Investigation of Molecular Structure and Thermal Properties of Thermo-Oxidative Aged SBS in Blends and Their Relations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Heavy Ends from Solvent Naphtha
2.3. Thermo-Oxidative Aging Procedure
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Thermal Analysis (TG-DTG)
3. Results and Discussion
3.1. Effect of Aging Temperature on the Molecular Structure of SBS
3.2. Effect of Aging Time on the Molecular Structure of SBS
3.3. Effect of Oxygen Concentration on the Molecular Structure of SBS
3.4. Thermal Properties
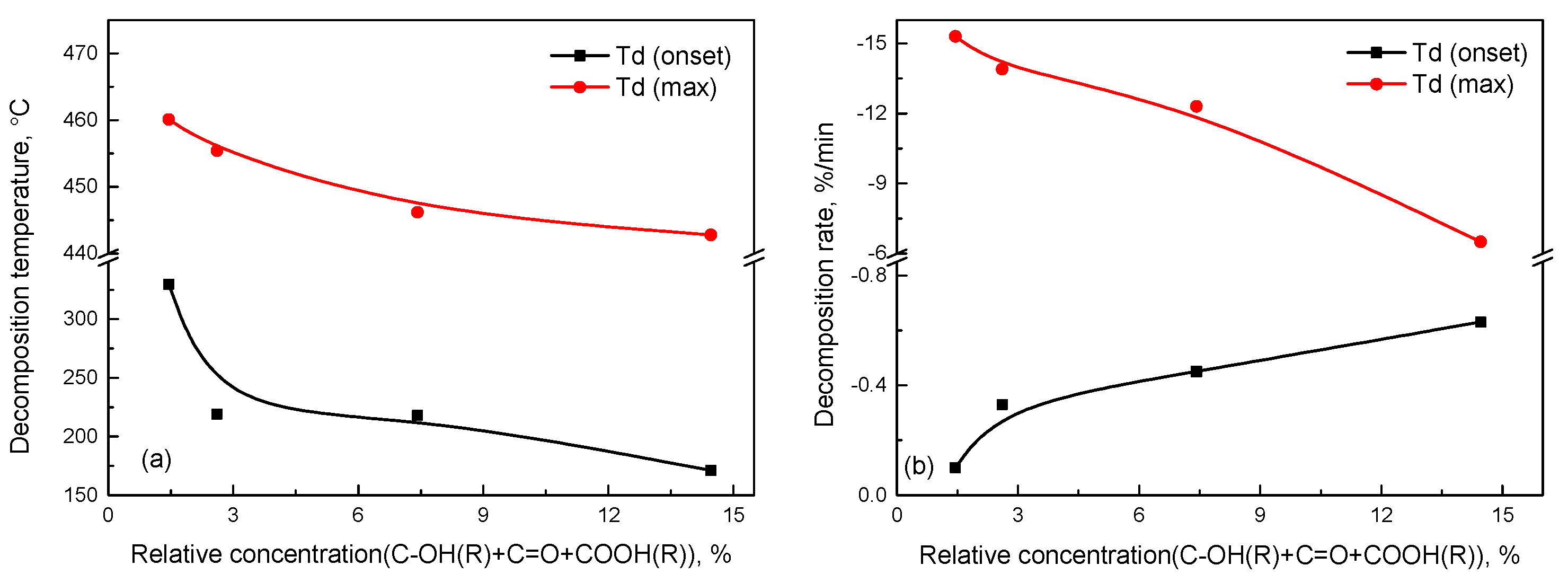
3.5. Relation Between Thermal Properties and Oxygen-Containing Groups of Aged SBS
4. Conclusions
- The FTIR and XPS spectra indicate that increments of aging temperature, aging time and oxygen concentration can accelerate the decomposition of tri-block structural SBS into bi-block structural SB- and the increasing concentration of oxygen-containing groups such as –OH, C=O, –COOH, etc. Increments of temperature or oxygen concentration have a significant effect on the structural destruction of SBS in short-term aging, while the rising oxygen concentration has no further effect on the molecular structure of SBS in long-term aging.
- The TG and DTG results illustrate that a large number of low-molecular weight substances were formed in the adverse thermo-oxidative environment, and the initial and maximum decomposition temperature of aged SBS both decreased. With the rising degree of aging of the SBS, the initial decomposition rate gradually increases at the beginning of thermal weightlessness, and the decomposition rate slows down when in comparison with the neat SBS.
