Carbon-Supported Pd and PdFe Alloy Catalysts for Direct Methanol Fuel Cell Cathodes
Abstract
:1. Introduction
2. Results
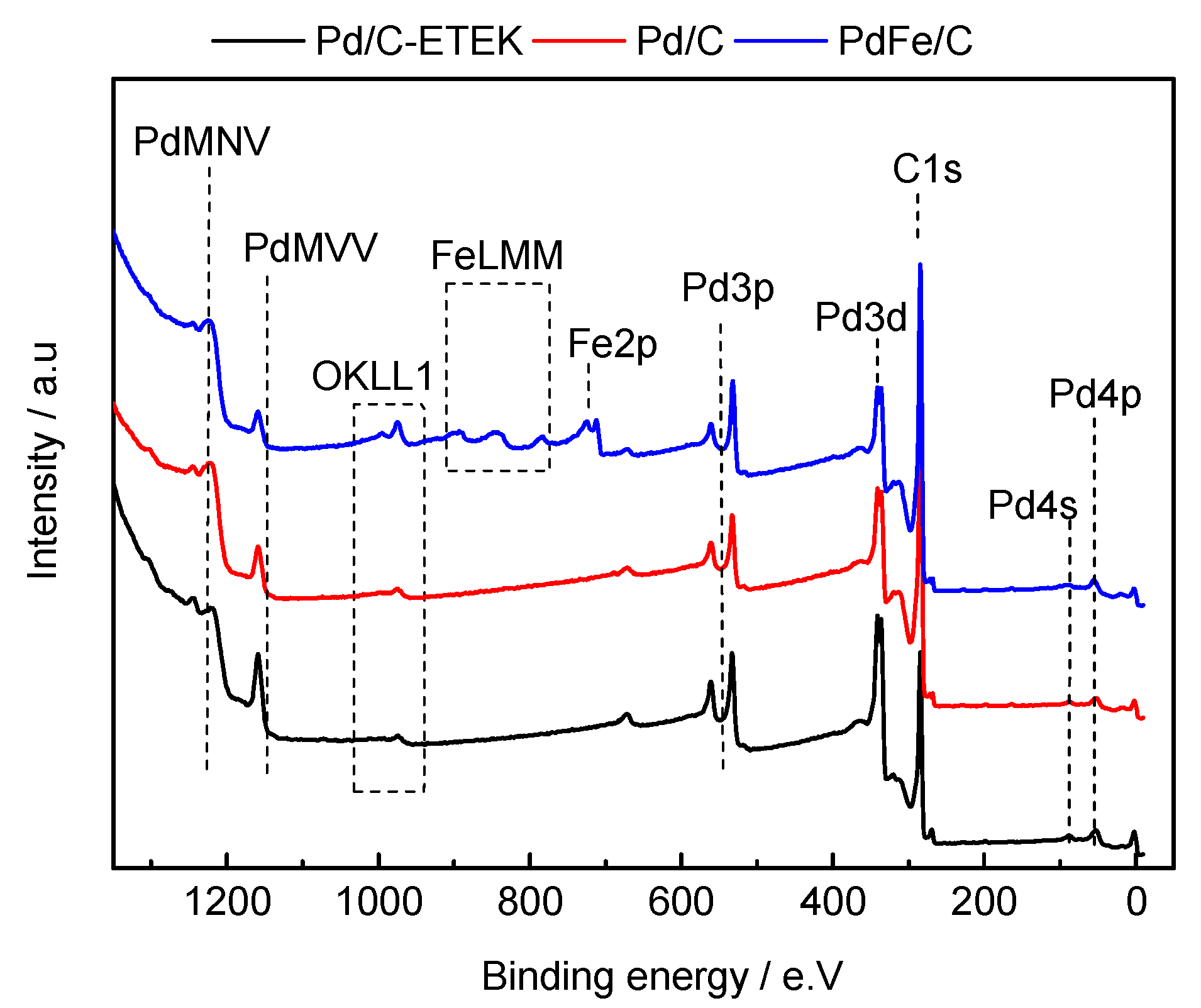
2.1. Physico-Chemical Characterization of the Synthesized Materials
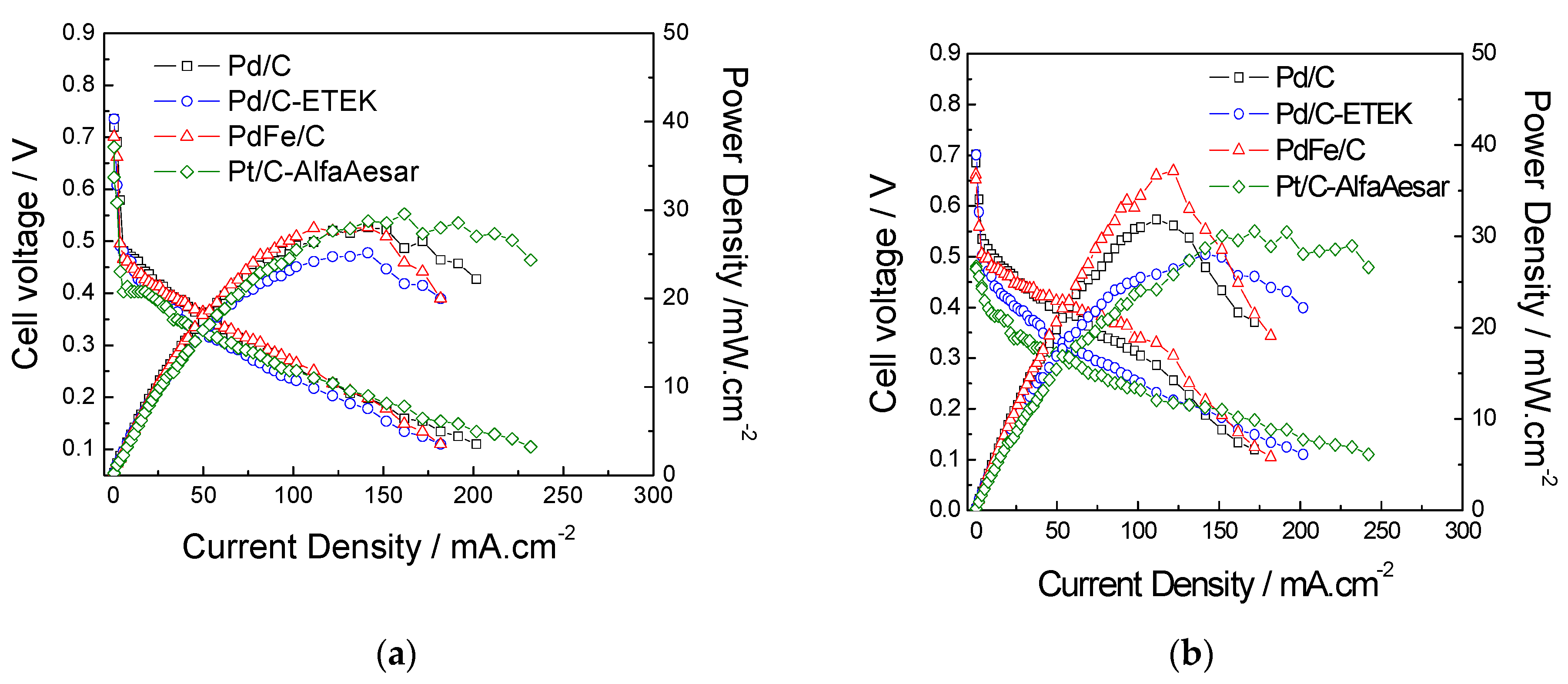
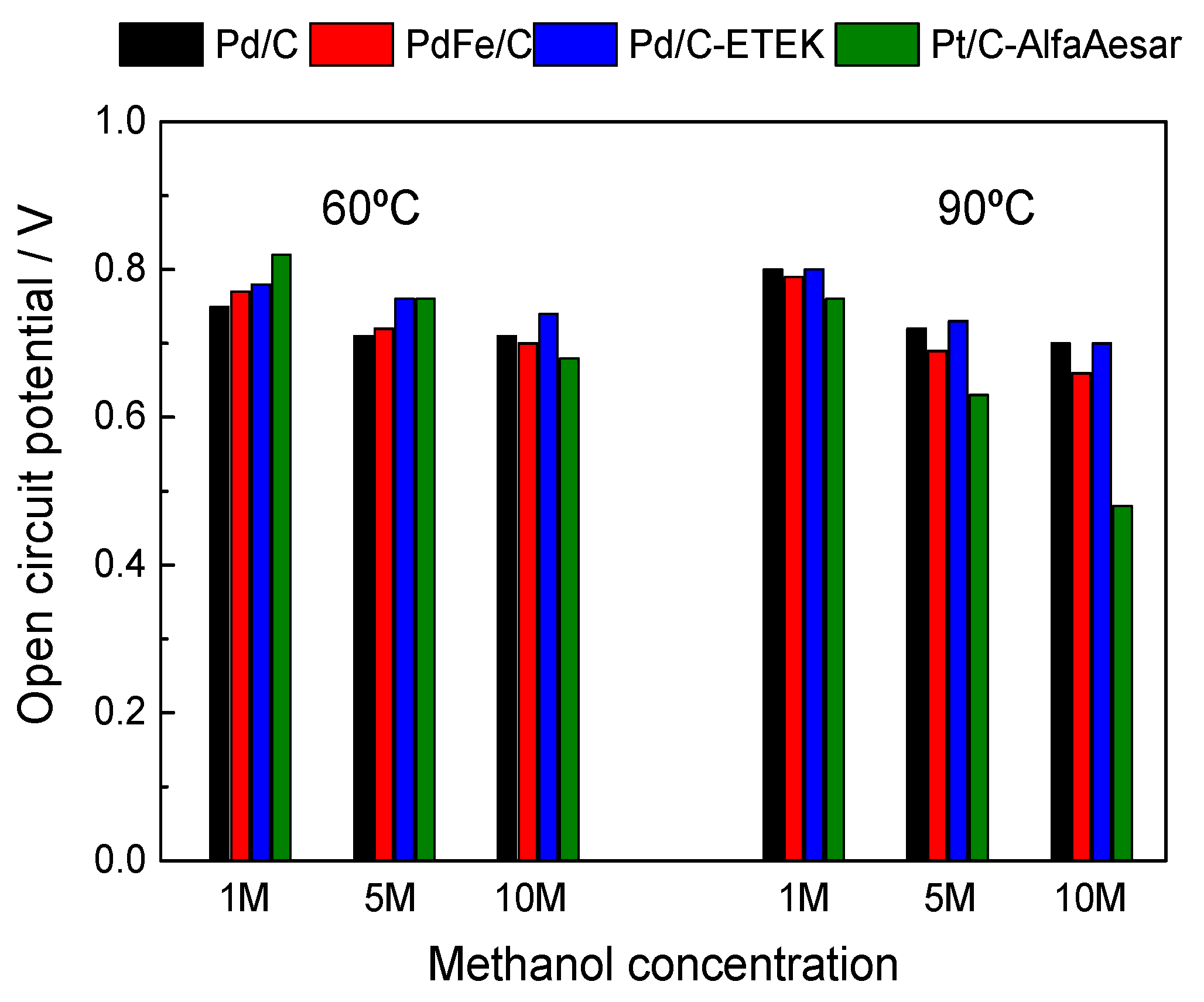
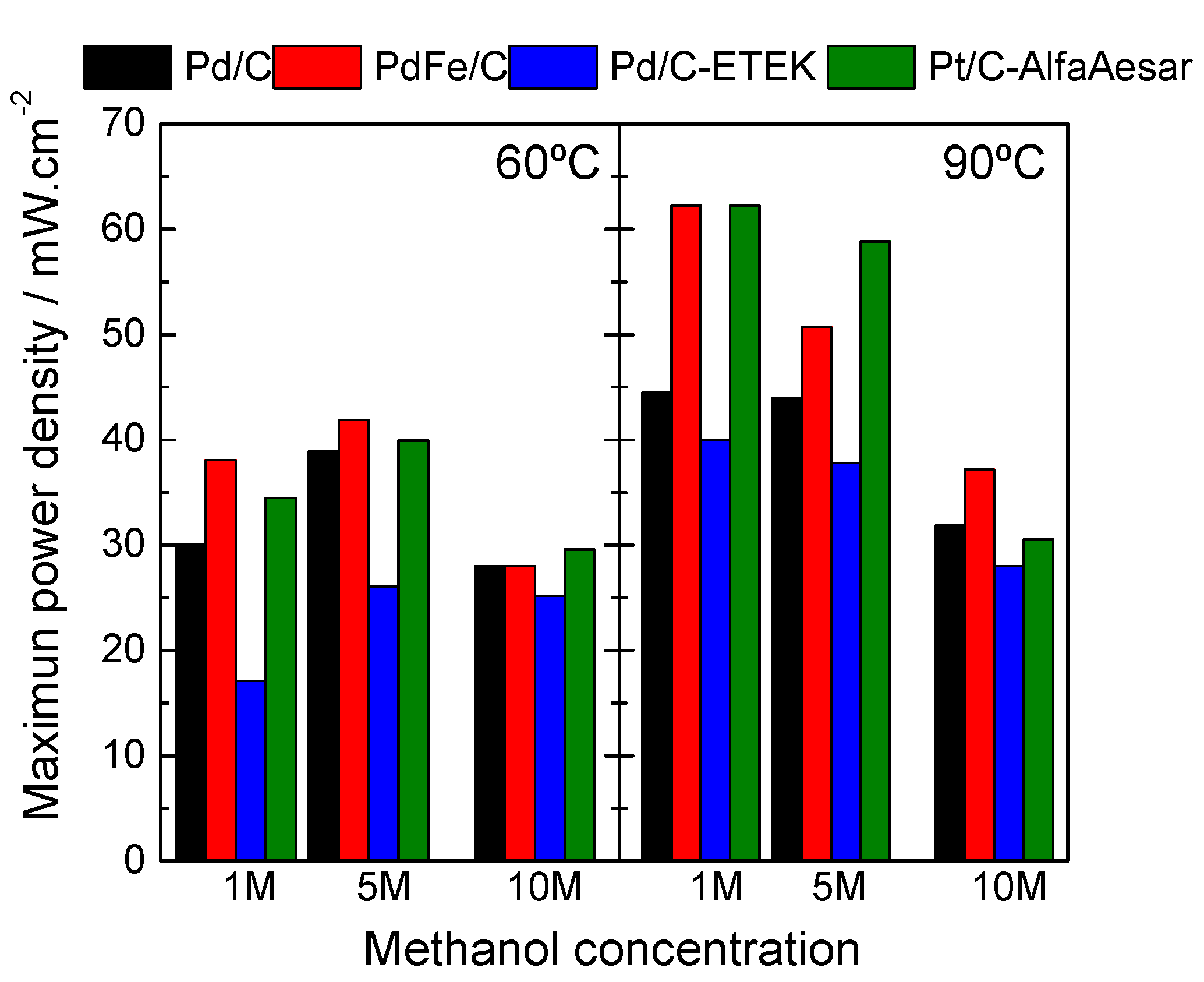
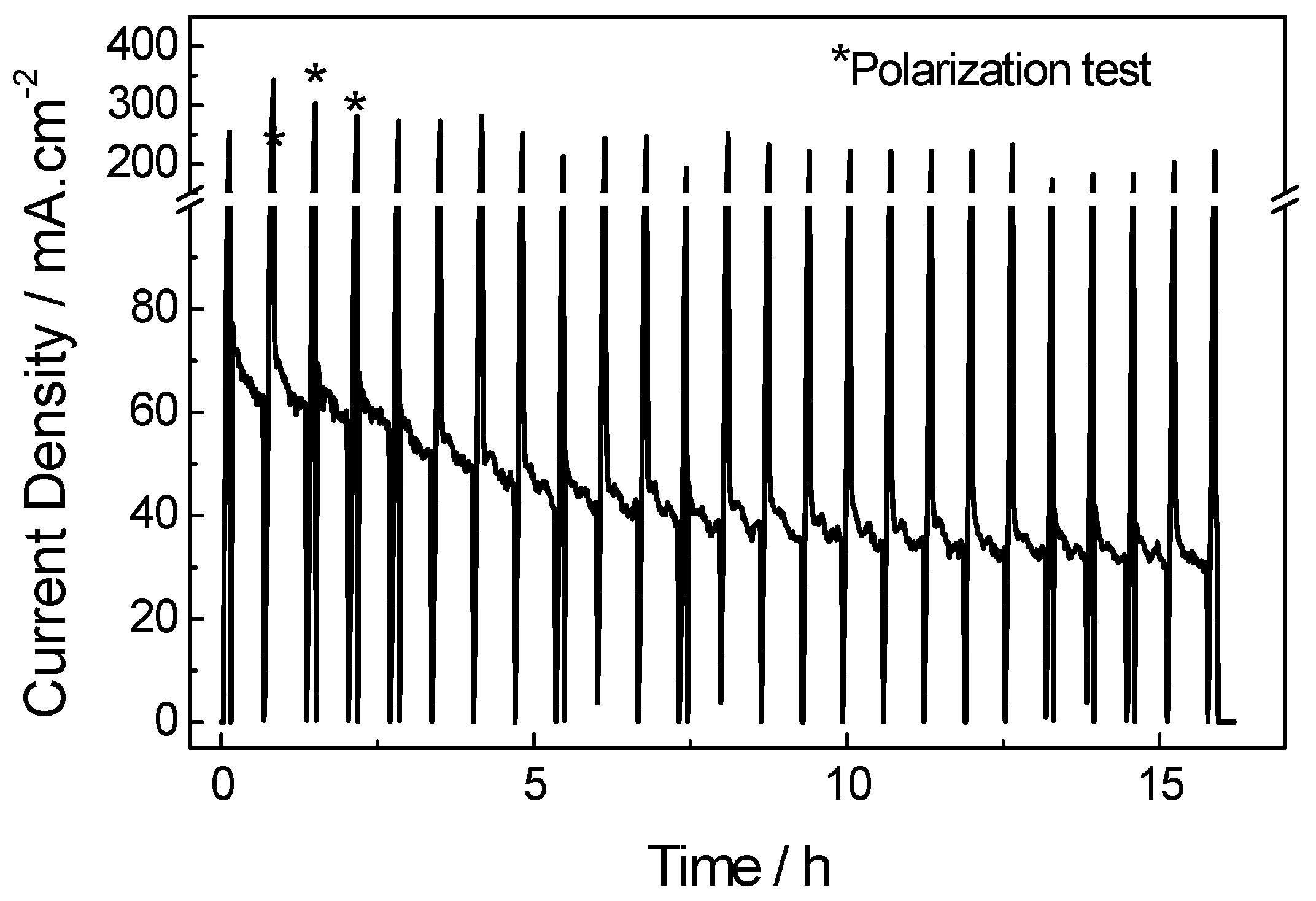
2.2. Electrochemical Performances of Pd-Based Catalysts in DMFC
3. Conclusions
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Physicochemical Characterization
4.3. Electrochemical Studies
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Andujar, J.M.; Segura, F. Fuel cells: History and updating.A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 2013, 226, 223–240. [Google Scholar] [CrossRef]
- Bahrami, H.; Faghri, A. Review and advances of direct methanol fuel cells: Part II: Modeling and numerical simulation. J. Power Sources 2013, 230, 303–320. [Google Scholar] [CrossRef]
- Aricò, A.S.; Srinivasan, S.; Antonucci, V. DMFCs: From Fundamental Aspects to Technology Development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Han, J.; Liu, H. Real time measurements of methanol crossover in a DMFC. J. Power Sources 2007, 164, 166–173. [Google Scholar] [CrossRef]
