Stability of an Electrodeposited Nanocrystalline Ni-Based Alloy Coating in Oil and Gas Wells with the Coexistence of H2S and CO2
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Coating Preparation
2.2. Potentiodynamic Polarization
2.3. Weight Loss Test
2.4. Adhesive Force Test of the Corrosion Scale
2.5. Morphological and Structural Characterization of the Ni-Based Alloy Coating and Corrosion Scale
3. Results and Discussion
3.1. Morphology and Structure of the Electrodeposited Ni-Based Alloy Coating
3.2. Potentiodynamic Polarization Curve
3.3. Corrosion Rate and Morphology
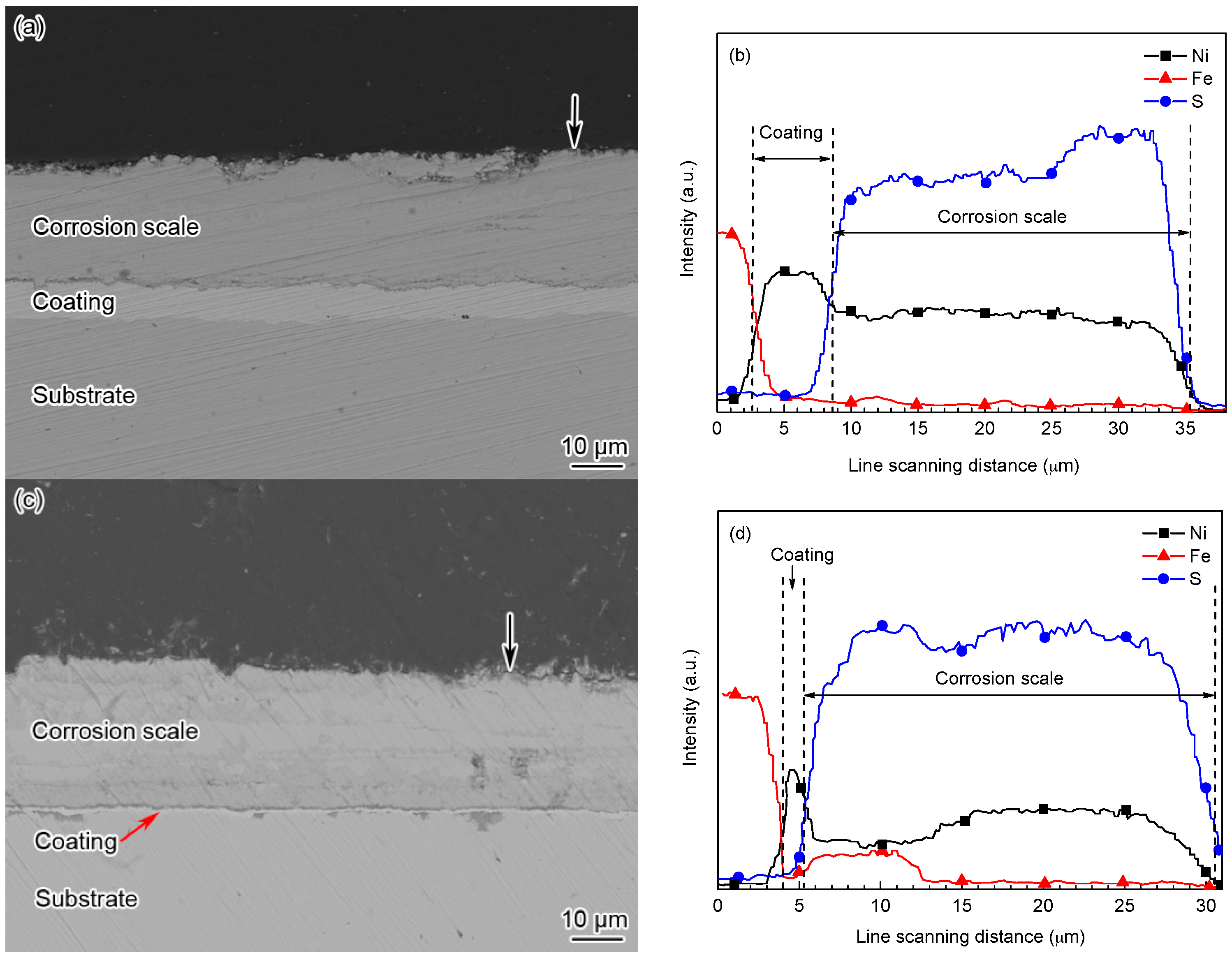
3.4. Effect of Temperature on the Characteristics of the Corrosion Scale
3.5. Effect of Immersion Time on the Characteristics of the Corrosion Scale
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, Z.H.; Zhang, S.C.; Zhou, L.Z.; Cai, R.; Xie, J.F.; Chang, Z.L. Applicability of Tungsten Nickel Alloy Coated Tubing. Corros. Protect. 2014, 35, 1256–1259. (In Chinese) [Google Scholar]
- Choi, Y.S.; Nesic, S.; Ling, S. Effect of H2S on the CO2 Corrosion of Carbon Steel in Acidic Solutions. Electrochim. Acta 2011, 56, 1752–1760. [Google Scholar] [CrossRef]
- Zhou, C.S.; Zheng, S.Q.; Chen, C.F.; Lu, G.W. The effect of the partial pressure of H2S on the permeation of hydrogen in low carbon pipeline steel. Corros. Sci. 2013, 67, 187–192. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.Z.; Liu, R.K.; Du, C.W.; Li, X.G. Electrochemical and sulfide stress corrosion cracking behaviors of tubing steels in a H2S/CO2 annular environment. J. Mater. Eng. Perform. 2014, 23, 1279–1287. [Google Scholar] [CrossRef]
- Sun, W.; Nesic, S.; Papavinasam, S. Kinetics of corrosion layer formation. Part 2—Iron sulfide and mixed iron sulfide/carbonate layers in carbon dioxide/hydrogen sulfide corrosion. Corrosion 2008, 64, 586–599. [Google Scholar] [CrossRef]
- Kvarekvål, J.; Svenningsen, G. Effect of High H2S Partial Pressures on Localized Corrosion of Carbon Steel. CORROSION 2015; NACE International: Houston, TX, USA, 2015; p. 5720. [Google Scholar]
- ISO Standard 15156-3. Petroleum and Natural Gas Industries—Materials for Use in H2S-Containing Environments in Oil and Gas Production—Part 3: Cracking-Resistant CRAs (Corrosion-Resistant Alloys) and Other Alloys; NACE International: Huston, TX, USA, 2003. [Google Scholar]
- Zhang, G.A.; Zang, Y.; Guo, X.P.; Jiang, F.; Shi, D.Y.; Chen, Z.Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Zhao, X.H.; Han, Y.; Bai, Z.Q.; Wei, B. The experiment research of corrosion behaviour about Ni-based alloys in simulant solution containing H2S/CO2. Electrochim. Acta 2011, 56, 7725–7731. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kapp, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Costa, J.D.; de Sousa, M.B.; Alves, J.J.N.; Campos, A.R.N.; Santana, R.A.C.; Prasad, S. Studies on electrodeposition and characterization of the Ni-W-Fe alloys coatings. J. Alloys Compd. 2015, 619, 697–703. [Google Scholar] [CrossRef]
