Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review
Abstract
:1. Introduction
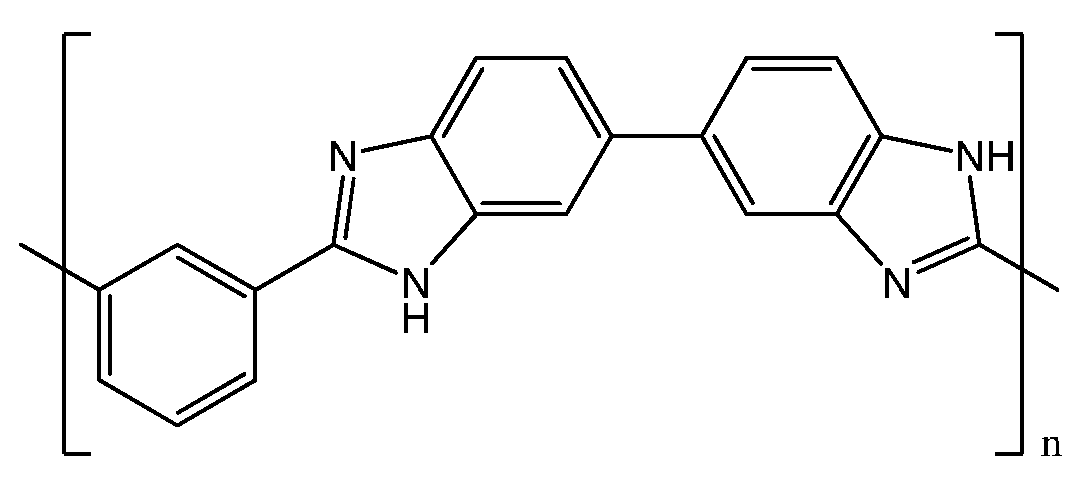
2. PBI-Based Membranes
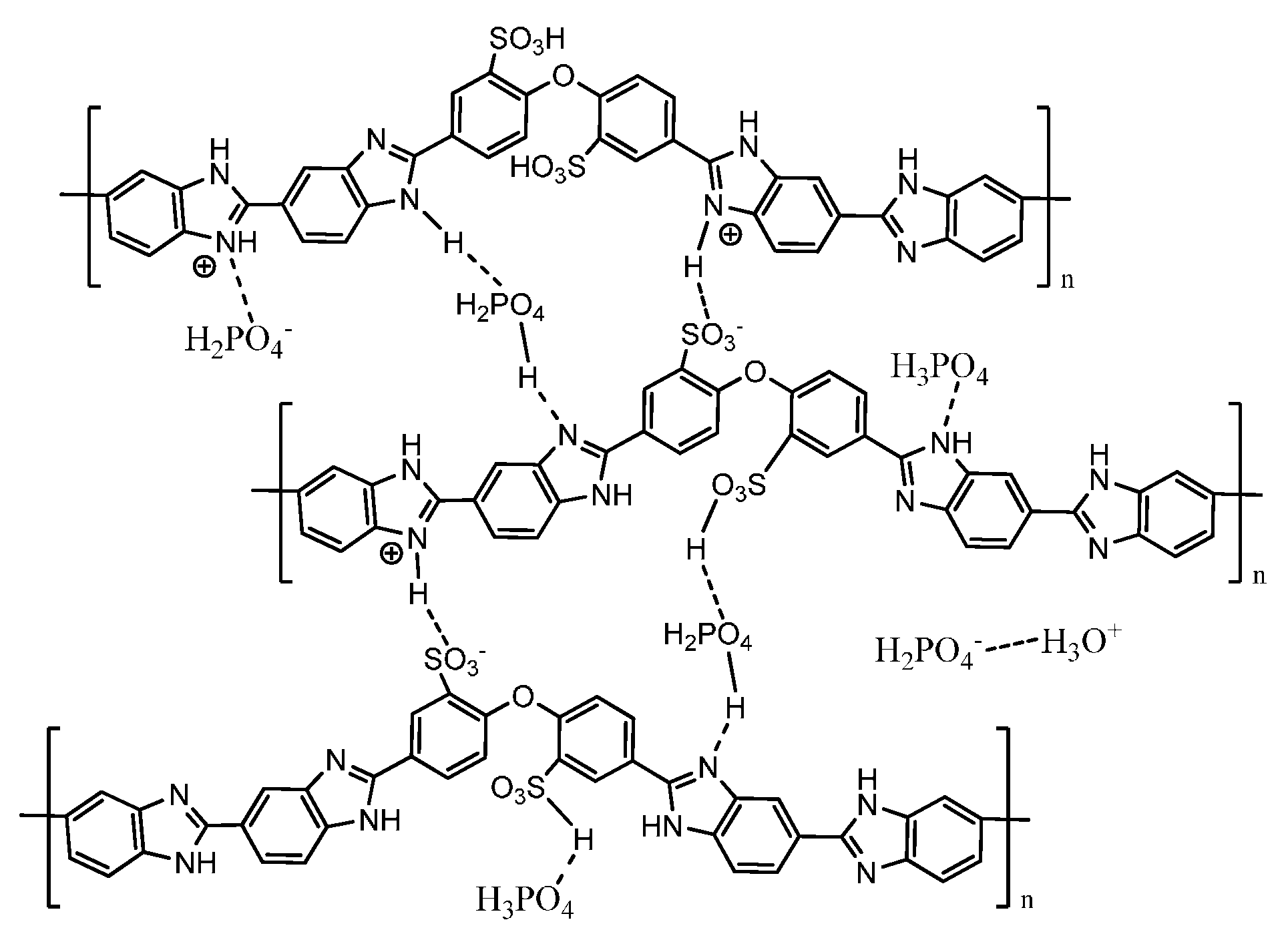
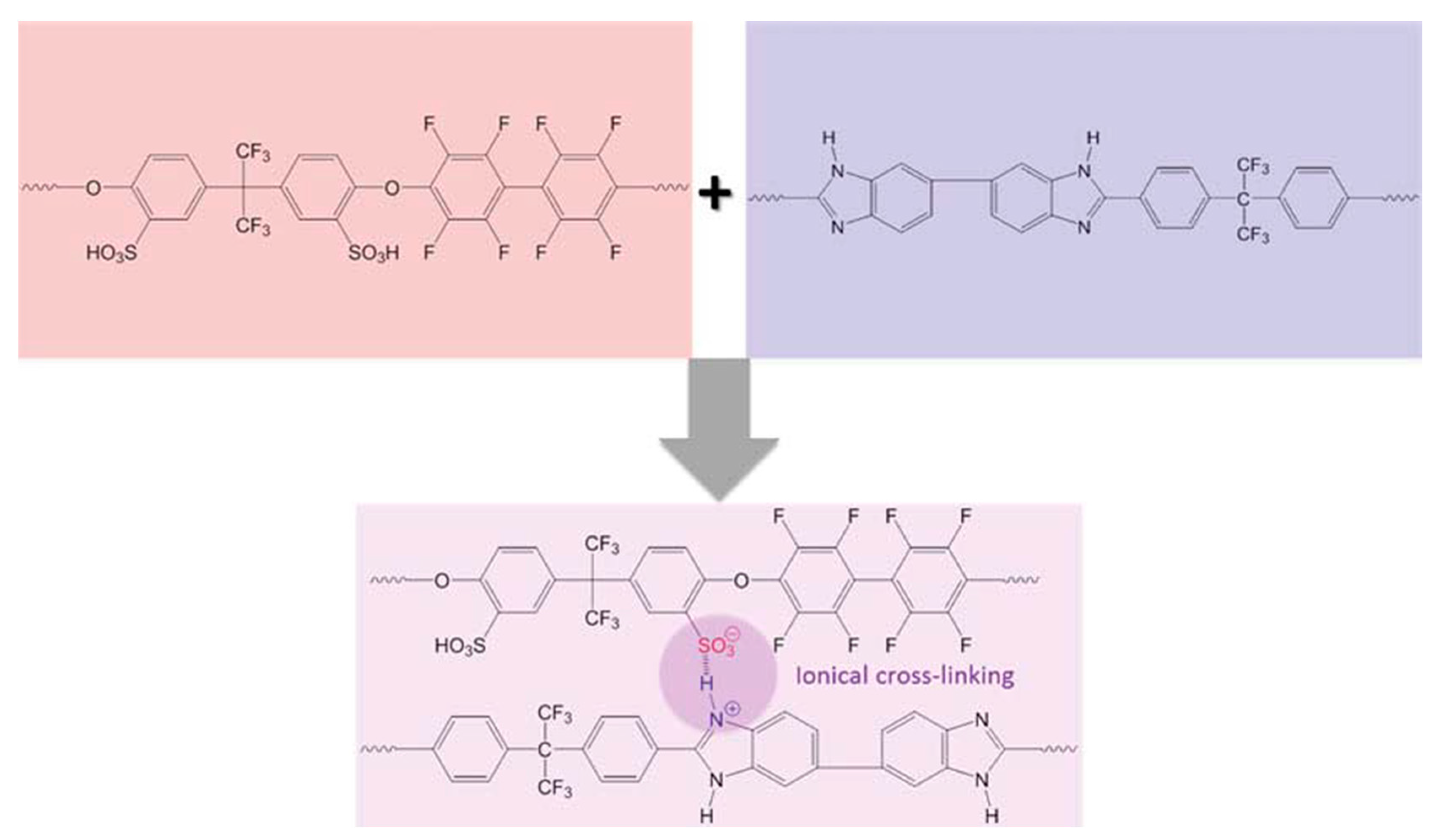
3. PBI-Based Acid-Base Blends
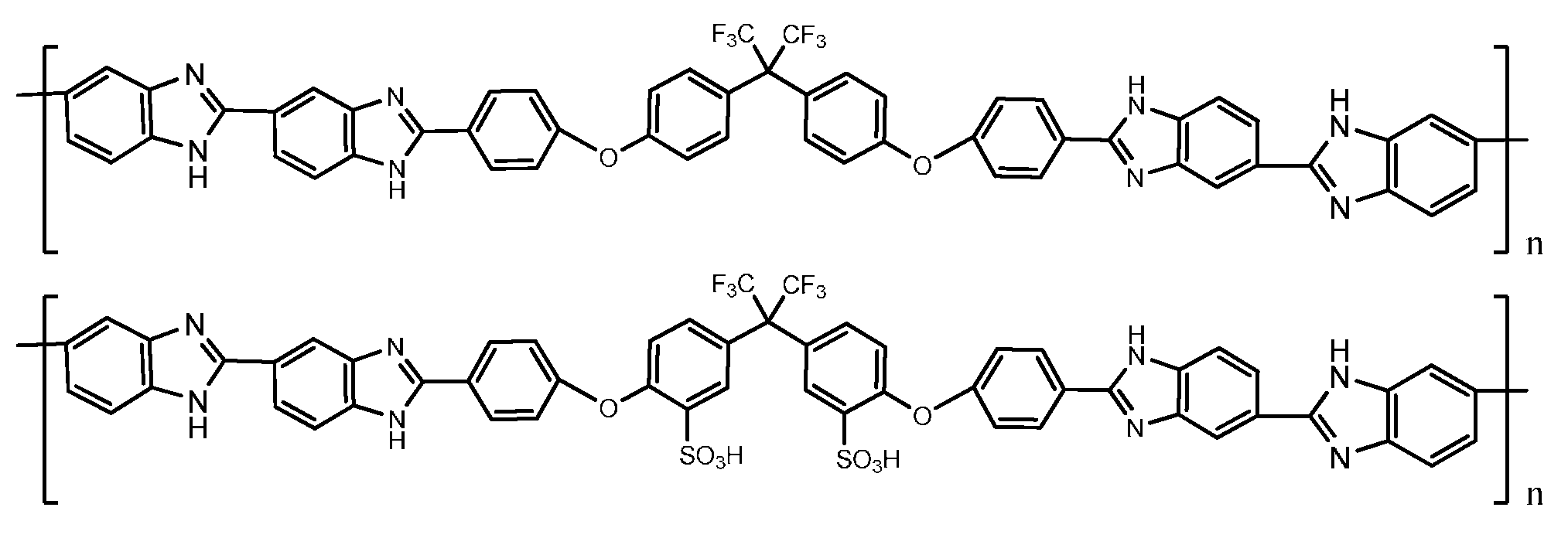
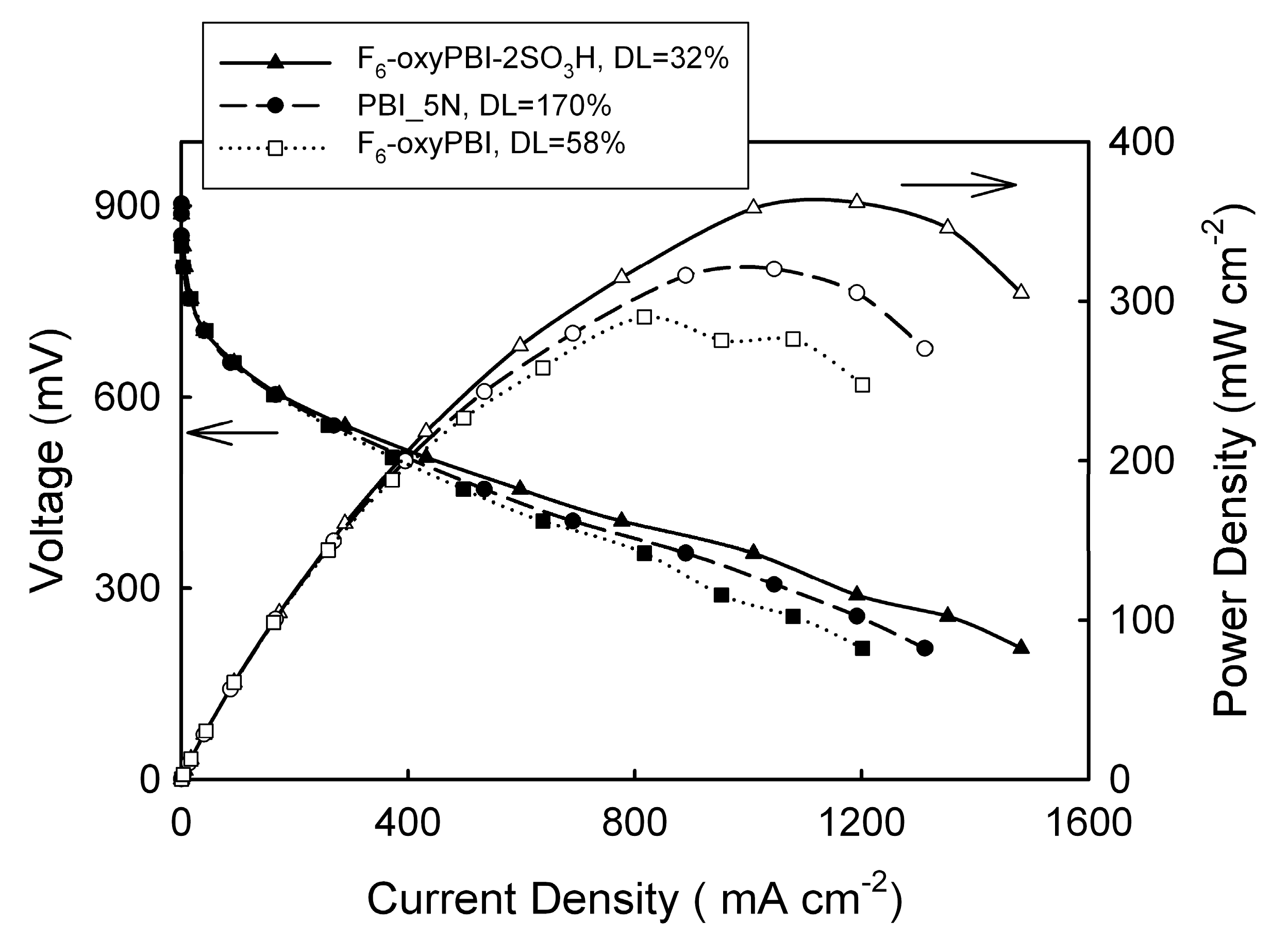
4. PBI Composite Membranes
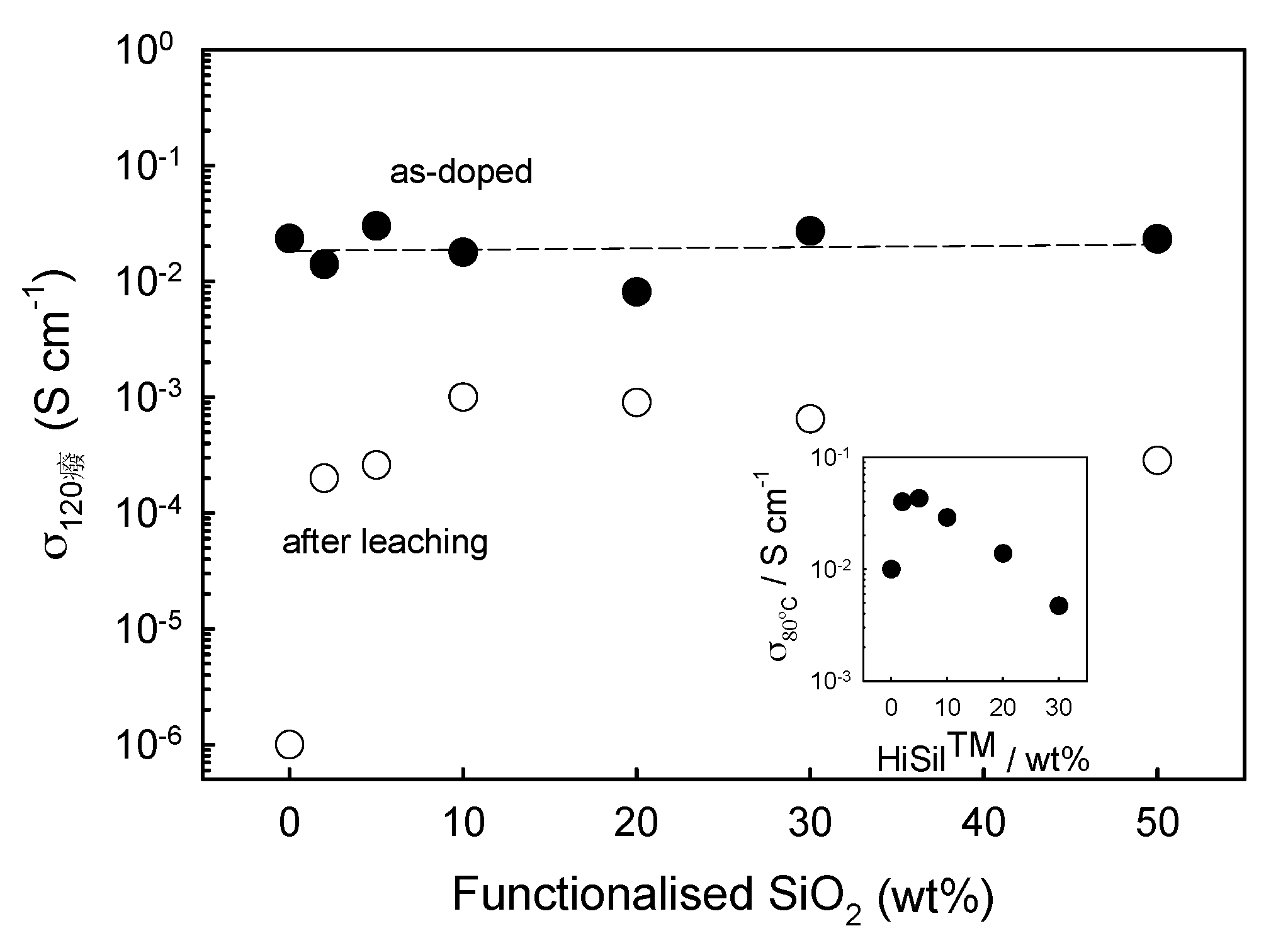
4.1. Hygroscopic and Non-Hygroscopic Oxides
4.2. Heteropolyacids, Salts, and Phosphates
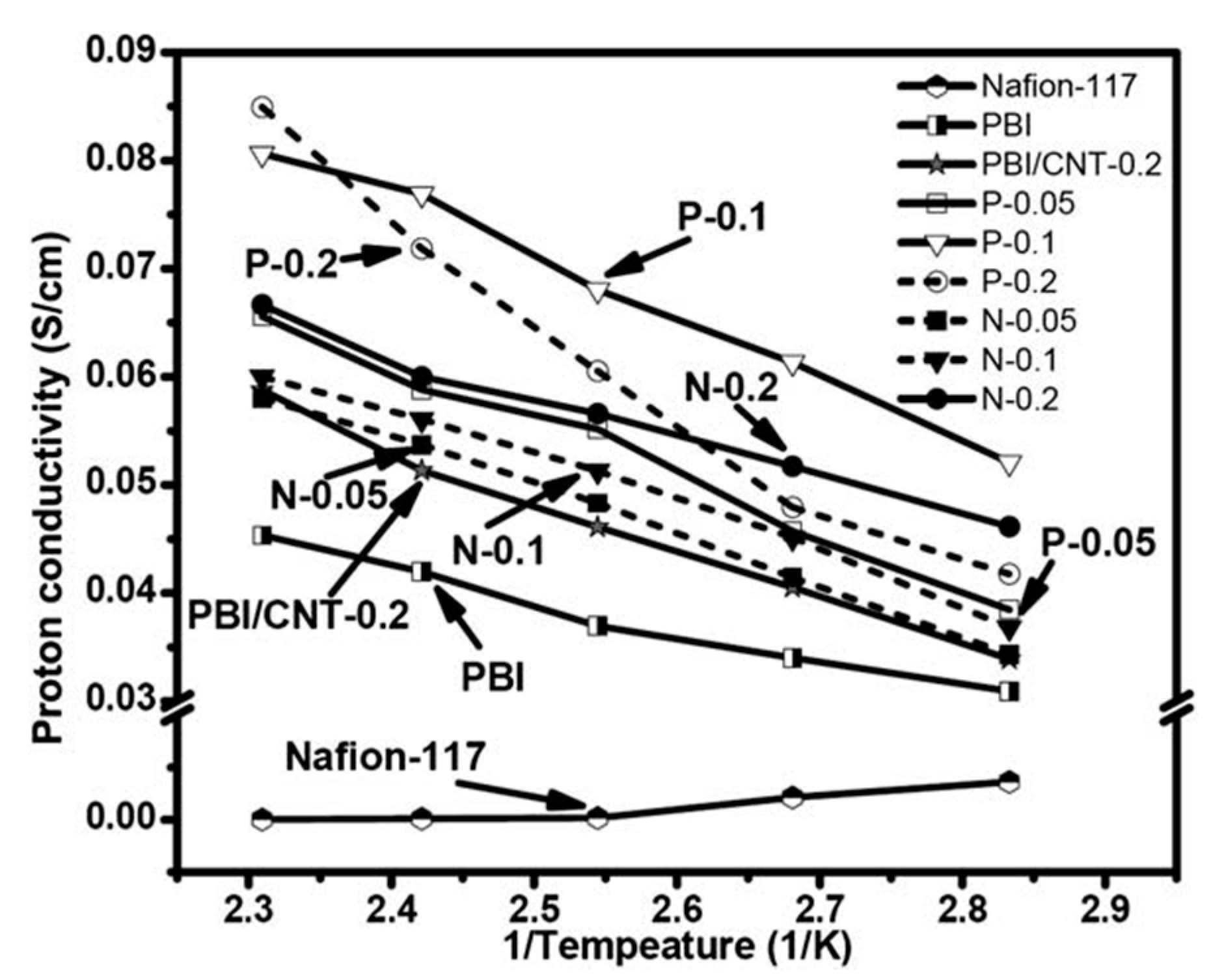
4.3. Carbon-Based Fillers
4.4. Polymer Phases
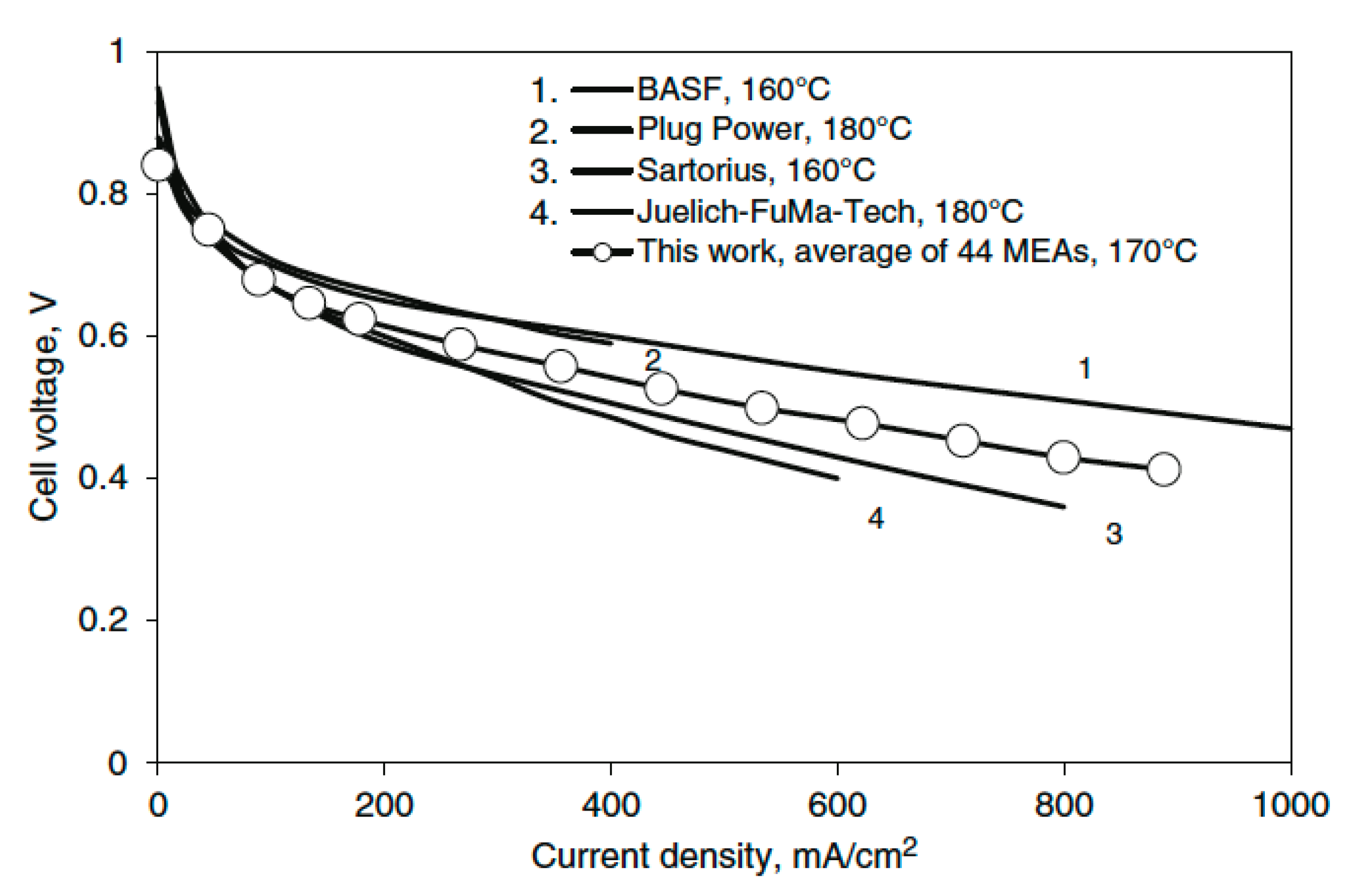
4.5. PBI Application in Stacks
5. Other Polymer Membranes
6. Proton Conductivity and Transport Mechanisms
- (1)
- direct hopping along the nitrogen sites of PBI chains, which is relevant only for non-doped PBI;
- (2)
- hopping from the N-H sites to phosphoric acid anions. This contribution is relevant for n = [H3PO4]/[BI] < 2;
- (3)
- hopping along the phosphoric acid anions (n > 2). This term is associated to free acid, and can contribute a strong increase of the conductivity;
- (4)
- hopping via water molecules, concurrent with the previous one, which is significant at high temperature.
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- EG&G Technical Services, Inc. Fuel Cell Handbook, 7th ed.; Under Contract No. DE-AM26-99FT40575; U.S. Department of Energy: Morgantown, WV, USA, 2004.
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sources 2016, 311, 91–102. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Peng, L.; Zhang, J.; Shao, Z.; Huang, J.; Sun, C.; Ouyang, M.; He, X. Recent progress on the key materials and components for proton exchange membrane fuel cells in vehicle applications. Energies 2016, 9, 603. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Gao, J.-A.; Jensen, J.O.; Bjerrum, N.J. The CO poisoning effect in PEMFCs operational at temperatures up to 200 °C. J. Electrochem. Soc. 2003, 150, 12. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sánchez, E.M.; Gómez-Romero, P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, J. PEM Fuel Cell Electrocatalyst and Catalyst Layers, Fundamentals and Applications; Springer: London, UK, 2008. [Google Scholar]
- Quartarone, E.; Mustarelli, P. Polymer fuel cells based on polybenzimidazole/H3PO4. Energy Environ. Sci. 2012, 5, 6436–6444. [Google Scholar] [CrossRef]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of proton conducting acid doped polybenzimidazole in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1225–1232. [Google Scholar] [CrossRef]