- Qi, Z.; Kaufman, A. Open circuit voltage and methanol crossover in DMFCs. J. Power Sources 2002, 110, 177–185. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Mench, M.M. Fuel Cell Engines; John Wiley & Sons, Inc.: Hoboquen, NJ, USA, 2008; p. 176. [Google Scholar]
- Sebastian, D.; Serov, A.; Artyushkova, K.; Atanassov, P.; Antonino, S.; Arico, A.S.; Baglio, V. Performance, methanol tolerance and stability of Fe-aminobenzimidazole derived catalyst for direct methanol fuel cells. J. Power Sources 2016, 319, 235–246. [Google Scholar] [CrossRef]
- Shao, M. Palladium-Based Electrocatalysts for Oxygen Reduction Reaction. In Electrocatalysis in Fuel Cells: A Non- and Low- Platinum Approach; Shao, M., Ed.; Springer: London, UK, 2013; pp. 513–527. [Google Scholar]
- Lo Vecchio, C.; Alegre, C.; Sebastián, D.; Stassi, A.; Aricò, A.S.; Baglio, V. Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance. Materials 2015, 8, 7997–8008. [Google Scholar] [CrossRef]
- Rivera Gavidia, L.M.; García, G.; Anaya, D.; Querejeta, A.; Francisco Alcaide, F.; Pastor, E. Carbon-supported Pt-free catalysts with high specificity and activity toward the oxygen reduction reaction in acidic medium. Appl. Catal. B Environ. 2016, 184, 12–19. [Google Scholar] [CrossRef]
