Reinforced Geopolymer
2.4. Tensile Strength Test of Abaca Fibers
2.5. Scanning Electron Microscopy—Energy Dispersive Spectroscopy (SEM-EDS) Measurements
2.6. X-ray Diffraction (XRD) Measurements
2.7. Fourier Transform Infrared (FTIR) Spectroscopy Measurements
2.8. Thermogravimetric Analysis (TGA) Measurements
2.9. Compressive Strength and Flexural Strength Test of Geopolymer Composites
3. Results and Discussion
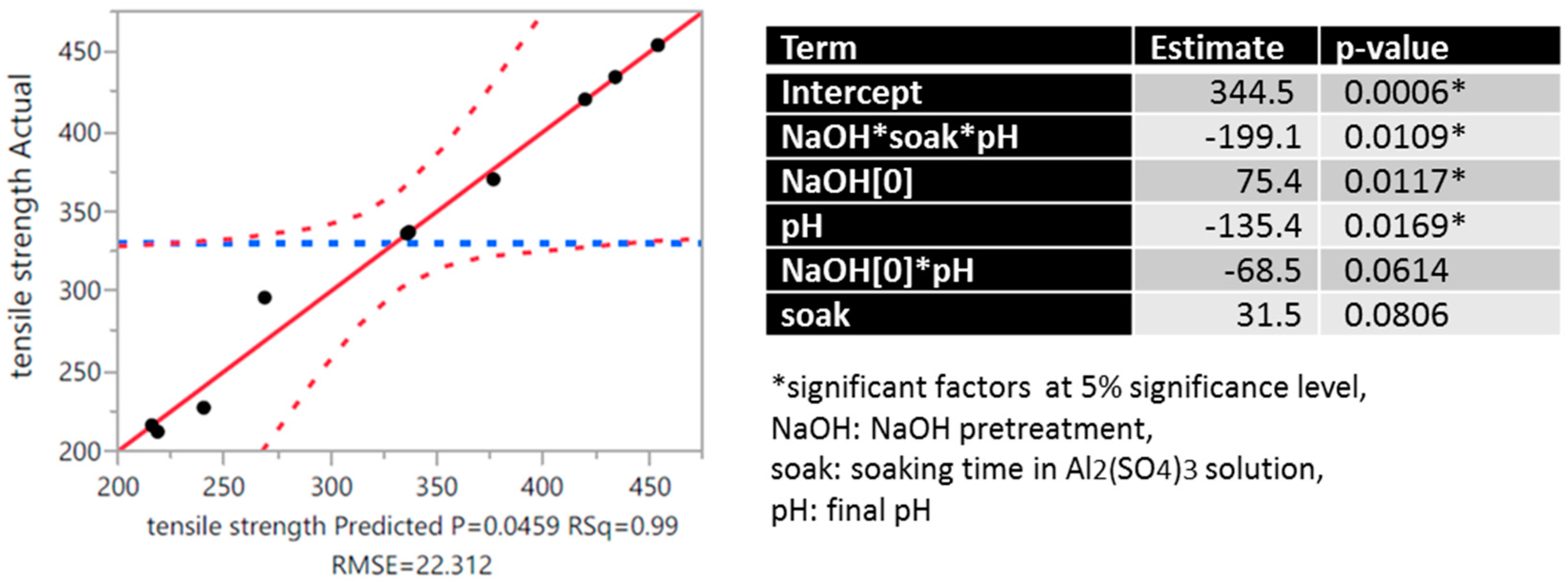
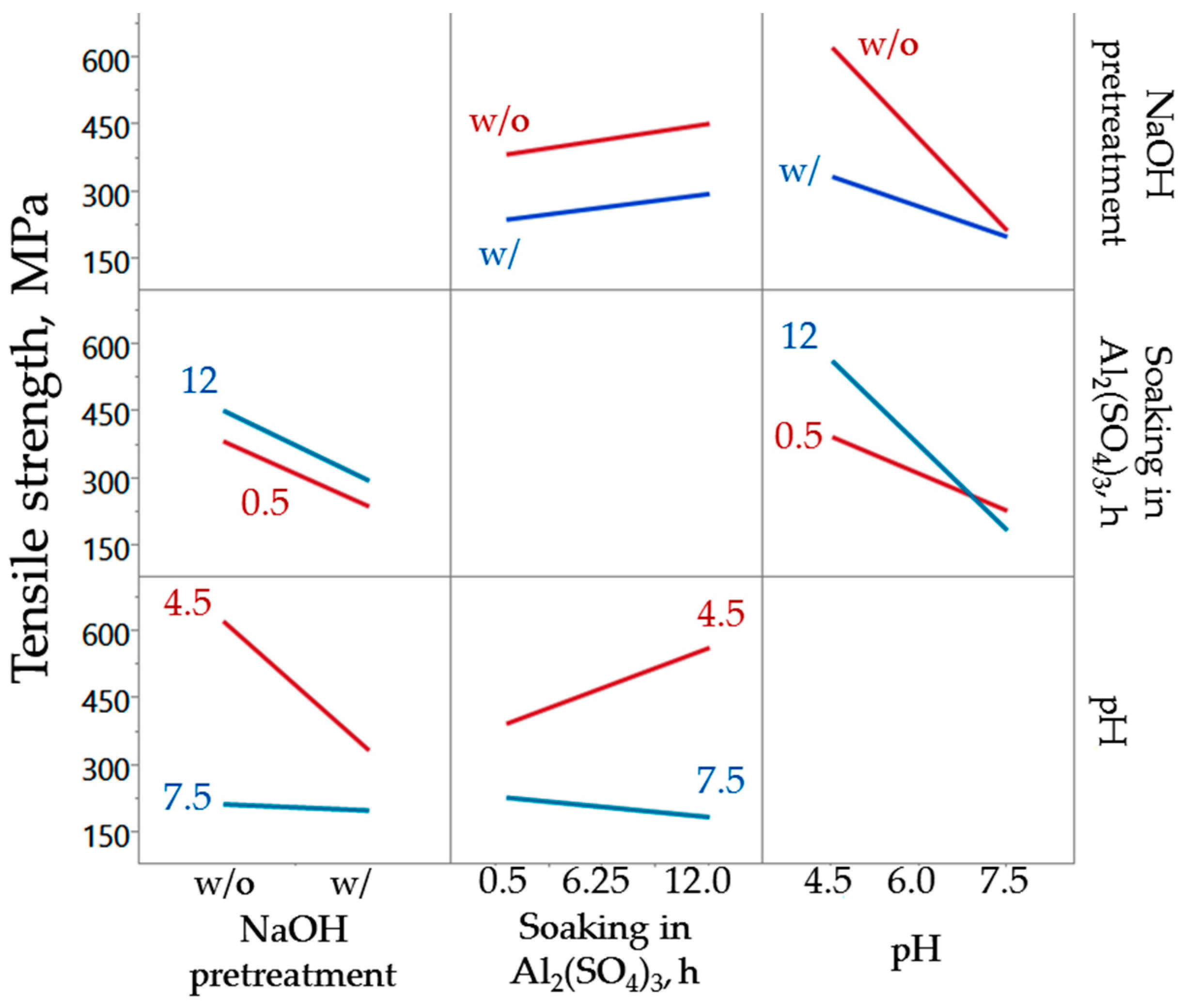
3.1. Tensile Strength of Chemically Treated Abaca Fiber