- Weng, D.; Wainright, J.S.; Landau, U.; Savinell, R.F. Electro-osmotic drag coefficient of water and methanol in polymer electrolytes at elevated temperatures. J. Electrochem. Soc. 1996, 143, 1260–1263. [Google Scholar] [CrossRef]
- Wang, J.-T.; Savinell, R.F.; Wainright, J.; Litt, M.; Yu, H. A H2/O2 fuel cell using acid doped polybenzimidazole as polymer electrolyte. Electrochim. Acta 1996, 41, 193–197. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. PBI-based polymer membranes for high temperature fuel cells—Preparation, characterization and fuel cell demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Membr. Sci. 2003, 226, 169–184. [Google Scholar] [CrossRef]
- Asensio, J.A.; Borrós, S.; Gómez-Romero, P. Polymer Electrolyte Fuel Cells Based on Phosphoric Acid-Impregnated Poly(2,5-benzimidazole) Membranes. J. Electrochem. Soc. 2004, 151, A304–A310. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, H.; Jana, T.; Scanlon, E.; Chen, R.; Choe, E.-W.; Ramanathan, L.S.; Yu, S.; Benicewicz, B.C. Synthesis and characterization of pyridine-based polybenzimidazoles for high temperature polymer electrolyte membrane fuel cell applications. Fuel Cells 2005, 5, 287–295. [Google Scholar] [CrossRef]
- Fisher, K.; Qian, G.; Benicewicz, B.C. PBI membranes via the PPA process. In High Temperature Polymer Membrane Fuel Cells; Li, Q., Aili, D., Hjuler, H.A., Jensen, J.O., Eds.; Springer: Berlin, Germany, 2016; pp. 217–238. [Google Scholar]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High Temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Angioni, S.; Villa, D.C.; Dal Barco, S.; Quartarone, E.; Righetti, P.P.; Tomasi, C.; Mustarelli, P. Polysulfonation of BI-based membranes for HT-PEMFCs: A possible way to maintain high proton transport at a low H3PO4 doping level. J. Mater. Chem. A 2014, 2, 663–671. [Google Scholar] [CrossRef]
- Villa, D.C.; Angioni, S.; Dal Barco, S.; Mustarelli, P.; Quartarone, E. Polysulfonated fluoro-oxyPBI membranes for PEMFCs: An efficient strategy to achieve good fuel cell perfomances with low H3PO4 doping levels. Adv. Energy Mater. 2014, 4, 1031949. [Google Scholar] [CrossRef]
- Thomas, O.D.; Peckham, T.J.; Thanganathan, U.; Yang, Y.; Holdcroft, S. Sulfonated polybenzimidazoles: Proton conduction and acid-base crosslinking. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3640–3650. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, G.; Li, J.; Hao, J.; Wei, F.; Li, W.; Zhang, J.; Shao, Z.-G.; Yi, B. Polybenzimidazole-crosslinked poly(vinylbenzyl chloride) with quaternary 1,4-diazabicyclo (2.2.2) octane groups as high-performance anion exchange membrane for fuel cells. J. Power Sources 2015, 296, 204–214. [Google Scholar] [CrossRef]