- Pang, S.-K. Why palladium cathodes can bear resistance to methanol but not platinum cathodes. Eletrochim. Acta 2015, 161, 420–426. [Google Scholar] [CrossRef]
- Ramanathan, M.; Ramani, V.; Prakash, J. Kinetics of the oxygen reduction reaction on Pd3 M (M = Cu, Ni, Fe) electrocatalysts synthesized at elevated annealing temperatures. Electrochim. Acta 2012, 75, 254–261. [Google Scholar] [CrossRef]
- Lee, K.; Savadogo, O.; Ishihara, A.; Mitsushima, S.; Kamiya, N.; Ota, K. Methanol-Tolerant Oxygen Reduction Electrocatalysts Based on Pd-3D Transition Metal Alloys for Direct Methanol Fuel Cells. J. Electrochem. Soc. 2006, 153, A20–A24. [Google Scholar] [CrossRef]
- Pires, F.I.; Villullas, H.M. Pd-based catalysts: Influence of the second metal on their stability and oxygen reduction activity. Int. J. Hydrogen Energy 2012, 37, 17052–17059. [Google Scholar] [CrossRef]
- Wang, J.M.; Inada, H.; Wu, L.; Zhu, Y.; Choi, Y.; Liu, P.; Zhou, W.-P.; Adzic, R.R. Oxygen Reduction on Well-Defined Core-Shell Nanocatalysts: Particle Size, Facet, and Pt Shell Thickness Effects. J. Am. Chem. Soc. 2009, 131, 17298–17302. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, M. Investigating the influence of Pd situation (as core or shell) in synthesized catalyst for ORR in PEMFC. Int. J. Hydrogen Energy 2015, 40, 6661–6671. [Google Scholar] [CrossRef]
