Synthesis and Properties of Carbon Nanotube-Grafted Silica Nanoarchitecture-Reinforced Poly(Lactic Acid)
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussion
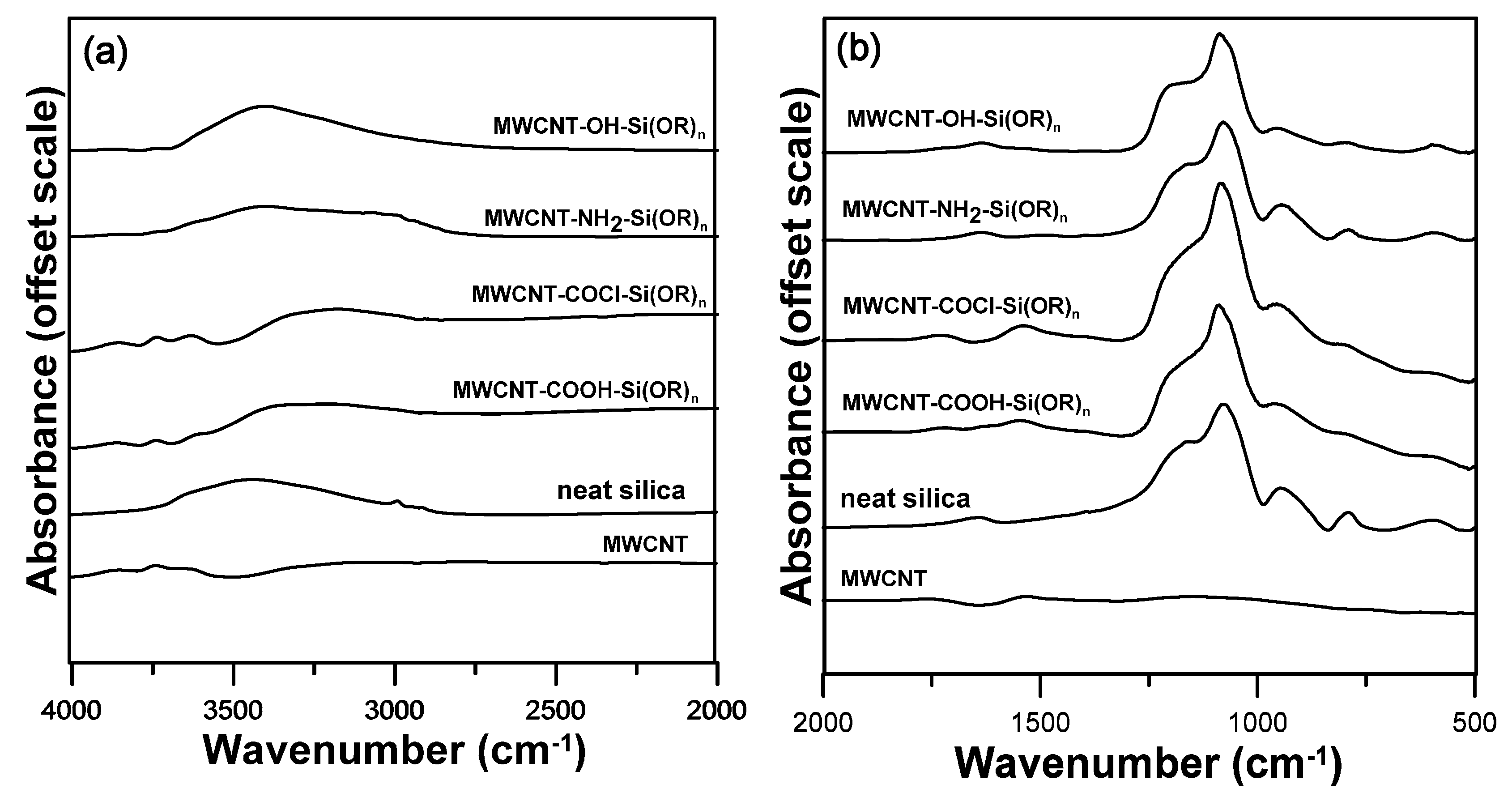