- Carollo, A.; Quartarone, E.; Tomasi, C.; Mustarelli, P.; Belotti, F.; Magistris, A.; Maestroni, F.; Parachini, M.; Garlaschelli, L.; Righetti, P.P. Developments of new proton conducting membranes based on different polybenzimidazole structures for fuel cells applications. J. Power Sources 2006, 160, 175–180. [Google Scholar] [CrossRef]
- Mustarelli, P.; Quartarone, E.; Grandi, S.; Angioni, S.; Magistris, A. Increasing the permanent conductivity of PBI membranes for HT-PEMs. Solid State Ion. 2012, 225, 228–231. [Google Scholar] [CrossRef]
- Peron, J.; Ruiz, E.; Jones, D.J.; Rozière, J. Solution sulfonation of a novel polybenzimidazole. A proton electrolyte for fuel cell application. J. Membr. Sci. 2008, 314, 247–256. [Google Scholar] [CrossRef]
- Mader, J.A.; Benicewicz, B.C. Sulfonated polybenzimidazoles for high temperature PEM fuel cells. Macromolecules 2010, 43, 6706–6715. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; Angioni, S.; Mustarelli, P.; Quartarone, E. Ion dynamics and mechanical properties of sulfonated polybenzimidazole membranes for high-temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2015, 119, 9745–9753. [Google Scholar] [CrossRef]
- Chuang, S.-W.; Hsu, S.L.-C. Synthesis and properties of a new fluorine-containing polybenzimidazole for high-temperature fuel-cell applications. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4508–4513. [Google Scholar] [CrossRef]
- Qian, G.; Benicewicz, B.C. Synthesis and characterization of high molecular weight hexafluoroisopropylidene-containing polybenzimidazole for high-temperature polymer electrolyte membrane fuel cells. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4064–4073. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Cleemann, L.N.; Jensen, J.O.; Pan, C.; Bjerrum, N.J.; He, R. Crosslinked hexafluoropropylidene polybenzimidazole membranes with chloromethyl polysulfone for fuel cell applications. Adv. Energy Mater. 2013, 3, 622–630. [Google Scholar] [CrossRef]
- Yu, S.; Xiao, L.; Benicewicz, B.C. Durability studies of PBI-based high temperature PEMFCs. Fuel Cells 2008, 8, 165–174. [Google Scholar] [CrossRef]
- Liao, J.H.; Li, Q.F.; Rudbeck, H.C.; Jensen, J.O.; Chromik, A.; Bjerrum, N.J.; Kerres, J.; Xing, W. Oxidative degradation of polybenzimidazole membranes as electrolytes for high temperature proton exchange membrane fuel cells. Fuel Cells 2011, 11, 745–755. [Google Scholar] [CrossRef]