- De Lima-Neto, P.; da Silva, G.P.; Correia, A.N. A comparative study of the physicochemical and electrochemical properties of Cr and Ni-W-P amorphous electrocoatings. Electrochim. Acta 2006, 51, 4928–4933. [Google Scholar] [CrossRef]
- He, F.J.; Yang, J.; Lei, T.X. Structure and properties of electrode-posited Fe-Ni-W alloys with different levels of tungsten content: A comparative study. Appl. Surf. Sci. 2007, 253, 7591–7598. [Google Scholar] [CrossRef]
- De Lima-Neto, P.; Correia, A.N.; Santana, R.A.C.; Colares, R.P.; Barros, E.B.; Casciano, P.N.S.; Vaz, G.L. Morphological, structural, microhardness and electrochemical characterisations of electrodeposited Cr and Ni-W coatings. Electrochim. Acta 2010, 55, 2078–2086. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Ganesh Sundara, S.; Raman, S.K. Corrosion behaviour of electro-deposited nanocrystalline Ni-W and Ni-Fe-W alloys. Mater. Sci. Eng. A 2007, 460–461, 39–45. [Google Scholar] [CrossRef]
- He, F.J.; Miao, W.; Xin, L. Properties of electrodeposited amorphous Fe-Ni-W alloy deposits. Trans. Nonferrous Met. Soc. China 2006, 16, 1289–1294. [Google Scholar] [CrossRef]
- Quiroga Arganaraz, M.P.; Ribotta, S.B.; Folqure, M.E.; Gassa, L.M.; Benitez, G.; Vela, M.E.; Salvarezza, R.C. Ni-W coatings electrodeposited on carbon steel: Chemical composition, mechanical properties and corrosion resistance. Electrochim. Acta 2011, 56, 5898–5903. [Google Scholar] [CrossRef]
- Alimadadi, H.; Ahmadi, M.; Aliofkhazraei, M.; Younesi, S.R. Corrosion properties of electrodeposited nanocrystalline and amorphous patterned Ni-W alloy. Mater. Des. 2009, 30, 1356–1361. [Google Scholar] [CrossRef]
- Chianpairot, A.; Lothongkum, G.; Schuh, C.A.; Boonyongmaneerat, Y. Corrosion of nanocrystalline Ni-W alloys in alkaline and acidic 3.5 wt.% NaCl solutions. Corros. Sci. 2011, 53, 1066–1071. [Google Scholar] [CrossRef]
- Chang, L.M.; Wang, Z.T.; Shi, S.Y.; Liu, W. Study on microstructure and properties of electrodeposited Ni-W alloy coating with glycolic acid system. J. Alloy. Compd. 2011, 509, 1501–1504. [Google Scholar] [CrossRef]
- Han, B.L.; Lu, X.C. Effect of nano-sized CeF3 on microstructure, mechanical, high temperature friction and corrosion behavior of Ni-W composite coatings. Surf. Coat. Technol. 2009, 203, 3656–3660. [Google Scholar] [CrossRef]
- Khalil, M.W.; Salah Eldin, T.A.; Hassan, H.B.; El-Sayed, K.; Abdel Hamid, Z. Electrodeposition of Ni-GNS-TiO2 nanocomposite coatings as anticorrosion scale for mild steel in neutral environment. Surf. Coat. Technol. 2015, 275, 98–111. [Google Scholar] [CrossRef]