- Baglio, V.; D’Urso, C.; Sebastian, D.; Stassi, A.; Aricò, A.S. PtCo catalyst with modulated surface characteristics for the cathode of direct methanol fuel cells. Int. J. Hydrogen Energy 2014, 39, 5399–5405. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Zou, Z.; Xia, B.; Yang, H. Low temperature preparation of carbon-supported Pd Co alloy electrocatalysts for methanol-tolerant oxygen reduction reaction. Electrochim. Acta 2008, 53, 6662–6667. [Google Scholar] [CrossRef]
- Dector, A.; Cuevas-Muniz, F.M.; Guerra-Balcazar, M.; Godinez, L.A.; Ledesma-Garcia, J.; Arriaga, L.G. Glycerol oxidation in a microfluidic fuel cell using Pd/C and Pd/MWCNT anodes electrodes. Int. J. Hydrogen Energy 2013, 38, 12617–12622. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; Tsiakaras, P.; Shen, P.K. Direct alcohol fuel cells: A novel non-platinum and alcohol inert ORR electrocatalyst. Appl. Catal. B Environ. 2008, 78, 381–387. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, F.; Wu, K.; Lu, Z.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Carbon supported Palladium e Iron nanoparticles with uniform alloy structure as methanol-tolerant electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2012, 37, 2993–3000. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. PdAg Alloy Nanowires: Facile One-Step Synthesis and High Electrocatalytic Activity for Formic Acid Oxidation. ACS Catal. 2012, 2, 84–90. [Google Scholar] [CrossRef]
- Alegre, C.; Gálvez, M.E.; Moliner, R.; Baglio, V.; Aricò, A.S.; Lázaro, M.J. Towards an optimal synthesis route for the preparation of highly mesoporous carbon xerogel-supported Pt catalysts for the oxygen reduction reaction. Appl. Catal. B Environ. 2014, 147, 947–957. [Google Scholar] [CrossRef]
- Amin, R.S.; El-Khatib, K.M.; Siracusano, S.; Baglio, V.; Stassi, A.; Aricò, A.S. Metal oxide promoters for methanol electro-oxidation. Int. J. Hydrogen Energy 2014, 39, 9782–9790. [Google Scholar] [CrossRef]
- Ma, H.-C.; Xue, X.-Z.; Liao, J.-H.; Liu, C.-P.; Xing, W. Effect of borohydride as reducing agent on the structures and electrochemical properties of Pt/C catalyst. Appl. Surf. Sci. 2006, 252, 8593–8597. [Google Scholar] [CrossRef]
- Liu, H.; Manthiram, A. Controlled synthesis and characterization of carbon-supported Pd4Co nanoalloy electrocatalysts for oxygen reduction reaction in fuel cells. Energy Environ. Sci. 2009, 2, 124–132. [Google Scholar] [CrossRef]