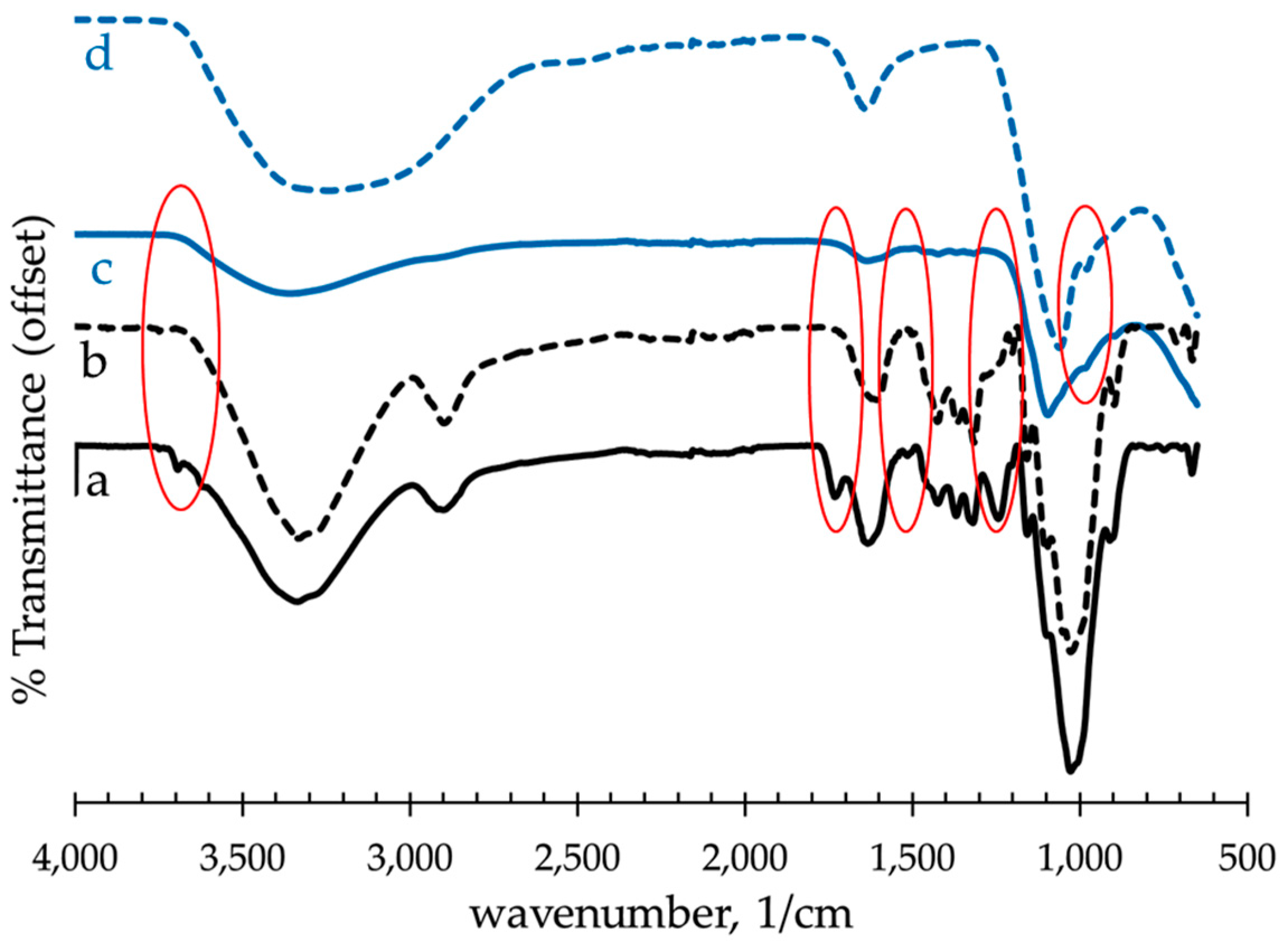
3.2. Fourier Transform Infrared Spectroscopy (FTIR) of Raw and Alkali-Treated Abaca Fiber
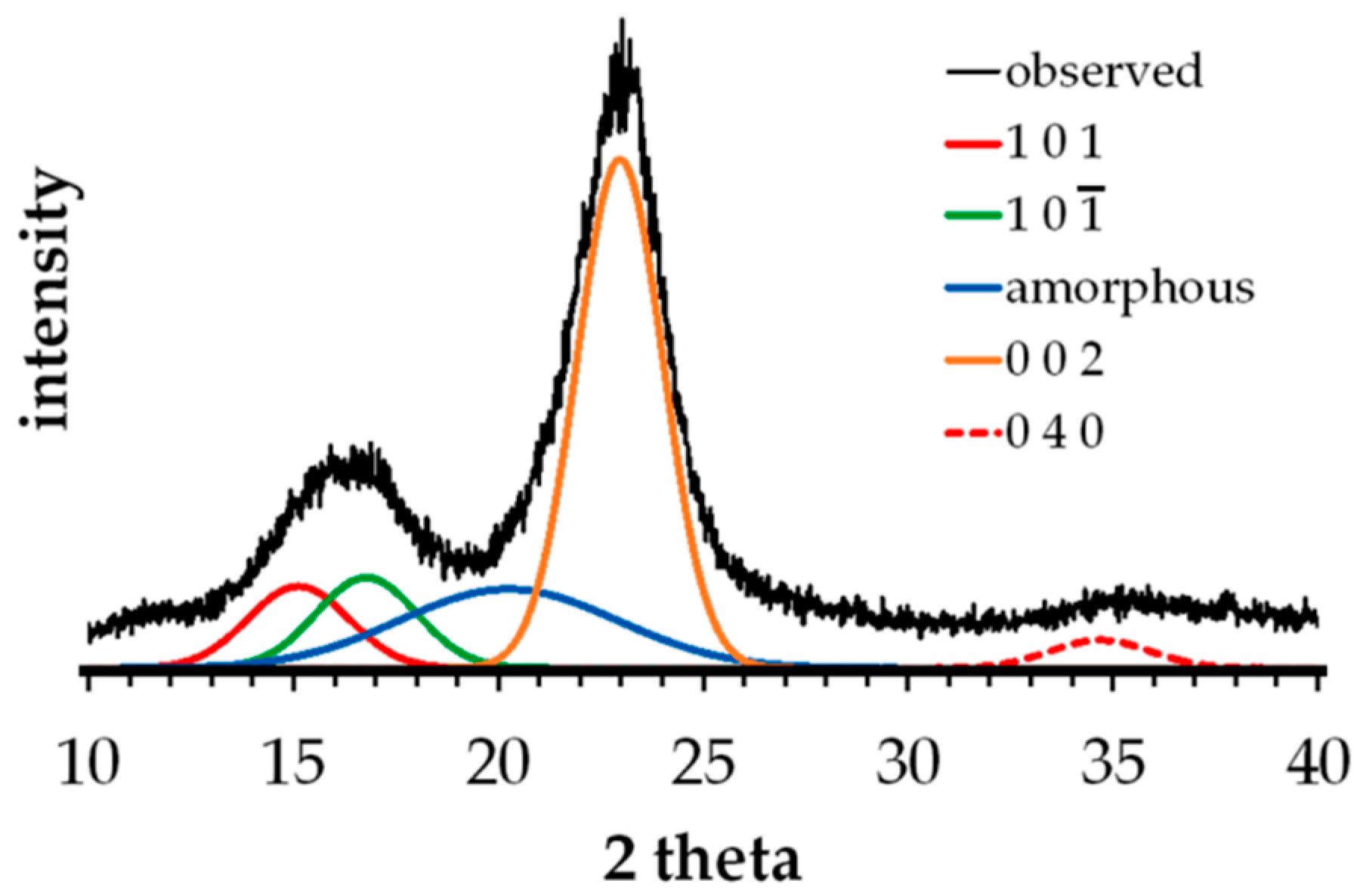
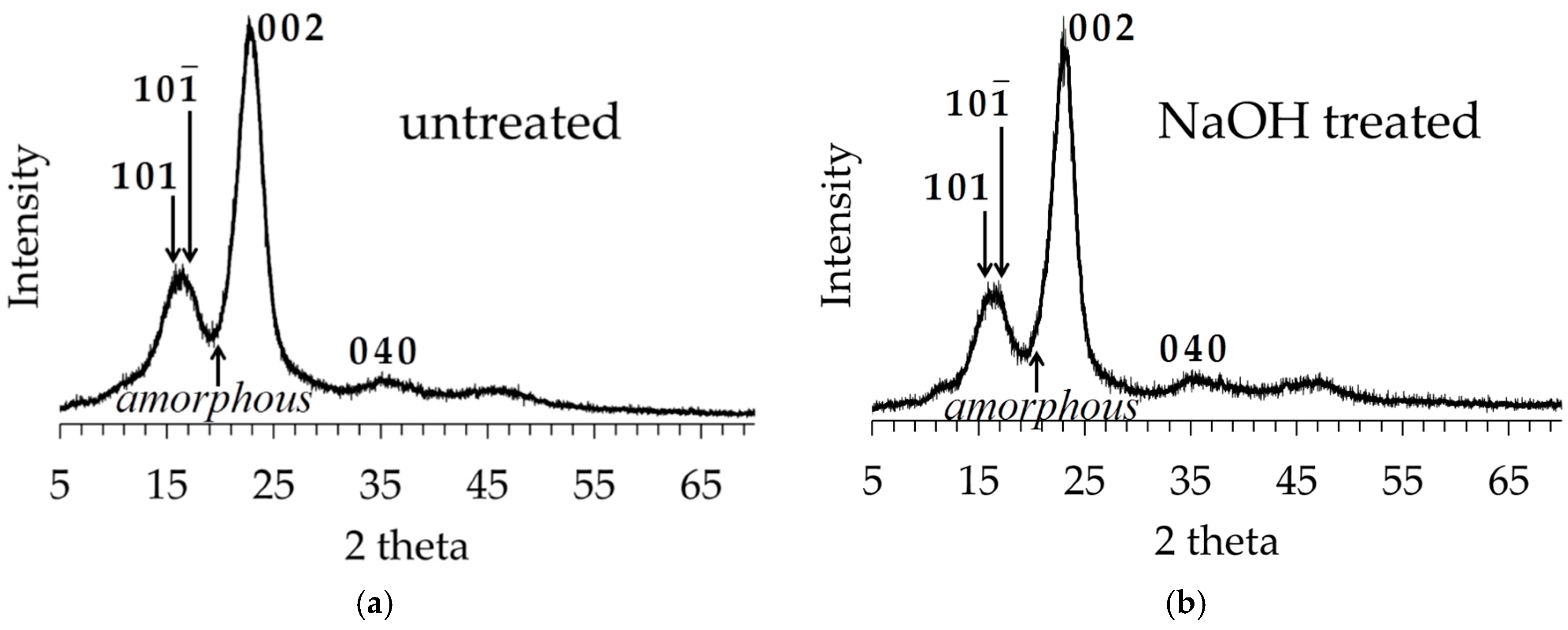
3.3. XRD Analysis of Untreated and Alkali-Treated Abaca Fibers
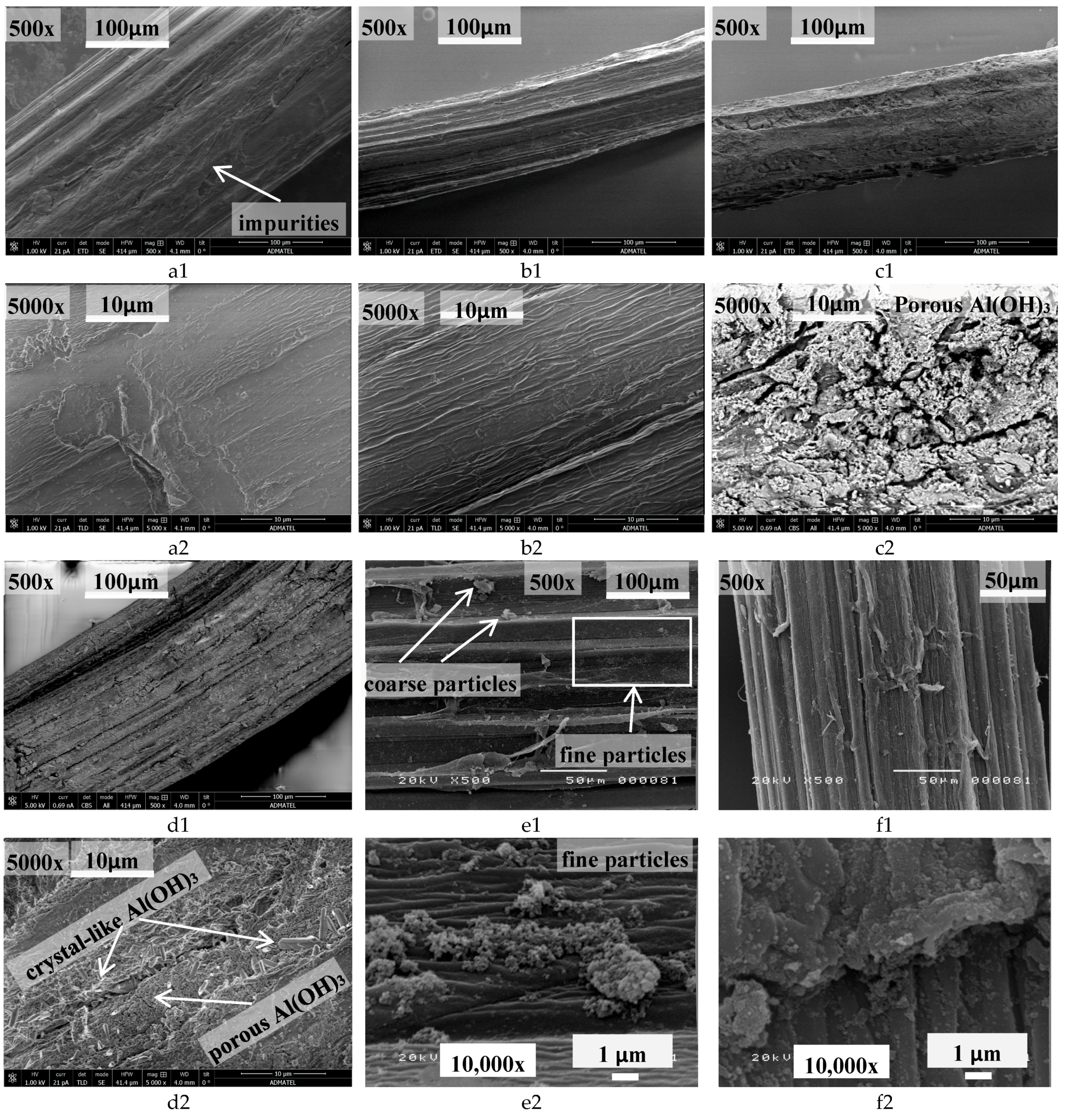
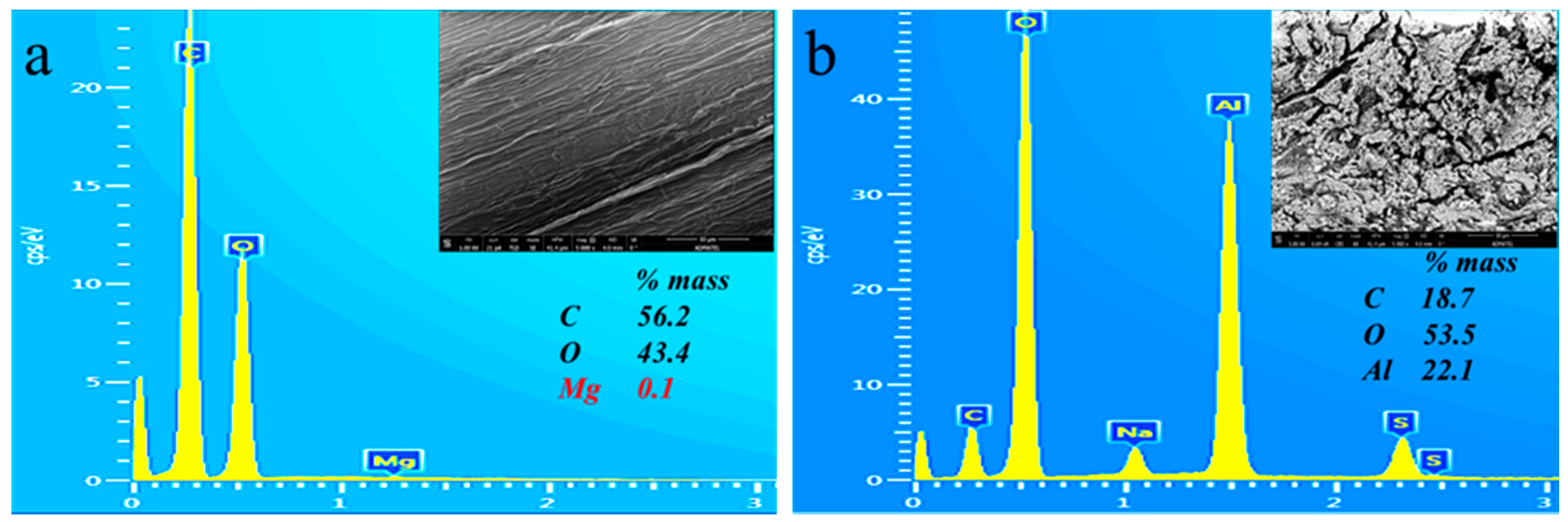
3.4. Morphology and Elemental Microanalysis of Untreated and Treated Abaca Fiber Surface through SEM-EDS
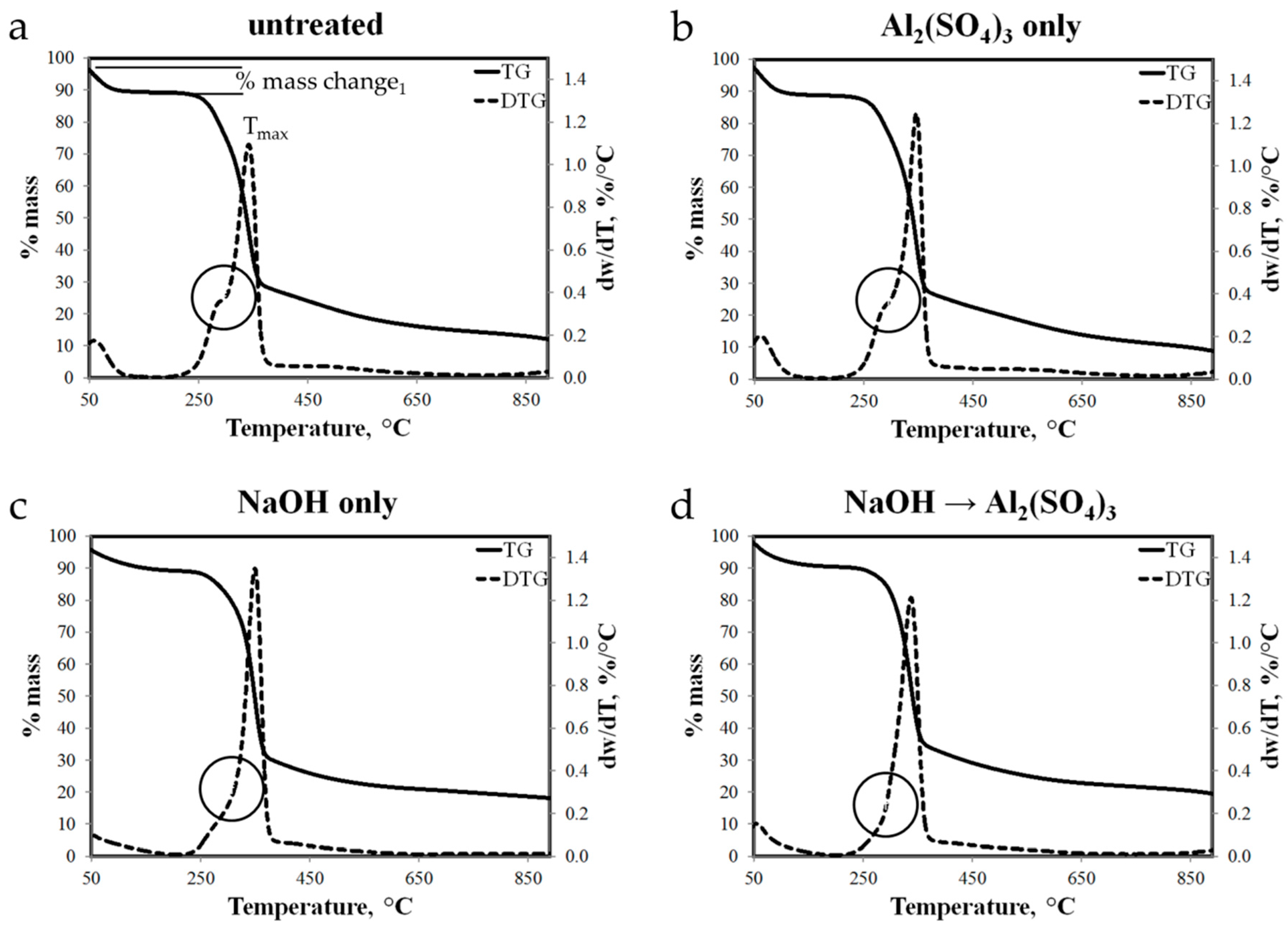
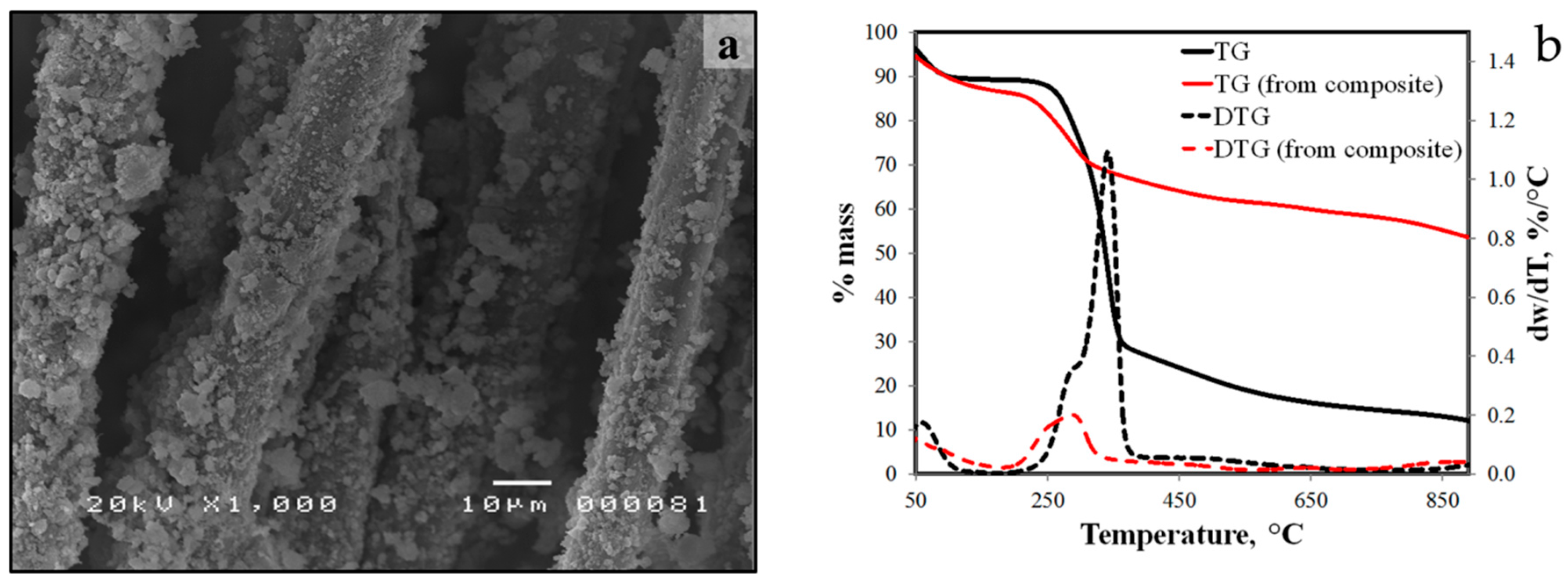