- Hou, K.H.; Chen, Y.C. Preparation and wear resistance of pulse electrodeposited Ni-W/Al2O3 composite coatings. Appl. Surf. Sci. 2011, 257, 6340–6346. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Albers, U.; Bade, K. Friction and wear characteristics of electrodeposited nanocrystalline nickel-tungsten alloy films. Wear 2008, 264, 106–112. [Google Scholar] [CrossRef]
- Donten, M.; Cesiulis, H.; Stojek, Z. Electrodeposition and properties of Ni-W, Fe-W, Fe-Ni-W amorphous alloys, a comparative study. Electrochim. Acta 2000, 45, 3389–3396. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Sun, J.B.; Zhao, W.M.; Dong, L.X.; Li, L.Y.; Meng, F.G. Erosion of behavior of arc sprayed FeTi/CrB MMC coating at elevated temperature. Surf. Coat. Technol. 2015, 262, 141–147. [Google Scholar] [CrossRef]
- Wu, Y.P.; Lin, P.H.; Chu, C.L.; Wang, Z.H.; Gao, M.; Hu, J.H. Cavitation erosion characteristics of a Fe-Cr-Si-B-Mn coating fabricated by high velocity oxy-fuel (HVOF) thermal spray. Mater. Lett. 2007, 61, 1867–1872. [Google Scholar]
- Wang, B.Q.; Shui, Z.R. The hot erosion behavior of HVOF chromium carbide-metal cermet coatings sprayed with different powders. Wear 2002, 253, 550–557. [Google Scholar] [CrossRef]
- Sun, F.Y.; Zhao, G.X.; Guo, Q.C.; Li, D.P. Effect of Ni-W alloy coating on the corrosion resistance of QT-900 coiled tubing. Surf. Technol. 2014, 43, 6–10. (In Chinese) [Google Scholar]
- Xu, W.G.; Wang, Y.D.; Bai, X.; Tan, Y.X. Application of tungalloy oil tube in oil field. Chem. Eng. Oil Gas 2009, 38, 430–433. (In Chinese) [Google Scholar]
- Shengli Oilfield Shengxin Antisepsis Co., Ltd. Anti-Corrosion Drill Rod for Well Drilling of Oilfield and Anti-Corrosion Treatment Process. China Patent Application No. 201310137819.8, 20 January 2016. [Google Scholar]
- Li, J.M.; Wang, D.D.; Cai, H.; Wang, A.J.; Zhang, J.M. Competitive deposition of elcetroless Ni-W-P coatings on mild steel via a dual-complexant plating bath composed of sodium citrate and lactic acid. Surf. Coat. Technol. 2015, 279, 9–15. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.X.; Lu, X.; Liu, C.M.; Gao, W. Parameter optimization for electroless Ni-W-P coating. Surf. Coat. Technol. 2015, 276, 195–201. [Google Scholar] [CrossRef]
- Banas, J.; Lelek-Borkowska, U.; Mazurkiewicz, B.; Solarski, W. Effect of CO2 and H2S on the composition and stability of passive film on iron alloys in geothermal water. Electrochim. Acta 2007, 52, 5704–5714. [Google Scholar] [CrossRef]
- Ueda, M. Effect of alloying elements and microstructure on stability of corrosion product in CO2 and/or H2S environments. Chem. Eng. Oil Gas 2005, 34, 43–52. (In Chinese) [Google Scholar]
- Yao, Y.W.; Yao, S.W.; Song, Z.X. Corrosion behavior of Ni-W alloy in Nacl solution. Mater. Eng. 2006, 9, 42–44. (In Chinese) [Google Scholar]