- Devivaraprasad, R.; Ramesh, R.; Naresh, N.; Kar, T.; Singh, R.K.; Neergat, M. Oxygen Reduction Reaction and Peroxide Generation on Shape-Controlled and Polycrystalline Platinum Nanoparticles in Acidic and Alkaline Electrolytes. Langmuir 2014, 30, 8995–9006. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation Physical Electronic Division: Eden Prairie, MN, USA, 1979; pp. p. 38, p. 39, p. 76, p. 77, p. 110 and p. 111. [Google Scholar]
- Baglio, V.; Sebastián, D.; D’Urso, C.; Stassi, A.; Amin, R.S.; El-Khatib, K.M.; Aricò, A.S. Composite anode electrode based on iridium oxide promoter for direct methanol fuel cells. Electrochim. Acta 2014, 128, 304–310. [Google Scholar] [CrossRef]
- Aricò, A.S.; Baglio, V.; Antonucci, V. Direct Methanol Fuel Cells: History, Status and Perspectives. In Electrocatalysis of Direct Methanol Fuel Cells. From Fundamentals to Applications; Liu, H., Zhang, J., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 1–70. [Google Scholar]
- Choi, B.; Nam, W.-H.; Chung, D.Y.; Park, I.-S.; Yoo, S.J.; Song, J.C.; Sung, Y.-E. Enhanced Methanol Tolerance of Highly Pd rich Pd-Pt Cathode Electrocatalysts in Direct Methanol Fuel Cells. Electrochim. Acta 2015, 164, 235–242. [Google Scholar] [CrossRef]