- Kerres, J. Applications of Acid-Base Blend Concepts to Intermediate Temperature Membranes. In High Temperature Polymer Membrane Fuel Cells; Li, Q., Aili, D., Hjuler, H.A., Jensen, J.O., Eds.; Springer: Berlin, Germany, 2016; pp. 217–238. [Google Scholar]
- Kerres, J.; Ullrich, A.; Meier, F.; Häring, T. Synthesis and characterization of novel acid-base polymer blends for application in membrane fuel cells. Solid State Ion. 1999, 125, 243–249. [Google Scholar] [CrossRef]
- Kerres, J.; Ullrich, A.; Häring, T. Engineering Ionomer Blends and Engineering Ionomer Blend Membranes. European Patent 1,076,676; U.S. Patent 6,723,757, 2004. [Google Scholar]
- Hasiotis, C.; Qingfeng, L.; Deimede, V.; Kallitsis, J.K.; Kontoyannis, C.G.; Bjerrum, N.J. Development and Characterization of Acid-Doped Polybenzimidazole/Sulfonated Polysulfone Blend Polymer Electrolytes for Fuel Cells. J. Electrochem. Soc. 2001, 148, A513–A519. [Google Scholar] [CrossRef]
- Li, Q.F.; Rudbeck, H.C.; Chromik, A.; Jensen, J.O.; Pan, C.; Steenberg, T.; Calverley, M.; Bjerrum, N.J.; Kerres, J. Properties, degradation and high temperature fuel cell test of different types of PBI and PBI blend membranes. J. Membr. Sci. 2010, 347, 260–270. [Google Scholar] [CrossRef]
- Mack, F.; Aniol, K.; Ellwein, C.; Kerres, J.; Zeis, R. Novel phosphoric acid-doped PBI-blends as membranes for high-temperature PEM fuel cells. J. Mater. Chem. A 2015, 3, 10864–10874. [Google Scholar] [CrossRef]
- Joseph, D.; Krishnan, N.N.; Henkensmeier, D.; Jang, J.H.; Choi, S.H.; Kim, H.-J.; Han, J.; Nam, S.W. Thermal crosslinking of PBI/sulfonated polysulfone based blend membranes. J. Mater. Chem. A 2017, 5, 409–417. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M. Composite membranes for medium-temperature PEM fuel cells. Annu. Rev. Mater. Res. 2003, 33, 129–154. [Google Scholar] [CrossRef]
- Mustarelli, P.; Quartarone, E.; Grandi, S.; Carollo, A.; Magistris, A. Polybenzimidazole-based membranes as a real alternative to nafion for fuel cells operating at low temperature. Adv. Mater. 2008, 20, 1339–1343. [Google Scholar] [CrossRef]
- Chuang, S.-W.; Hsu, S.L.-C.; Hsu, C.-L. Synthesis and properties of fluorine-containing polybenzimidazole/montmorillonite nanocomposite membranes for direct methanol fuel cell applications. J. Power Sources 2007, 168, 172–177. [Google Scholar] [CrossRef]