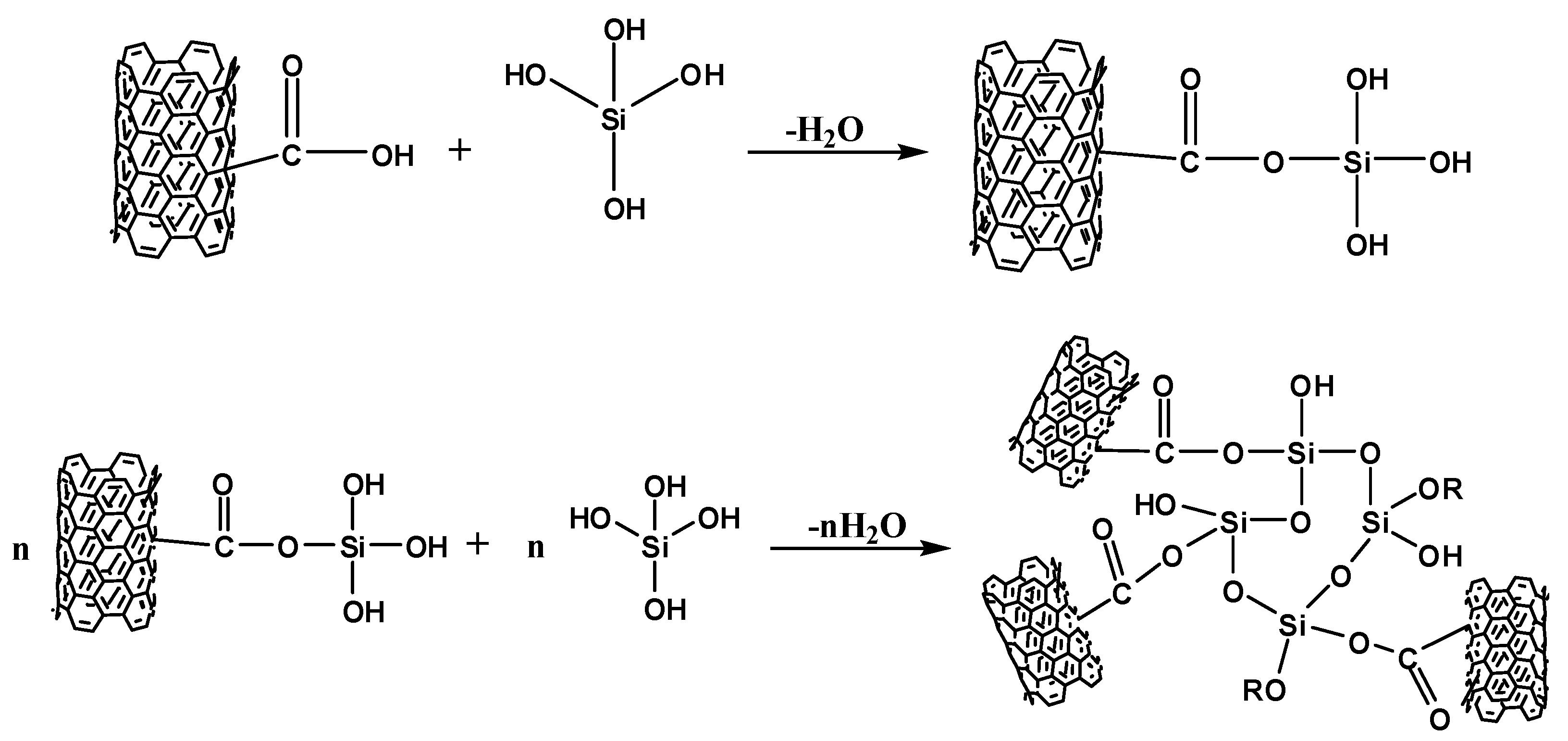
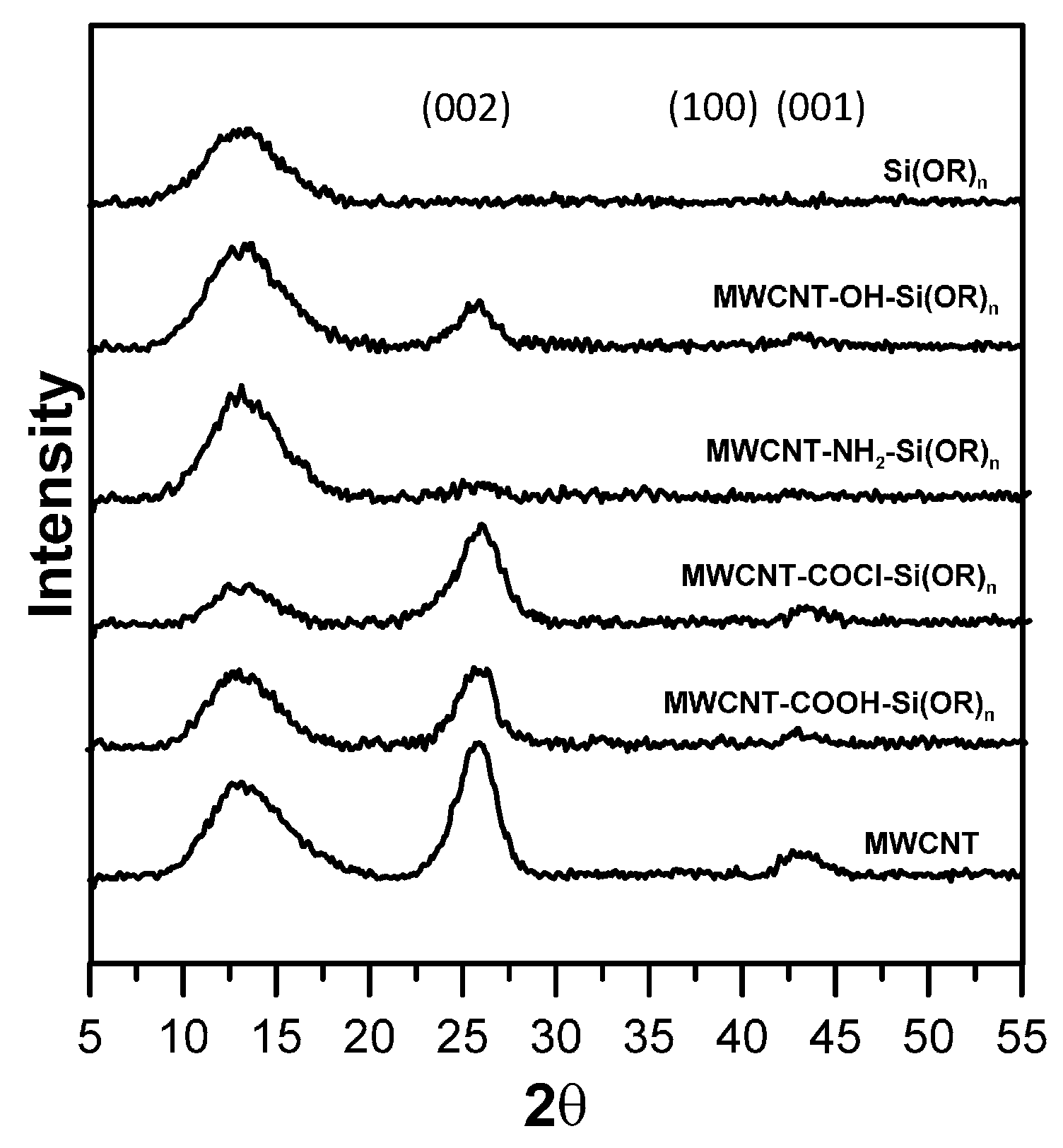