- Based upon the relation of the relative concentration of oxygen-containing groups and the thermal properties of aged SBS, the initial thermal stability of SBS rapidly reduces as the relative concentration of the oxygen-containing groups accumulates around 3%. When the relative concentration of the oxygen-containing groups is more than 3%, the maximum decomposition temperature slowly decreases due to the difficult destruction of strong bonds on the molecular structure of aged SBS.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ouyang, C.F.; Wang, S.F.; Zhang, Y.; Zhang, Y.X. Improving the aging resistance of styrene-butadiene- styrene tri-block copolymer modified asphalt by addition of antioxidants. Polym. Degrad. Stab. 2006, 91, 795–804. [Google Scholar] [CrossRef]
- Kok, B.V.; Yilmaz, M.; Sengoz, B.; Sengur, A.; Avci, E. Investigation of complex modulus of base and SBS modified bitumen with artificial neural networks. Expert Syst. Appl. 2010, 37, 7775–7780. [Google Scholar] [CrossRef]
- Wen, G.; Zhang, Y.; Zhang, Y.; Sun, K.; Fan, Y. Rheological characterization of storage-stable SBS-modified asphalts. Polym. Test. 2002, 21, 295–302. [Google Scholar] [CrossRef]
- Zhang, D.M.; Zhang, H.L.; Zhu, C.Z.; Shi, C.J. Synergetic effect of multi-dimensional nanomaterials for anti-aging properties of SBS modified bitumen. Constr. Build. Mater. 2017, 144, 423–431. [Google Scholar] [CrossRef]
- Nciri, N.; Kim, N.; Cho, N. New insights into the effects of styrene-butadiene-styrene polymer modifier on the structure, properties, and performance of asphalt binder: The case of AP-5 asphalt and solvent deasphalting pitch. Mater. Chem. Phys. 2017, 193, 477–495. [Google Scholar] [CrossRef]
- Dong, F.Q.; Zhao, W.Z.; Zhang, Y.Z.; Wei, J.M.; Fan, W.Y.; Yu, Y.J. Influence of SBS and asphalt on SBS dispersion and the performance of modified asphalt. Constr. Build. Mater. 2014, 62, 1–7. [Google Scholar] [CrossRef]
- Costa, P.; Ribeiro, S.; Botelho, G.; Machado, A.V.; Mendez, S.L. Effect of butadiene/styrene ratio, block structure and carbon nanotube content on the mechanical and electrical properties of thermoplastic elastomers after UV ageing. Polym. Test. 2015, 42, 225–233. [Google Scholar] [CrossRef]
- Liu, G.; Nielsen, E.; Komacka, J.; Greet, L.; Ven, M.V.D. Rheological and chemical evaluation on the ageing properties of SBS polymer modified bitumen: From the laboratory to the field. Constr. Build. Mater. 2014, 51, 244–248. [Google Scholar] [CrossRef]
- Geng, J.G.; Li, H.B.; Sheng, Y.P. Changing regularity of SBS in the aging process of polymer modified Asphalt Binder Based on GPC analysis. Int. J. Pavement Res. Technol. 2014, 7, 77–82. [Google Scholar]
- Cortizo, M.S.; Larsen, D.O.; Bianchetto, H.; Alessandrini, J.L. Effect of the thermal degradation of SBS copolymers during the ageing of modified asphalts. Polym. Degrad. Stab. 2004, 86, 275–282. [Google Scholar] [CrossRef]
- Wu, S.P.; Pang, L.; Mo, L.T.; Chen, Y.C.; Zhu, G.J. Influence of aging on the evolution of structure, morphology and rheology of base and SBS modified bitumen. Constr. Build. Mater. 2009, 23, 1005–1010. [Google Scholar] [CrossRef]
- Rabek, J.F.; Ranby, B. Studies on the photo-oxidative mechanism of polymers. VII. The role of singlet oxygen in the dye-photosensitized oxidation of cis-1, 4- and 1, 2-polybutadienes and butadiene-styrene copolymers. J. Appl. Polym. Sci. 1979, 23, 2481–2491. [Google Scholar] [CrossRef]
- Luengo, C.; Allen, N.S.; Edge, M.; Wilkinson, A.; Parellada, M.D.; Barrio, J.A.; Santa, V.R. Photo-oxidative degradation mechanisms in styrene-ethylene-butadiene-styrene (SEBS) triblock copolymer. Polym. Degrad. Stab. 2006, 91, 947–956. [Google Scholar] [CrossRef]