- Quartarone, E.; Magistris, A.; Mustarelli, P.; Grandi, S.; Carollo, A.; Zukowska, G.Z.; Garbarczyk, J.E.; Nowinski, J.L.; Gerbaldi, C.; Bodoardo, S. Pyridine-based PBI composite membranes for PEMFCs. Fuel Cells 2009, 9, 349–355. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P.; Carollo, A.; Grandi, S.; Magistris, A.; Gerbaldi, C. PBI composite and nanocomposite membranes for PEMFCs: The role of the filler. Fuel Cells 2009, 9, 231–236. [Google Scholar] [CrossRef]
- Kurdakova, V.; Quartarone, E.; Mustarelli, P.; Magistris, P.; Caponetti, E.; Saladino, M.L. PBI-based composite membranes for polymer fuel cells. J. Power Sources 2010, 195, 7765–7769. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Úbeda, D.; Pinar, F.J. Promising TiOSO4 composite polybenzimidazole-based membranes for high temperature PEMFCs. ChemSusChem 2011, 4, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Úbeda, D.; Pinar, F.J. Enhancement of the fuel cell performance of a high temperature proton exchange membrane fuel cell running with titanium composite polybenzimidazole-based membranes. J. Power Sources 2011, 196, 8265–8271. [Google Scholar] [CrossRef]
- Pinar, F.J.; Cañizares, P.; Rodrigo, M.A.; Ubeda, D.; Lobato, J. Titanium composite PBI-based membranes for high temperature polymer electrolyte membrane fuel cells. Effect on titanium dioxide amount. RSC Adv. 2012, 2, 1547–1556. [Google Scholar] [CrossRef]
- Namazi, H.; Ahmadi, H. Improving the proton conductivity and water uptake of polybenzimidazole-based proton exchange nanocomposite membranes with TiO2 and SiO2 nanoparticles chemically modified surfaces. J. Power Sources 2011, 196, 2573–2583. [Google Scholar] [CrossRef]
- Staiti, P.; Minutoli, M.; Hocevar, S. Membranes based on phosphotungstic acid and polybenzimidazole for fuel cell application. J. Power Sources 2000, 90, 231–235. [Google Scholar] [CrossRef]
- Gomez-Romero, P.; Asensio, J.A.; Borros, S. Hybrid proton-conducting membranes for polymer PBI-Based Composite Membranes electrolyte fuel cells: Phosphomolybdic acid doped Poly(2,5-benzimidazole)—(ABPBI-H3PMo12O40). Electrochim. Acta 2005, 50, 4715–4720. [Google Scholar] [CrossRef]
- Staiti, P. Proton conductive membranes based on silicotungstic acid/silica and polybenzimidazole. Mater. Lett. 2001, 47, 241–246. [Google Scholar] [CrossRef]
- Gatto, I.; Saccà, A.; Carbone, A.; Pedicini, R.; Urbani, F.; Passalacqua, E. CO-tolerant electrodes developed with PhosphoMolybdic Acid for Polymer Electrolyte Fuel Cell (PEFCs) application. J. Power Sources 2007, 171, 540–545. [Google Scholar] [CrossRef]