3.5. Thermogravimetric Analysis of Raw and Treated Abaca Fiber
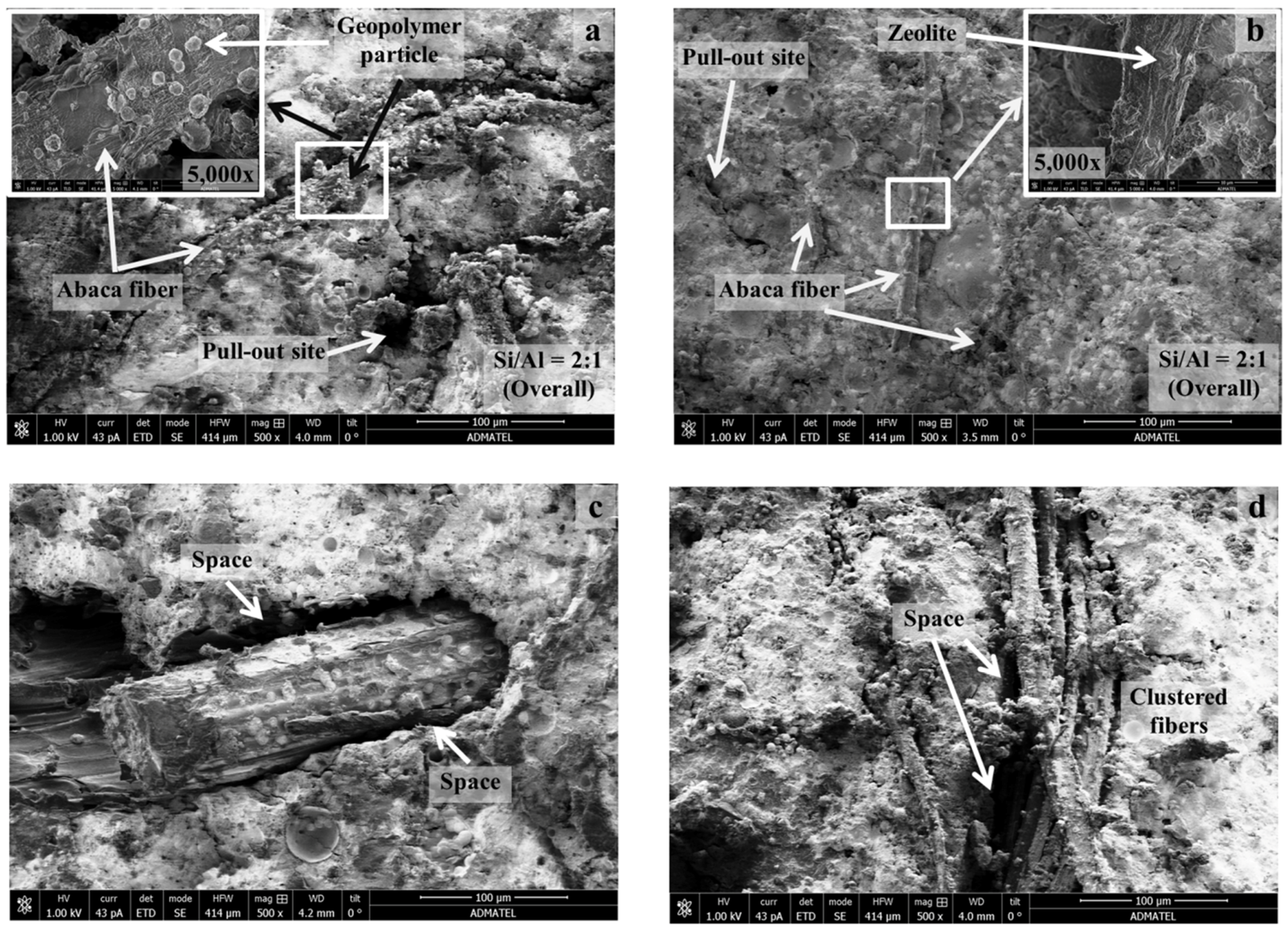
3.6. Preliminary Characterization of Fiber-Reinforced Geopolymers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khalil, H.P.; Bhat, A.H.; Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Balasubramanian, M. Composite Materials and Processing; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 2–4. [Google Scholar]
- Chandrasekar, M.; Ishak, M.R.; Sapuan, S.M.; Leman, Z.; Jawaid, M. A review on the characterisation of