- Zeng, W.B.; Wu, S.P.; Wen, J.; Chen, Z.W. The temperature effects in aging index of asphalt during UV aging process. Constr. Build. Mater. 2015, 93, 1125–1131. [Google Scholar] [CrossRef]
- Xiao, F.P.; Newton, D.; Putman, B.; Amirkhanian, S.N. A long-term ultraviolet aging procedure on foamed WMA mixtures. Mater. Struct. 2013, 46, 1987–2001. [Google Scholar] [CrossRef]
- Kim, T.W.; Baek, J.; Lee, H.J.; Ji, Y.C. Fatigue performance evaluation of SBS modified mastic asphalt mixtures. Constr. Build. Mater. 2014, 48, 908–916. [Google Scholar] [CrossRef]
- Liang, M.; Liang, P.; Fan, W.; Qian, C.; Xin, X.; Shi, J.T.; Nan, G.Z. Thermo-rheological behavior and compatibility of modified asphalt with various styrene–butadiene structures in SBS copolymers. Mater. Des. 2015, 88, 177–185. [Google Scholar] [CrossRef]
- Serrano, E.; Zubeldia, A.; Larrañaga, M.; Remiro, P.; Mondragon, I. Effect of different thermal treatments on the self-assembled nanostructures of a styrene–butadiene–styrene star block copolymer. Polym. Degrad. Stab. 2004, 83, 495–507. [Google Scholar] [CrossRef]
- Shirini, B.; Imaninasab, R. Performance evaluation of rubberized and SBS modified porous asphalt mixtures. Constr. Build. Mater. 2016, 107, 165–171. [Google Scholar] [CrossRef]
- Cong, P.L.; Chen, S.F.; Yu, J.Y.; Wu, S.P. Effects of aging on the properties of modified asphalt binder with flame retardants. Constr. Build. Mater. 2010, 24, 2554–2558. [Google Scholar] [CrossRef]
- Cong, P.L.; Wang, X.; Xu, P.J.; Liu, J.F.; He, R.; Chen, S.F. Investigation on properties of polymer modified asphalt containing various antiaging agents. Polym. Degrad. Stab. 2013, 98, 2627–2634. [Google Scholar] [CrossRef]
- Xiao, F.P.; Amirkhanian, S.N.; Shen, J.N.; Putman, B. Influences of crumb rubber size and type on reclaimed asphalt pavement (RAP) mixtures. Constr. Build. Mater. 2009, 23, 1028–1034. [Google Scholar] [CrossRef]
- Moghaddam, T.B.; Baaj, H. The use of rejuvenating agents in production of recycled hot mix asphalt: A systematic review. Constr. Build. Mater. 2016, 114, 805–816. [Google Scholar] [CrossRef]
- Presti, D.L.; Carrión, A.J.D.B.; Airey, G. Towards 100% recycling of reclaimed asphalt in road surface courses: Binder design methodology and case studies. J. Clean. Product. 2016, 131, 43–51. [Google Scholar] [CrossRef]
- Liu, G.; Leegwater, G.; Nielsen, E.; Komacka, J.; Ven, M.V.D. Evaluating the rheological properties of PMB-containing RA binders from surface-layer asphalt mixtures to be recycled. Constr. Build. Mater. 2013, 49, 8–14. [Google Scholar] [CrossRef]
- Chen, M.Z.; Xiao, F.P.; Putman, B.; Leng, B.B.; Wu, S.P. High temperature properties of rejuvenating recovered binder with rejuvenator, waste cooking and cotton seed oils. Constr. Build. Mater. 2014, 59, 10–16. [Google Scholar] [CrossRef]
- Somé, S.C.; Gaudefroy, V.; Delaunay, D. Effect of vegetable oil additives on binder and mix properties: Laboratory and field investigation. Mater. Struct. 2016, 49, 2197–2208. [Google Scholar] [CrossRef]
- Cong, P.L.; Luo, W.H.; Xu, P.J.; Zhao, H. Investigation on recycling of SBS modified asphalt binders containing fresh asphalt and rejuvenating agents. Constr. Build. Mater. 2015, 91, 225–231. [Google Scholar] [CrossRef]
- Su, T.T.; Jiang, H.; Gong, H. Thermal stabilities and thermal degradation kinetics of a styrene-butadiene-styrene star block copolymer. Polym.-Plast. Technol. Eng. 2009, 48, 535–541. [Google Scholar] [CrossRef]
- Xu, J.B.; Zhang, A.M.; Zhou, T.; Cao, X.J.; Xie, Z.N. A study on thermal oxidation mechanism of styrene–butadiene–styrene block copolymer (SBS). Polym. Degrad. Stab. 2007, 92, 1682–1691. [Google Scholar] [CrossRef]
- Chiantore, O.; Tripodi, S.; Sarmoria, C.; Vallés, E. Mechanism and molecular weight model for thermal oxidation of linear ethylene–butene copolymer. Polymer 2001, 42, 3981–3987. [Google Scholar] [CrossRef]