- Chu, M.S.; Wu, S.K. Improvement in the oxidation resistance of α2-Ti3Al by sputtering Al film and subsequent interdiffusion treatment. Surf. Coat. Technol. 2004, 179, 257–264. [Google Scholar] [CrossRef]









| Reagent | Concentration (g/L) | Operational Parameters |
|---|---|---|
| Nickel sulphate (NiSO4·6H2O) | 130–230 | Current density: 30–100 mA/cm2 Deposition time: 75 min Temperature: 50–80 °C pH: 3.4–5.4 |
| Nickel Carbonate (NiCO3) | 35–55 | |
| Ammonium sulfate ((NH4)2SO4) | 5–15 | |
| Hydroxyethylidene (C2H8O7P2) | 3–11 | |
| Sodium Tungstate Dihydrate (Na2WO4·2H2O) | 10–30 | |
| Sodium allylsulfonate (C3H5SO3Na) | 10–55 | |
| 1,4-butynediol (C4H6O2) | 5–15 | |
| Sodium citrate (Na3C6H5O7·2H2O) | 32–66 | |
| Sulfuric acid (H2SO4) | 2–6 | |
| Citric Acid (C6H8O7) | 6–11 | |
| Sodium dodecyl sulfate (C12H25SO4Na) | 24–38 | |
| Sodium hypophosphite (NaH2PO2·H2O) | 8–26 |
| Composition | NaCl | KCl | CaCl2 | BaCl2·2H2O | MgCl2·6H2O | SrCl2·6H2O | NaBr |
|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 4.41 | 0.47 | 17.2 | 0.28 | 33.4 | 0.75 | 0.11 |
| Condition | Material | Temperature (°C) | Test Time (h) | CO2 (MPa) | H2S (MPa) | Flow Velocity (m/s) |
|---|---|---|---|---|---|---|
| 1 | Ni-based alloy coating | 60 | 168 | 1.75 | 0.55 | 1.5 |
| 2 | Ni-based alloy coating, BG90SS | 90 | 168 | |||
| 3 | Ni-based alloy coating | 140 | 168 | |||
| 4 | Ni-based alloy coating | 140 | 360 |
| Material | Ecorr (mV vs. SCE) | icorr (μA/cm2) | ba (mV/decade) | bc (mV/decade) |
|---|---|---|---|---|
| Ni-based alloy coating | −525 | 2.48 | 97.6 | −389 |
| BG90SS steel | −704 | 14.3 | 64.9 | −251 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, Y.; Sun, C.; Sun, J.; Pu, B.; Ren, W.; Zhao, W. Stability of an Electrodeposited Nanocrystalline Ni-Based Alloy Coating in Oil and Gas Wells with the Coexistence of H2S and CO2. Materials 2017, 10, 632. https://doi.org/10.3390/ma10060632
Sui Y, Sun C, Sun J, Pu B, Ren W, Zhao W. Stability of an Electrodeposited Nanocrystalline Ni-Based Alloy Coating in Oil and Gas Wells with the Coexistence of H2S and CO2. Materials. 2017; 10(6):632. https://doi.org/10.3390/ma10060632
Chicago/Turabian StyleSui, Yiyong, Chong Sun, Jianbo Sun, Baolin Pu, Wei Ren, and Weimin Zhao. 2017. "Stability of an Electrodeposited Nanocrystalline Ni-Based Alloy Coating in Oil and Gas Wells with the Coexistence of H2S and CO2" Materials 10, no. 6: 632. https://doi.org/10.3390/ma10060632
APA StyleSui, Y., Sun, C., Sun, J., Pu, B., Ren, W., & Zhao, W. (2017). Stability of an Electrodeposited Nanocrystalline Ni-Based Alloy Coating in Oil and Gas Wells with the Coexistence of H2S and CO2. Materials, 10(6), 632. https://doi.org/10.3390/ma10060632