natural fibres and their composites after alkali treatment and water absorption. Plast. Rubber Compos. Process. Appl. 2017, 46, 119–136. [Google Scholar] [CrossRef]
- Achour, A.; Ghomari, F.; Belayachi, N. Properties of cementitious mortars reinforced with natural fibers. J. Adhes. Sci. Technol. 2017, 31, 1938–1962. [Google Scholar] [CrossRef]
- Coutts, R.; Warden, P. Air-cured abaca reinforced cement composites. Int. J. Cem. Compos. 1987, 9, 69–73. [Google Scholar] [CrossRef]
- Punyamurthy, R.; Sampathkumar, D.; Ranganagowda, R.P.; Bennehalli, B.; Srinivasa, C.V. Mechanical properties of abaca fiber reinforced polypropylene composites: Effect of chemical treatment by benzenediazonium chloride. J. King Saud Univ. Eng. Sci. 2015. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Jaszkiewicz, A.; Erdmann, K. Polypropylene composites with enzyme modified abaca fibre. Compos. Sci. Technol. 2010, 70, 854–860. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Takagi, H.; Yang, Z.; Wang, D. Effect of chemical treatments on transverse thermal conductivity of unidirectional abaca fiber/epoxy composite. Compos. Part A Appl. Sci. Manuf. 2014, 66, 227–236. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Faruk, O.; Sperber, V.E. Cars from Bio-Fibres. Macromol. Mater. Eng. 2006, 291, 449–457. [Google Scholar] [CrossRef]
- Philippine Statistics Authority, Republic of the Philippines. Selected Statistics on Agriculture; PSA: Quezon City, Philippines, 2016; pp. 13–18. ISSN 2012-0362. Available online: https://psa.gov.ph/sites/default/files/Selected%20Statistics%20on%20Agriculture%202016.pdf (accessed on 15 March 2017).