- Xu, C.; Wu, X.; Wang, X.; Mamlouk, M.; Scott, K. Composite membranes of polybenzimidazole and caesium-salts-of-heteropolyacids for intermediate temperature fuel cells. J. Mater. Chem. 2011, 21, 6014–6019. [Google Scholar] [CrossRef]
- Di, S.; Yan, L.; Han, S.; Yue, B.; Feng, Q.; Xie, L.; Chen, J.; Zhang, D.; Sun, C. Enhancing the high-temperature proton conductivity of phosphoric acid doped poly(2,5-benzimidazole) by preblending boron phosphate nanoparticles to the raw materials. J. Power Sources 2012, 211, 161–168. [Google Scholar] [CrossRef]
- Jin, Y.C.; Nishida, M.; Kanematsu, W.; Hibino, T. An H3PO4-doped polybenzimidazole/Sn 0.95Al0.05P2O7 composite membrane for high-temperature proton exchange membrane fuel cells. J. Power Sources 2011, 196, 6042–6047. [Google Scholar] [CrossRef]
- Wu, X.; Mamlouk, M.; Scott, K. A PBI-Sb0.2Sn0.8P2O7-H3PO4 composite membrane for intermediate temperature fuel cells. Fuel Cells 2011, 11, 620–625. [Google Scholar] [CrossRef]
- Li, N.; Zhang, F.; Wang, J.; Li, S.; Zhang, S. Dispersions of carbon nanotubes in sulfonated poly[bis(benzimidazobenzisoquinolinones)] and their proton-conducting composite membranes. Polymer 2009, 50, 3600–3608. [Google Scholar] [CrossRef]
- Kannan, R.; Kagalwala, H.N.; Chaudhari, H.D.; Kharul, U.K.; Kurungot, S.; Pillai, V.K. Improved performance of phosphonated carbon nanotube-polybenzimidazole composite membranes in proton exchange membrane fuel cells. J. Mater. Chem. 2011, 21, 7223–7231. [Google Scholar] [CrossRef]
- Suryani; Chang, C.-M.; Liu, Y.-L.; Lee, Y.M. Polybenzimidazole membranes modified with polyelectrolyte-functionalized multiwalled carbon nanotubes for proton exchange membrane fuel cells. J. Mater. Chem. 2011, 21, 7480–7486. [Google Scholar] [CrossRef]
- Xue, C.; Zou, J.; Sun, Z.; Wang, F.; Han, K.; Zhu, H. Graphite oxide/functionalized graphene oxide and polybenzimidazole composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2014, 39, 7931–7939. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Ukihashi, H.; Asawa, T.; Gunjima, T. Cation Exchange Membrane of Fluorinated Polymer Containing Polytetrafluoroethylene Fibrils for Electrolysis and Preparation Thereof. U.S. Patent 4,218,542, 1980. [Google Scholar]
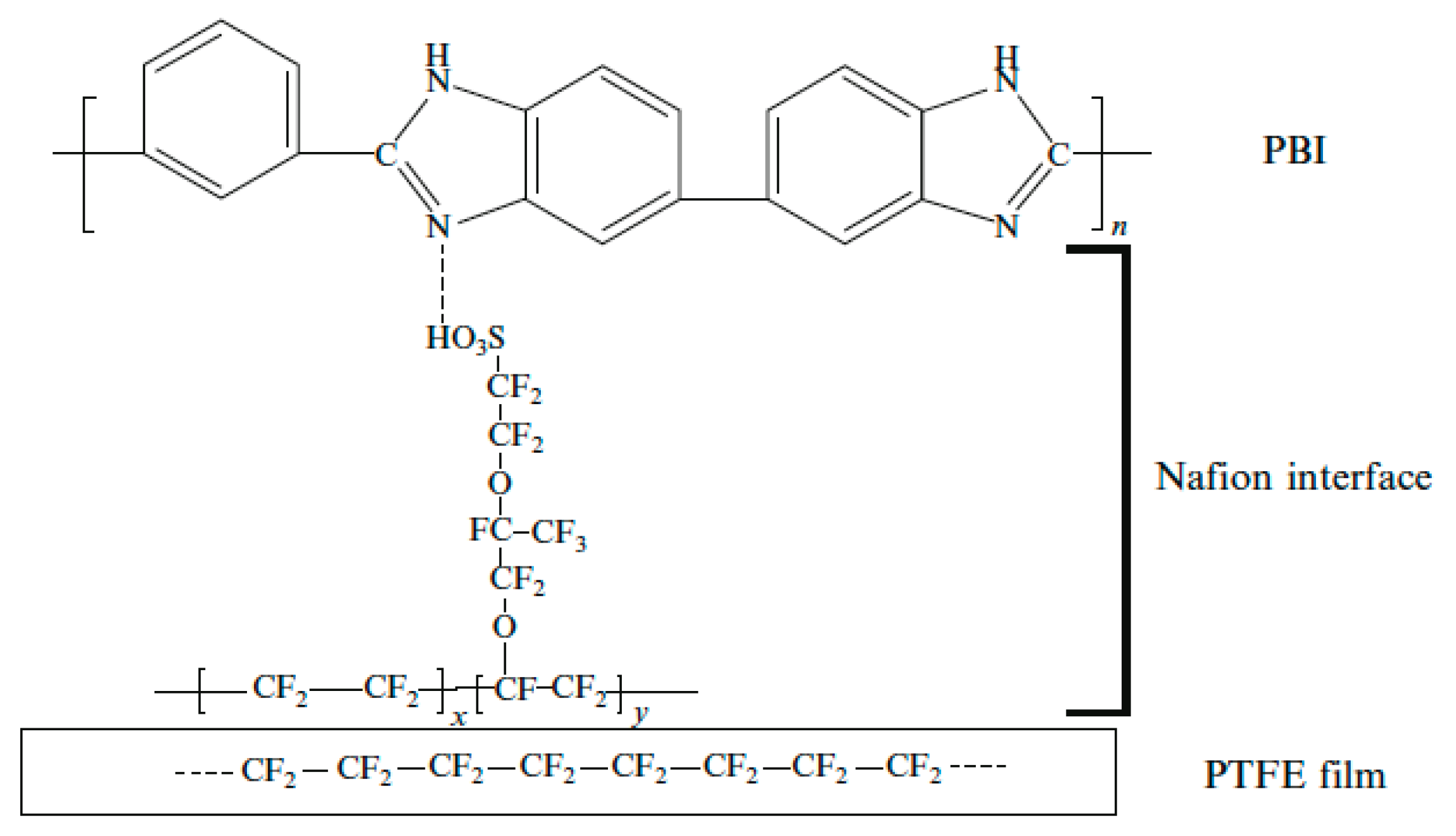
- Liu, F.; Yi, B.; Xing, D.; Yu, J.; Zhang, H. Nafion/PTFE composite membranes for fuel cell applications. J. Membr. Sci. 2003, 212, 213–223. [Google Scholar] [CrossRef]