- Aricò, A.S.; Sebastian, D.; Schuster, M.; Bauer, B.; D’Urso, C.; Lufrano, F.; Baglio, V. Selectivity of direct methanol fuel cell membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xin, Q.; Li, W.; Zhou, Z.; Jiang, L.; Yanga, S.; Sun, G. An improved palladium-based DMFCs cathode catalyst. Chem. Commun. 2004, 2776–2777. [Google Scholar] [CrossRef] [PubMed]
- Stassi, A.; D’Urso, C.; Baglio, V.; Di Blasi, A.; Antonucci, V.; Aricò, A.S.; Castro Luna, A.M.; Bonesi, A.; Triaca, W.E.J. Electrocatalytic behaviour for oxygen reduction reaction of small nanostructured crystalline bimetallic Pt–M supported catalysts. J. Appl. Electrochem. 2006, 36, 1143–1149. [Google Scholar] [CrossRef]
- Zago, M.; Bisello, A.; Baricci, A.; Rabissi, C.; Brightman, E.; Hinds, G.; Casalegno, A. On the actual cathode mixed potential in direct methanol fuel cells. J. Power Sources 2016, 325, 714–722. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, T.S.; Liu, J.G. Effect of transient hydrogen evolution/oxidation reactions on the OCV of direct methanol fuel cells. Electrochem. Solid State Lett. 2005, 8, A549–A553. [Google Scholar] [CrossRef]
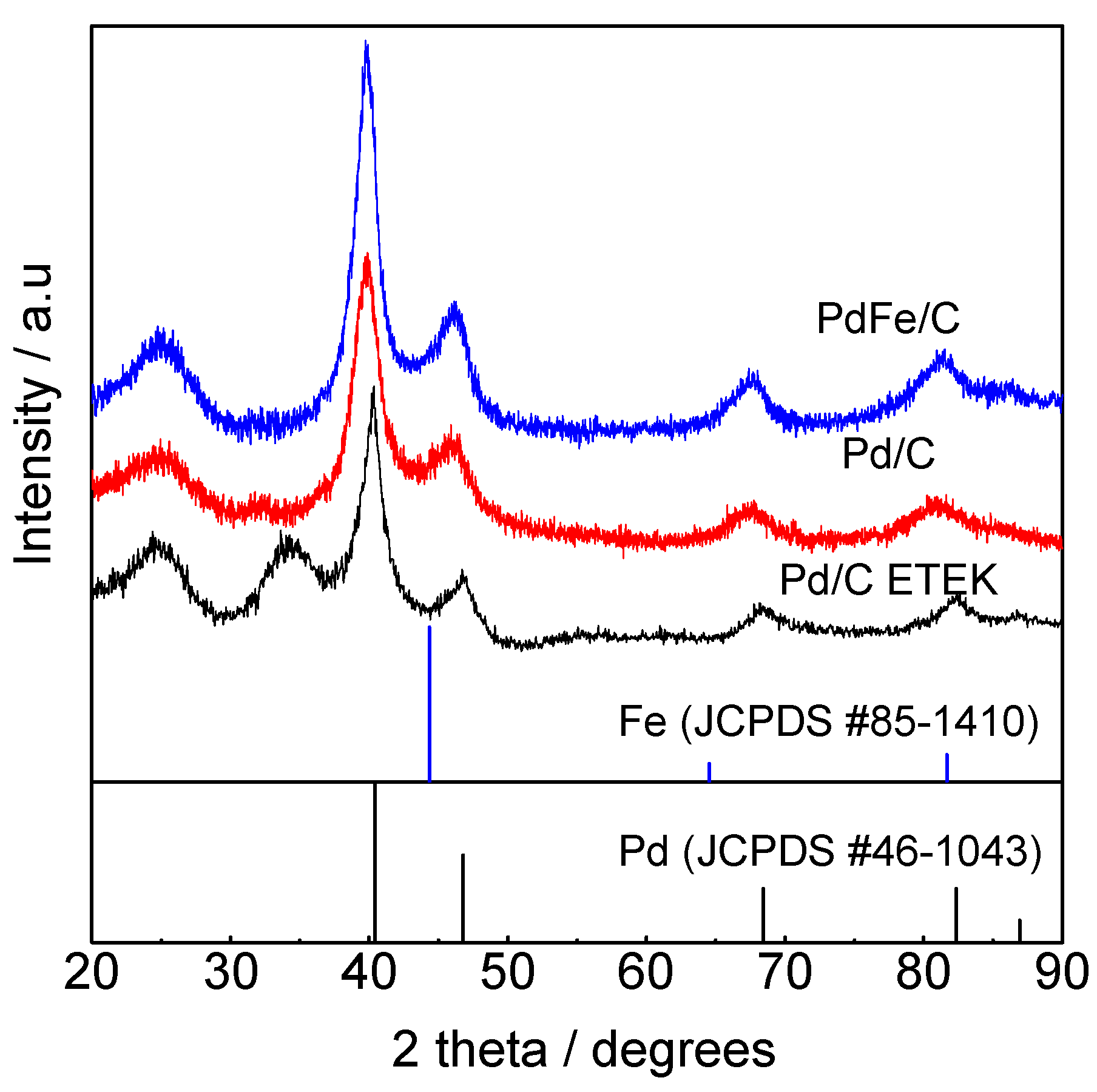
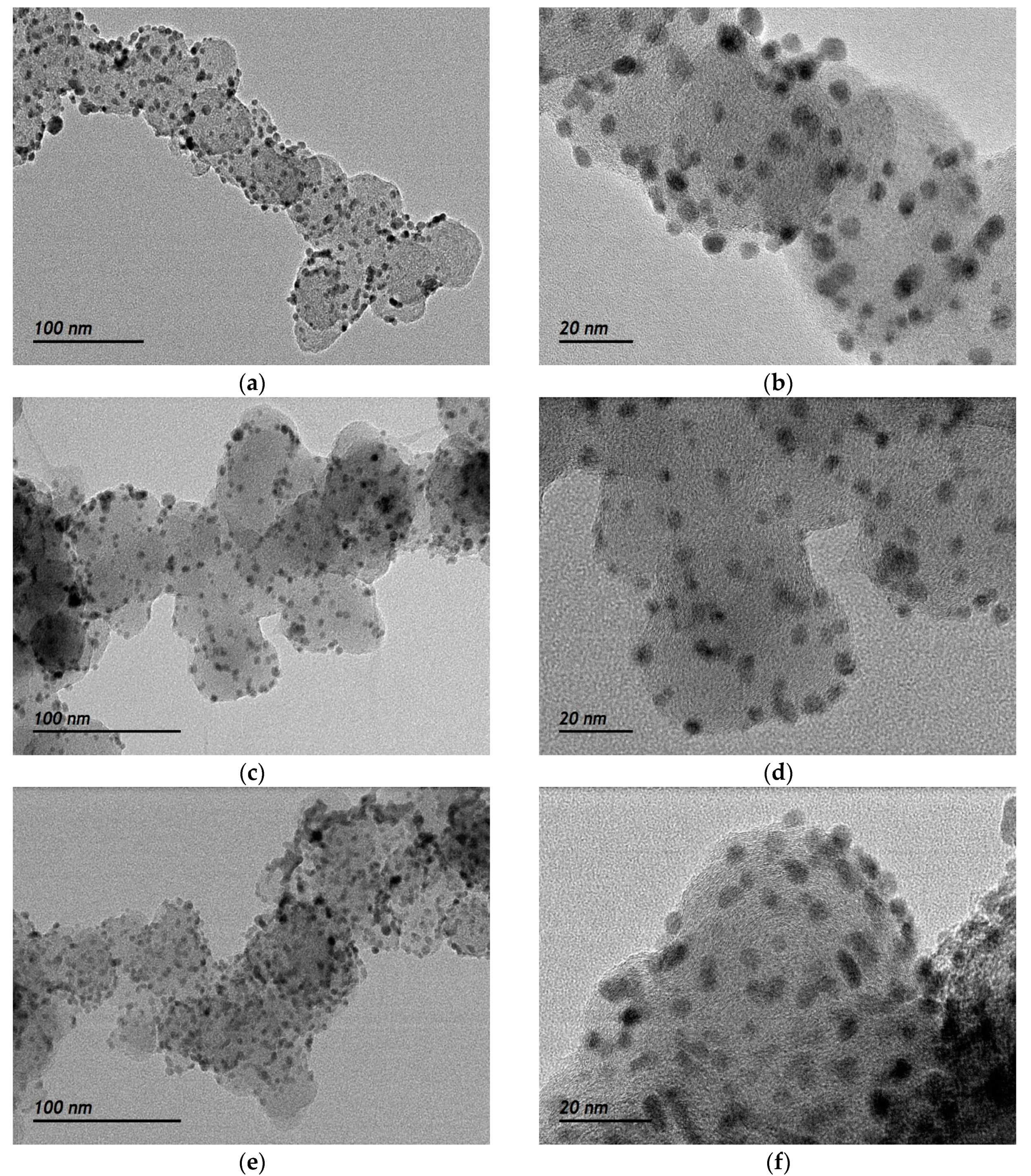
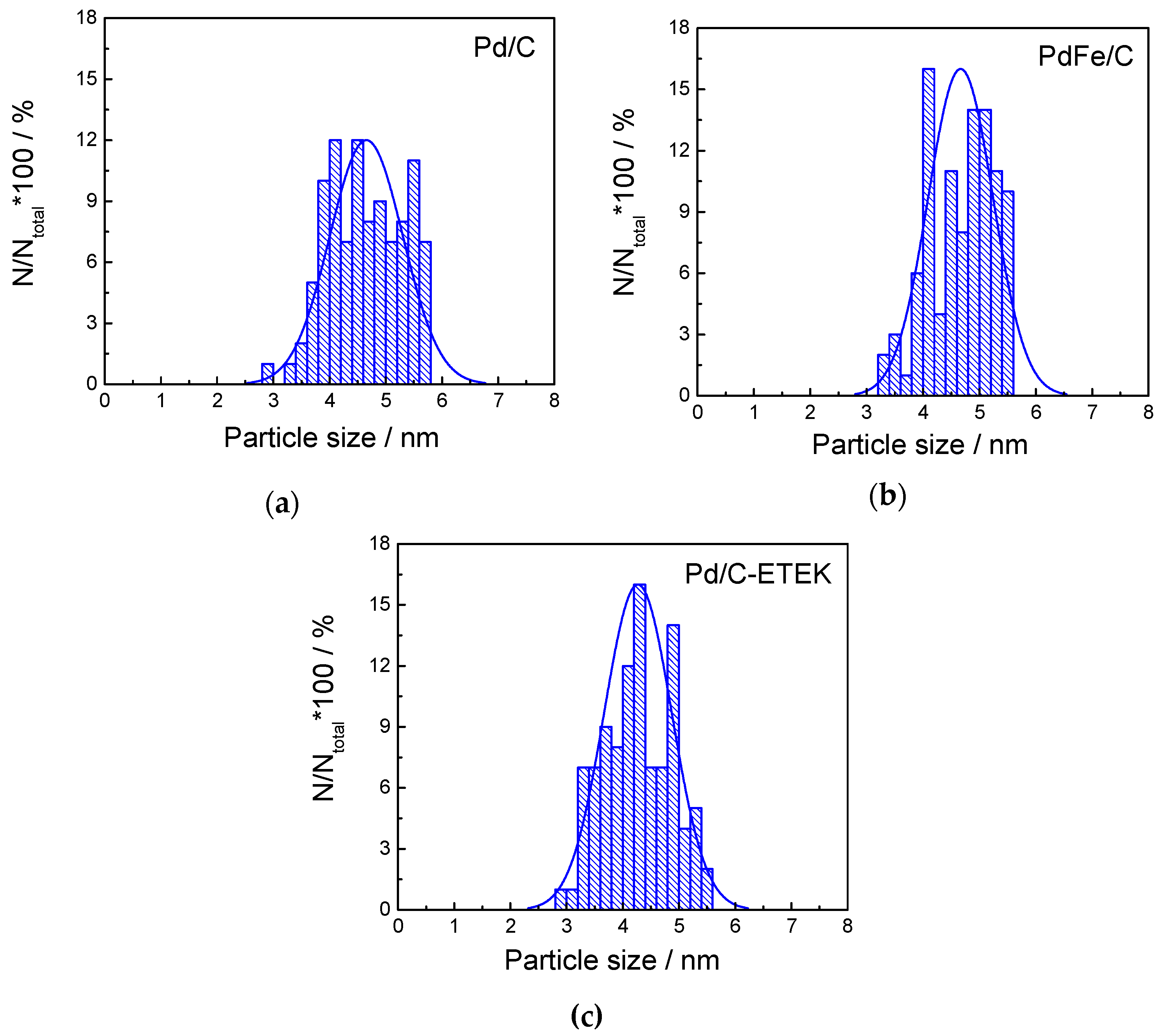
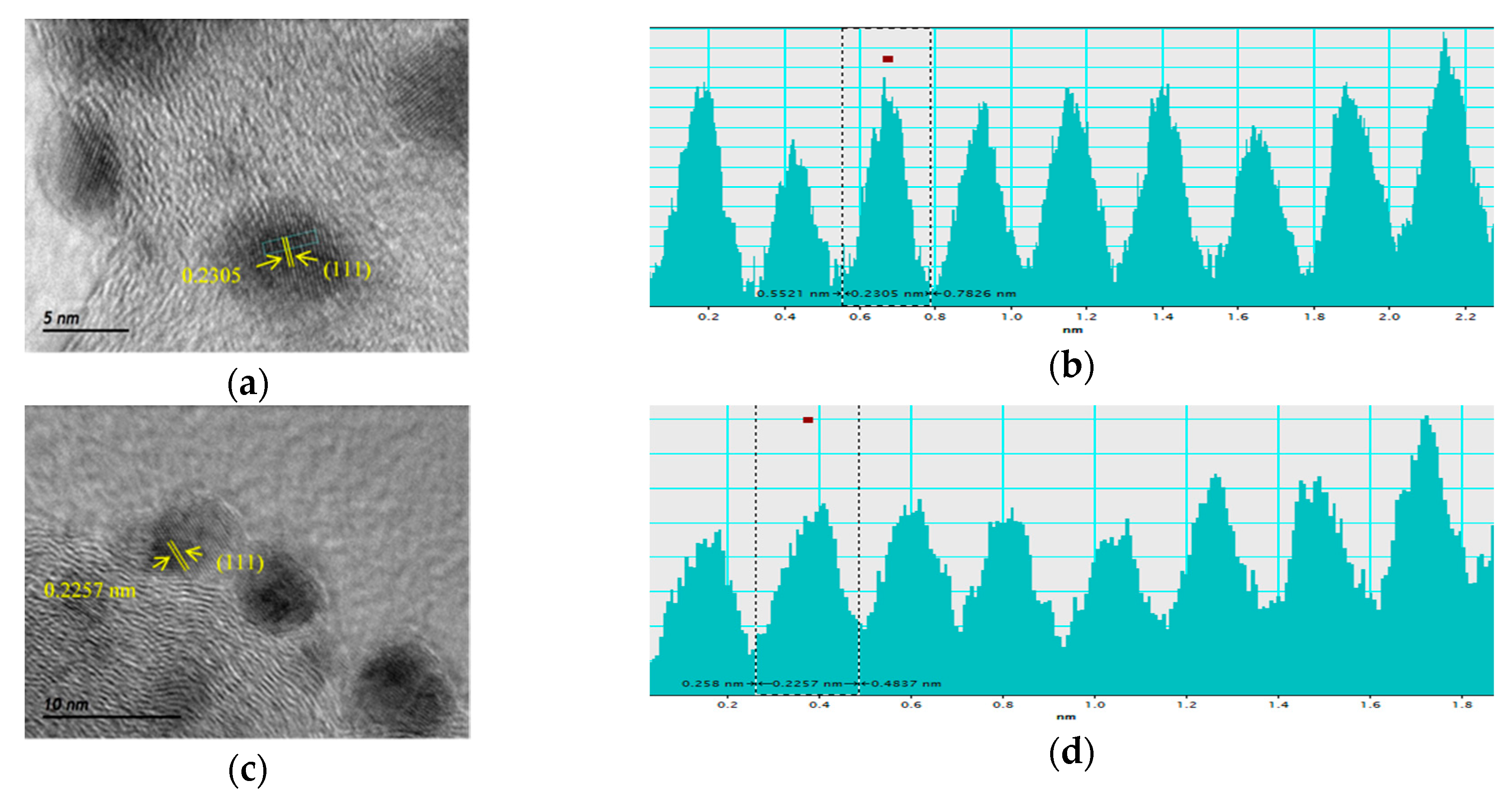


| Catalysts | Crystallite Size 1 (nm) | Interplanar Spacing 1 (Å) | Lattice Parameter 1 (Å) | Particle Size 2 (nm) | Interplanar Spacing 2 (Å) | Pd:Fe Atomic Ratio 3 (at %) | Metal Loading 3 (wt %) |