- Philippine Fiber Industry Development Authority, Department of Agriculture, Republic of the Phillipines. Available online: http://fida.da.gov.ph/index.php/archived-articles/19-philippine-abaca-helps-in-global-environment-conservation (accessed on 15 March 2017).
- Dungani, R.; Karina, M.S.; Sulaeman, A.; Hermawan, D.; Hadiyane, A. Agricultural waste fibers towards sustainability and advanced utilization: A review. Asian J. Plant Sci. 2016, 15, 42–55. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Ribeiro, M.G.; Sankar, K.; Kriven, W.M. Geopolymer-bamboo composite—A novel sustainable construction material. Constr. Build. Mater. 2016, 123, 501–507. [Google Scholar] [CrossRef]
- Musil, S.S.; Keane, P.F.; Kriven, W.M. Green Composite: Sodium-based Geopolymer Reinforced with Chemically Extracted Corn Husk Fibers. In Development in Strategic Materials and Computational Design IV; The American Ceramic Society; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 123–133. [Google Scholar] [CrossRef]
- Alzeer, M.; Mackenzie, K.J.D. Synthesis and mechanical properties of novel composites of inorganic polymers (geopolymers) with unidirectional natural flax fibres (phormiumtenax). Appl. Clay Sci. 2013, 75–76, 148–152. [Google Scholar] [CrossRef]
- Yan, L.; Kasal, B.; Huang, L. A review of recent research on the use of cellulosic fibres, their fibre fabric reinforced cementitious, geo-polymer and polymer composites in civil engineering. Compos. Part B Eng. 2016, 92, 94–132. [Google Scholar] [CrossRef]
- Davidovits, J. Reinforced Geopolymer Composites: A Critical Review. Available online: http://www.materialstoday.com/polymers-soft-materials/features/reinforced-geopolymer-composites-a-critical-review/ (accessed on 28 March 2017).
- Alzeer, M.; Mackenzie, K.J. Synthesis and mechanical properties of new fibre-reinforced composites of inorganic polymers with natural woolfibres. J. Mater. Sci. 2012, 47, 6958–6965. [Google Scholar] [CrossRef]
- Korniejenko, K.; Frączek, E.; Pytlak, E.; Adamski, M. Mechanical properties of geopolymer composites reinforced with natural fibers. Procedia Eng. 2016, 151, 388–393. [Google Scholar] [CrossRef]
- Mallick, P.K. Fiber-Reinforced Composites—Materials, Manufacturing and Design, 3rd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 19–126. [Google Scholar]
- Pickering, K.L.; AruanEfendy, M.G.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Jabbar, A.; Militký, J.; Wiener, J.; Javaid, M.U.; Rwawiire, S. Tensile, surface and thermal characterization of jute fibres after novel treatments. Indian J. Fibre Text. Res. 2016, 41, 249–254. [Google Scholar]
- Ramadevi, P.; Sampathkumar, D.; Srinivasa, C.; Bennehalli, B. Effect alkali treatment on water absorption of single cellulosic. BioResources 2012, 7, 3515–3524. [Google Scholar]
- Onuaguluchi, O.; Banthia, N. Plant-based natural fibre reinforced cement composites: A review. Cem. Concr. Compos. 2016, 68, 96–108. [Google Scholar] [CrossRef]
- Kabir, M.M.; Aravinthan, H.; Wang, H.; Cardona, T.; Lau, K.T. Effects of natural fiber surface on composite properties: A review. In Proceedings of the 1st International Postgraduate Conference on Engineering, Designing and Developing the Built Environment for Sustainable Wellbeing, Darling Heights, Australia, 27–29 April 2011; pp. 94–99. [Google Scholar]
- Izani, M.N.; Paridah, M.; Anwar, U.; Nor, M.M.; H’Ng, P. Effects of fiber treatment on morphology, tensile and thermogravimetric analysis of oil palm empty fruit bunches fibers. Compos. Part B Eng. 2013, 45, 1251–1257. [Google Scholar] [CrossRef]
- Ramadevi, P.; Sampathkumar, D.; Ranganagowda, R.; Bennehalli, B.; Badyankal, P.; Venkateshappa, S.C. Surface modification of abaca fiber by benzene diazonium chloride treatment and its influence on tensile properties of abaca fiber reinforced polypropylene composites. Ciênc. Tecnol. Mater. 2014, 26, 142–149. [Google Scholar] [CrossRef]
- Cai, M.; Takagi, H.; Nakagaito, A.N.; Li, Y.; Waterhouse, G.I. Effect of alkali treatment on interfacial bonding in abaca fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 589–597. [Google Scholar] [CrossRef]
- Lima, H.C.; Willrich, F.L.; Barbosa, N.P.; Rosa, M.A.; Cunha, B.S. Durability analysis of bamboo as concrete reinforcement. Mater. Struct. 2007, 41, 981–989. [Google Scholar] [CrossRef]
- Cook, J.T.; Schoggen, H.L.; Bell, R.I.; Smith, D.J.; Morton, G.H. Cementitious Material Reinforced with Chemically Treated Cellulose Fiber. U.S. Patent No. US7357833, 2008. [Google Scholar]