- Li, M.; Scott, K. A polymer electrolyte membrane for high temperature fuel cells to fit vehicle applications. Electrochim. Acta 2010, 55, 2123–2128. [Google Scholar] [CrossRef]
- Lin, H.-L.; Huang, J.-R.; Chen, Y.-T.; Su, P.-H.; Yu, T.L.; Chan, S.-H. Polybenzimidazole/poly(tetrafluoro ethylene) composite membranes for high temperature proton exchange membrane fuel cells. J. Polym. Res. 2012, 19, 9875. [Google Scholar] [CrossRef]
- Pinar, F.J.; Cañizares, P.; Rodrigo, M.A.; Úbeda, D.; Lobato, J. Long-term testing of a high-temperature proton exchange membrane fuel cell short stack operated with improved polybenzimidazole-based composite membranes. J. Power Sources 2015, 274, 177–185. [Google Scholar] [CrossRef]
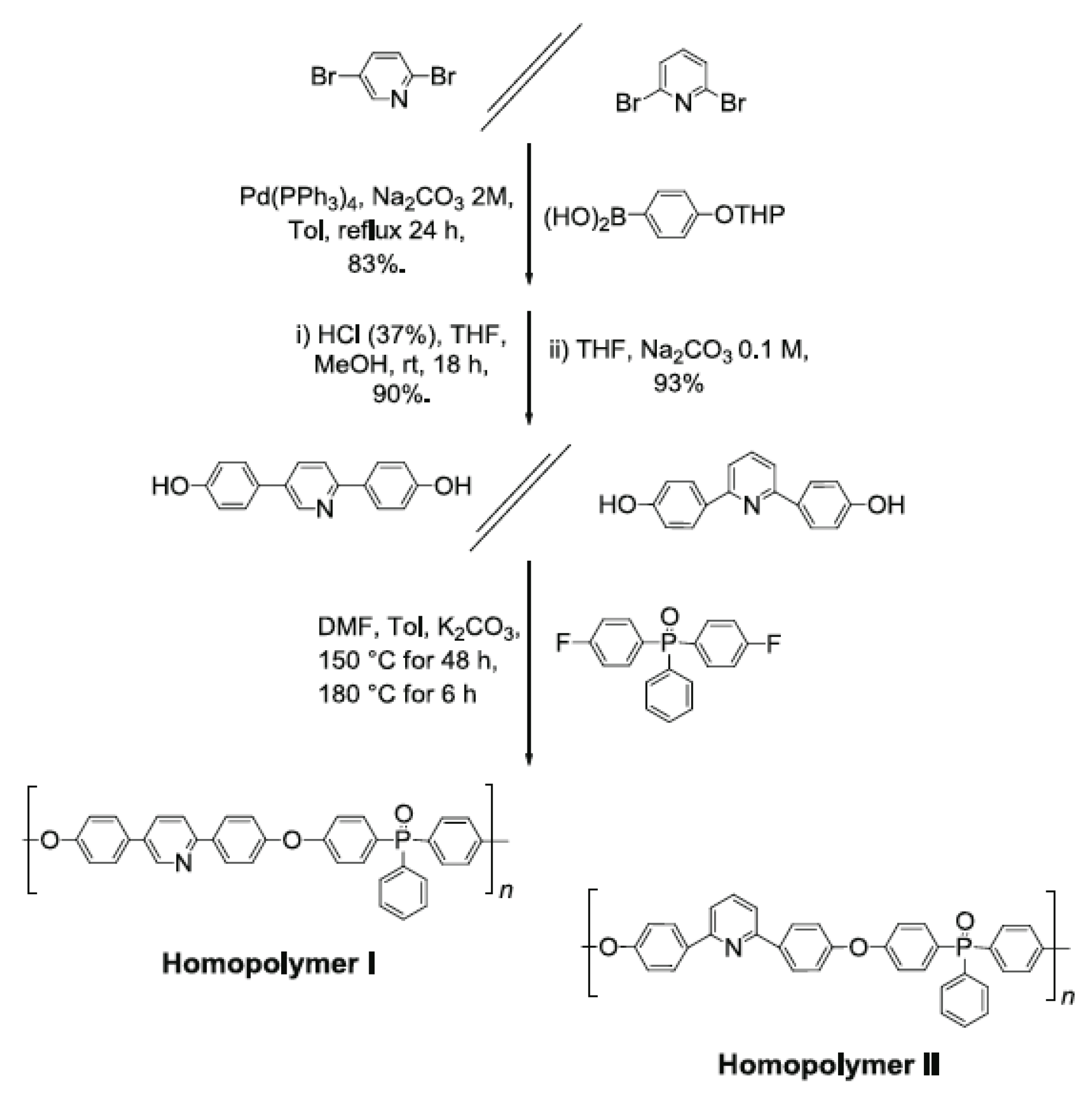
- Gourdoupi, N.; Andreopoulou, A.K.; Deimede, V.; Kallitsis, J.K. Novel Proton-Conducting Polyelectrolyte Composed of an Aromatic Polyether Containing Main-Chain Pyridine Units for Fuel Cell Applications. Chem. Mater. 2003, 15, 5044–5050. [Google Scholar]
- Kallitsis, J.K.; Andreopoulou, A.K. Pyridine containing aromatic polyether membranes. In High Temperature Polymer Membrane Fuel Cells; Li, Q., Aili, D., Hjuler, H.A., Jensen, J.O., Eds.; Springer: Berlin, Germany, 2016; pp. 91–126. [Google Scholar]
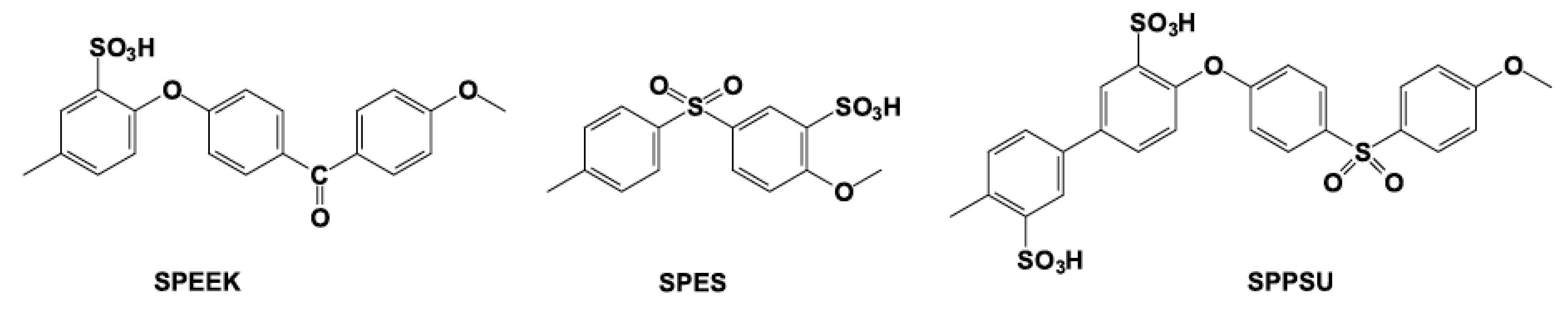
- Di Vona, M.L.; Knauth, P. Sulfonated Aromatic Polymers as Proton-Conducting Solid Electrolytes for Fuel Cells: A Short Review. Z. Phys. Chem. 2013, 227, 595–614. [Google Scholar] [CrossRef]
- Liu, B.; Guiver, M.D. Proton Conductivity of Aromatic Polymers. In Solid State Proton Conductors: Properties and Applications in Fuel Cells; Wiley: Chichester, UK, 2012. [Google Scholar]
- Marrony, M.; Rozière, J.; Jones, D.J.; Lindheimer, A. Multilayer sulfonated polyaromatic PEMFC membranes. Fuel Cells 2005, 5, 412–428. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric proton conducting membranes for medium temperature fuel cells (110–160C). J. Membr. Sci. 2001, 185, 73–81. [Google Scholar] [CrossRef]
- Mecheri, B.; D’Epifanio, A.; Di Vona, M.L.; Traversa, E.; Licoccia, S.; Miyayama, M. Sulfonated polyether ether ketone-based composite membranes doped with a tungsten-based inorganic proton conductor for fuel cell applications. J. Electrochem. Soc. 2006, 153, A463–A467. [Google Scholar] [CrossRef]
- Licoccia, S.; Di Vona, M.L.; D’Epifanio, A.; Marani, D.; Vittadello, M.; Jayakody, J.R.P.; Greenbaum, S.G. Ormosil/sulfonated polyetheretherketone-based hybrid composite proton conducting membranes. J. Electrochem. Soc. 2006, 153, A1226–A1231. [Google Scholar] [CrossRef]