|---|---|---|---|---|---|---|---|
| Pd/C | 3.9 | 2.273 | 3.949 | 4.7 ± 0.7 | 2.305 | - | 20 |
| PdFe/C | 3.8 | 2.261 | 3.922 | 4.6 ± 0.5 | 2.257 | 76:24 | 19 |
| Pd/C ETEK | 3.4 | 2.241 | 3.883 | 4.3 ± 0.6 | 2.271 | - | 30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera Gavidia, L.M.; Sebastián, D.; Pastor, E.; Aricò, A.S.; Baglio, V. Carbon-Supported Pd and PdFe Alloy Catalysts for Direct Methanol Fuel Cell Cathodes. Materials 2017, 10, 580. https://doi.org/10.3390/ma10060580
Rivera Gavidia LM, Sebastián D, Pastor E, Aricò AS, Baglio V. Carbon-Supported Pd and PdFe Alloy Catalysts for Direct Methanol Fuel Cell Cathodes. Materials. 2017; 10(6):580. https://doi.org/10.3390/ma10060580
Chicago/Turabian StyleRivera Gavidia, Luis M., David Sebastián, Elena Pastor, Antonino S. Aricò, and Vincenzo Baglio. 2017. "Carbon-Supported Pd and PdFe Alloy Catalysts for Direct Methanol Fuel Cell Cathodes" Materials 10, no. 6: 580. https://doi.org/10.3390/ma10060580
APA StyleRivera Gavidia, L. M., Sebastián, D., Pastor, E., Aricò, A. S., & Baglio, V. (2017). Carbon-Supported Pd and PdFe Alloy Catalysts for Direct Methanol Fuel Cell Cathodes. Materials, 10(6), 580. https://doi.org/10.3390/ma10060580