- Jones, B.; Nachtsheim, C.J. A class of screening designs robust to active second-order effects. J. Qual. Technol. 2010, 105–112. [Google Scholar] [CrossRef]
- Jones, B.; Nachtsheim, C.J. Definitive screening designs with added two-level categorical factors. J. Qual. Technol. 2013, 45, 121–129. [Google Scholar]
- Mwaikambo, L.Y.; Ansell, M.P. Hemp fibre reinforced cashew nut shell liquid composites. Compos. Sci. Technol. 2003, 63, 1297–1305. [Google Scholar] [CrossRef]
- Sedan, D.; Pagnoux, A.; Smith, A.; Chotard, T. Mechanical properties of hemp fibre reinforced cement: Influence of the fibre/matrix interaction. J. Eur. Ceram. Soc. 2008, 28, 183–192. [Google Scholar] [CrossRef]
- Peden, M.E.; Amankwah, S.A.; Keller, B.J.; Krug, E.C.; Peden, J.M. Evaluation of Aluminum Speciation Using Synthetics and Natural Samples: Final Report; SWS Contract Report 463; Illinois State Water Survey Division: Champaign, IL, USA, 1989; pp. 13–22. [Google Scholar]
- Kalaw, E.M.; Culaba, A.; Hinode, H.; Kurniawan, W.; Gallardo, S.; Promentilla, M.A. Optimizing and Characterizing Geopolymers from Ternary Blend of Philippine Coal Fly Ash, Coal Bottom Ash and Rice Hull Ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Ouajai, S.; Shanks, R. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stab. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- Le Troëdec, M.L.; Peyratout, C.S.; Smith, A.; Chotard, T. Influence of various chemical treatments on the interactions between hemp fibres and a lime matrix. J. Eur. Ceram. Soc. 2009, 29, 1861–1868. [Google Scholar] [CrossRef]
- Goudarzi, M.; Ghanbari, D.; Salavati-Niasari, M.; Ahmadi, A. Syntheis and characterization of Al(OH)3, Al2O3 Nanoparticles and Polymeric Nanocomposites. J. Clust. Sci. 2016, 27, 25–38. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. High tensile strength fly ash based geopolymer composite using copper coated micro steel fiber. Constr. Build. Maters. 2016, 112, 629–638. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.C.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Brigida, A.I.S.; Calado, V.M.A.; Goncalves, L.R.B.; Coelho, M.A.Z. Effect of chemical treatments on properties of green coconut fiber. Carbohydr. Polym. 2010, 79, 832–838. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Wang, D.; Zhang, R.; Liu, G.; Cheng, G. Thermogravimetric analysis of lignocellulosic biomass with ionic liquid pretreatment. Bioresour. Technol. 2014, 153, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, T.D.; Peijs, T. Aluminum triohydroxide in combination with ammonium polyphosphate as flame retardant for unsaturated polyester resin. Express Polym. Lett. 2009, 3, 743–751. [Google Scholar] [CrossRef]


| Oxide | SiO2 | Fe2O3 | CaO | Al2O3 | MgO | K2O | Others |
|---|---|---|---|---|---|---|---|
| Mass % | 33.9 | 26.5 | 13.6 | 13.5 | 7.9 | 1.2 | 3.4 |
| Factor/Level (Coded) | −1 | 0 | +1 |
|---|---|---|---|
| NaOH pretreatment | - | No | Yes |
| Soaking time in Al2(SO4)3, hrs | 0.5 | 6.25 | 12 |
| Final pH | 4.5 | 6.0 | 7.5 |
| NaOH Pretreatment | Soaking Time (h) | Final pH | Tensile Strength (MPa) |
|---|---|---|---|
| No | 12 | 6.0 | 450 ± 160 |
| No | 0.5 | 7.5 | 430 ± 130 |
| No | 6.25 | 6.0 | 420 ± 140 |
| Yes | 12 | 7.5 | 370 ± 82 |
| No | 0.5 | 4.5 | 340 ± 97 |
| Yes | 6.25 | 4.5 | 340 ± 140 |
| Yes | 6.25 | 6.0 | 300 ± 82 |
| Yes | 0.5 | 6.0 | 230 ± 82 |
| No | 6.25 | 7.5 | 220 ± 69 |
| Yes | 12 | 4.5 | 210 ± 83 |
| Wavenumber (cm−1) | Vibration | Source |
|---|---|---|
| 3690 | Free OH | moisture |
| 3350 | O–H linked stretching | Polysaccharide |
| 2920 | C–H stretching | Cellulose, Hemicellulose |
| 1740 | C=O stretching | Hemicellulose |
| 1630 | OH in H2O, bending | Moisture |
| 1510 | C=C aromatic symmetrical stretching | Lignin |
| 1430 | CH2 symmetric bending | Pectin, lignin |
| 1250 | C–O aryl group | Lignin |
| 980 | Al–O stretching | Al(OH)3 |
| 900 | Glycosidic bonds symmetric ring stretching | Polysaccharide |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malenab, R.A.J.; Ngo, J.P.S.; Promentilla, M.A.B. Chemical Treatment of Waste Abaca for Natural Fiber-Reinforced Geopolymer Composite. Materials 2017, 10, 579. https://doi.org/10.3390/ma10060579
Malenab RAJ, Ngo JPS, Promentilla MAB. Chemical Treatment of Waste Abaca for Natural Fiber-Reinforced Geopolymer Composite. Materials. 2017; 10(6):579. https://doi.org/10.3390/ma10060579
Chicago/Turabian StyleMalenab, Roy Alvin J., Janne Pauline S. Ngo, and Michael Angelo B. Promentilla. 2017. "Chemical Treatment of Waste Abaca for Natural Fiber-Reinforced Geopolymer Composite" Materials 10, no. 6: 579. https://doi.org/10.3390/ma10060579