- Nagao, M.; Takeuchi, A.; Heo, P.; Hibino, T.; Sano, M.; Tomita, A. A proton-conducting In3+-doped SnP2O7 electrolyte for intermediate-temperature fuel cells. Electrochem. Solid-State Lett. 2006, 9, A105–A109. [Google Scholar] [CrossRef]
- Lee, K.-S.; Spendelow, J.S.; Choe, Y.-K.; Fujimoto, C.; Kim, Y.S. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs. Nat. Energy 2016, 1, 16120. [Google Scholar]
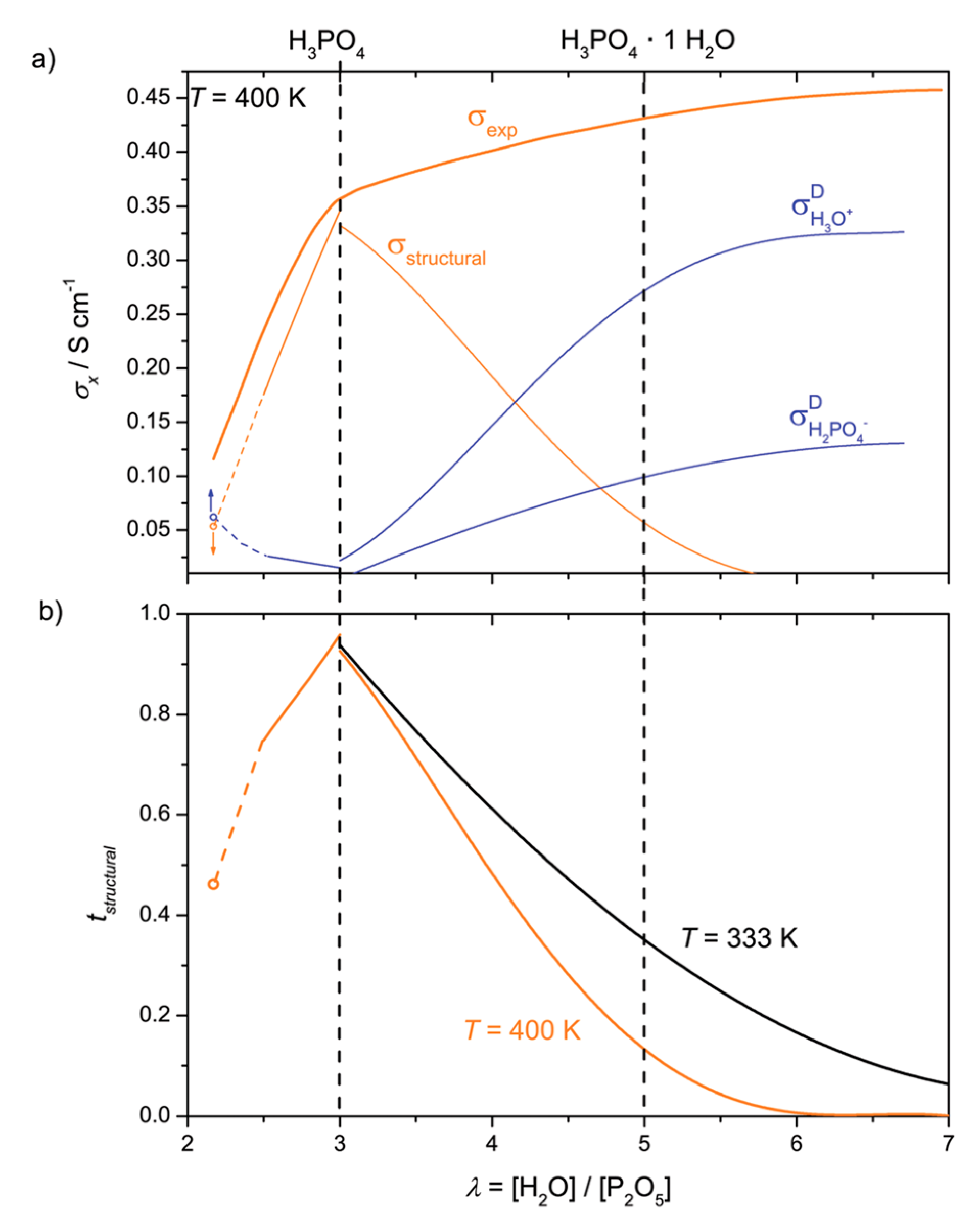
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [PubMed]
- Di Noto, V.; Piga, M.; Giffin, G.A.; Quartarone, E.; Righetti, P.; Mustarelli, P.; Magistris, A. Structure-property interplay of proton conducting membranes based on PBI5N, SiO2-Im and H3PO4 for high temperature fuel cells. Phys. Chem. Chem. Phys. 2011, 13, 12146–12154. [Google Scholar] [CrossRef] [PubMed]
- Vilčiauskas, L.; Tuckerman, M.E.; Bester, G.; Paddison, S.J.; Kreuer, K.-D. The mechanism of proton conduction in phosphoric acid. Nat. Chem. 2012, 4, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Melchior, J.-P.; Kreuer, K.-D.; Maier, J. Proton conduction mechanisms in the phosphoric acid-water system H4P2O7-H3PO4.2H2O: A 1H, 31P and 17O PFG-NMR and conductivity study. Phys. Chem. Chem. Phys. 2017, 19, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.A.; Vilčiauskas, L.; Melchior, J.-P.; Bester, G.; Kreuer, K.-D. Mechanism of Efficient Proton Conduction in Diphosphoric Acid Elucidated via First-Principles Simulation and NMR. J. Phys. Chem. B 2015, 119, 15866–15875. [Google Scholar] [CrossRef] [PubMed]
- Melchior, J.-P.; Majer, G.; Kreuer, K.-D. Why do proton conducting polybenzimidazole phosphoric acid membranes perform well in high-temperature PEM fuel cells? Phys. Chem. Chem. Phys. 2017, 19, 601–612. [Google Scholar] [CrossRef] [PubMed]
| Polymer | η (dL/g) | MWmon | DP | MWpol | |
|---|---|---|---|---|---|
| PBI_4N |  | 0.7 | 308 | 98 | 30,352 |
| PBI_5N_2,6 |  | 1.7 | 309 | 300 | 92,840 |
| PBI_5N_2,5 |  | 0.5 | 309 | 64 | 19,864 |
| PBI_6N_bipy |  | 1.0 | 386 | 123 | 47,574 |
| PBI_6N_pyra |  | n.a. | 298 | n.a. | n.a. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials 2017, 10, 687. https://doi.org/10.3390/ma10070687
Quartarone E, Angioni S, Mustarelli P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials. 2017; 10(7):687. https://doi.org/10.3390/ma10070687
Chicago/Turabian StyleQuartarone, Eliana, Simone Angioni, and Piercarlo Mustarelli. 2017. "Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review" Materials 10, no. 7: 687. https://doi.org/10.3390/ma10070687