- Wang, S.M.; Chang, J.R.; Tsiang, C.C. Infrared studies of thermal oxidative degradation of polystyrene- block, polybutadiene-block-polystyrene thermoplastic elastomers. Polym. Degrad. Stab. 1996, 52, 51–57. [Google Scholar] [CrossRef]
- Munteanu, S.B.; Brebu, M.; Vasile, C. Thermal and thermo-oxidative behaviour of butadiene–styrene copolymers with different architectures. Polym. Degrad. Stab. 2005, 89, 501–512. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, J.Y.; Feng, K.; Xue, L.H.; Xie, D. Synthesis and characterization of triethoxyvinylsilane surface modified layered double hydroxides and application in improving UV aging resistance of bitumen. Appl. Clay Sci. 2016, 120, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, J.Y.; Xue, L.H.; Sun, Y.B. Investigation of γ-(2,3-Epoxypropoxy) propyltrimethoxy silane surface modified layered double hydroxides improving UV ageing resistance of asphalt. Materials 2017, 10, 78. [Google Scholar] [CrossRef]
- Liu, P.; Mullins, M.; Bremner, T.; Browne, J.A.; Sue, H.J. Hygrothermal behavior of polybenzimidazole. Polymer 2016, 93, 88–98. [Google Scholar] [CrossRef]
- Priyadarshani1, N.; Benzine, C.W.; Cassidy, B.; Suriboot, J.; Liu, P.; Sue, H.J.; Bergbreiter, D.E. Polyolefin soluble polyisobutylene oligomer-bound metallophthalocyanine and azo dye additives. J. Polym. Sci. Pol. Chem. 2014, 52, 545–551. [Google Scholar] [CrossRef]
- Xu, X.; Yu, J.Y.; Zhang, C.L.; Xu, S.; Xue, L.H.; Xie, D. Investigation of aging behavior and thermal stability of styrene-butadiene-styrene tri-block copolymer in blends. Polymer 2016, 40, 947–953. [Google Scholar] [CrossRef]








| Samples | Relative Functional Group Index | ||||
|---|---|---|---|---|---|
| IC=C | IC–OH(R) | IC=O | |||
| I1639 | I967 | I3446 | I1050~1300 | I1695~1720 | |
| Neat SBS | 0.135 | 0.523 | 0 | 0.011 | 0 |
| Aged SBS (150 °C) | 0.128 | 0.509 | 0 | 0.029 | 0.009 |
| Aged SBS (180 °C) | 0.099 | 0.382 | 0.032 | 0.041 | 0.035 |
| Samples | Relative Concentration, % | ||||
|---|---|---|---|---|---|
| C–C(H) | C=C | C–OH(R) | C=O | COOH(R) | |
| 284.8 eV | 285.5 eV | 286.8 eV | 288.0 eV | 289.0 eV | |
| Neat SBS | 91.30 | 7.25 | 1.45 | 0 | 0 |
| Aged SBS (150 °C) | 90.22 | 7.17 | 2.25 | 0.24 | 0.12 |
| Aged SBS (180 °C) | 86.19 | 6.39 | 4.29 | 1.88 | 1.25 |
| Chemical State | Binding Energy (eV) | Relative Concentration (%) | ||
|---|---|---|---|---|
| Neat SBS | Aged SBS (12 h) | Aged SBS (48 h) | ||
| C–C(H) | 284.8 ± 0.1 | 91.30 | 90.14 | 85.11 |
| C=C | 285.5 ± 0.1 | 7.25 | 1.02 | 0.43 |
| C–O–H(R) | 286.8 ± 0.1 | 1.45 | 5.44 | 7.22 |
| C=O | 288.0 ± 0.1 | 0 | 2.58 | 4.68 |
| COOH(R) | 289.0 ± 0.1 | 0 | 0.82 | 2.56 |
| Samples | Tonset, °C | Td (max), °C | Ronset, %/min | Rd (max), %/min |
|---|---|---|---|---|
| Neat SBS | 329.8 | 460.1 | −0.10 | −15.3 |
| Aged SBS1 | 219.1 | 455.4 | −0.33 | −13.9 |
| Aged SBS2 | 217.9 | 446.2 | −0.45 | −12.3 |
| Aged SBS3 | 171.1 | 442.8 | −0.63 | −8.39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Yu, J.; Xue, L.; Zhang, C.; Zha, Y.; Gu, Y. Investigation of Molecular Structure and Thermal Properties of Thermo-Oxidative Aged SBS in Blends and Their Relations. Materials 2017, 10, 768. https://doi.org/10.3390/ma10070768
Xu X, Yu J, Xue L, Zhang C, Zha Y, Gu Y. Investigation of Molecular Structure and Thermal Properties of Thermo-Oxidative Aged SBS in Blends and Their Relations. Materials. 2017; 10(7):768. https://doi.org/10.3390/ma10070768
Chicago/Turabian StyleXu, Xiong, Jianying Yu, Lihui Xue, Canlin Zhang, Yagang Zha, and Yi Gu. 2017. "Investigation of Molecular Structure and Thermal Properties of Thermo-Oxidative Aged SBS in Blends and Their Relations" Materials 10, no. 7: 768. https://doi.org/10.3390/ma10070768