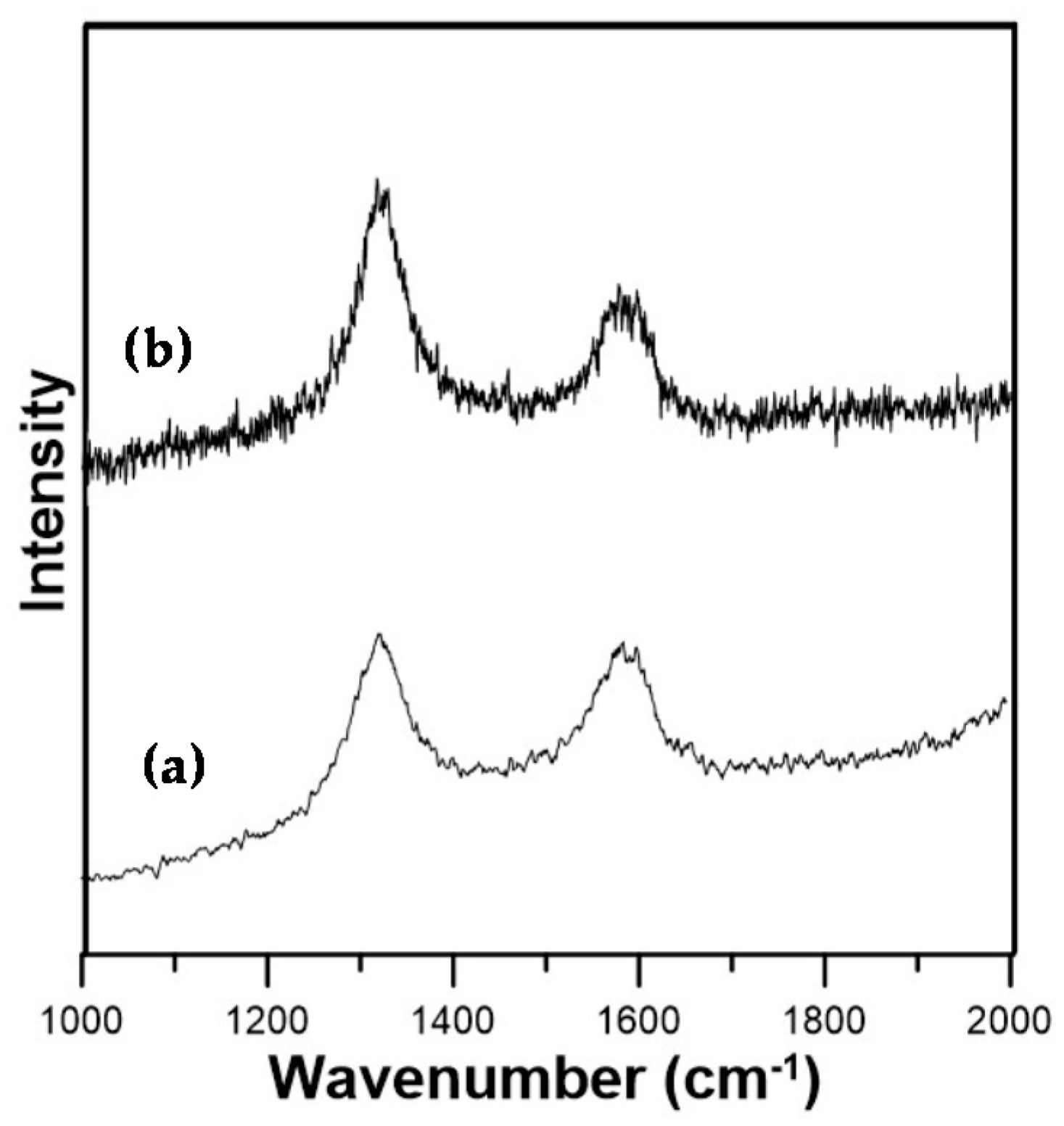
3.1. Analysis of Surface-Functionalized MWCNTs
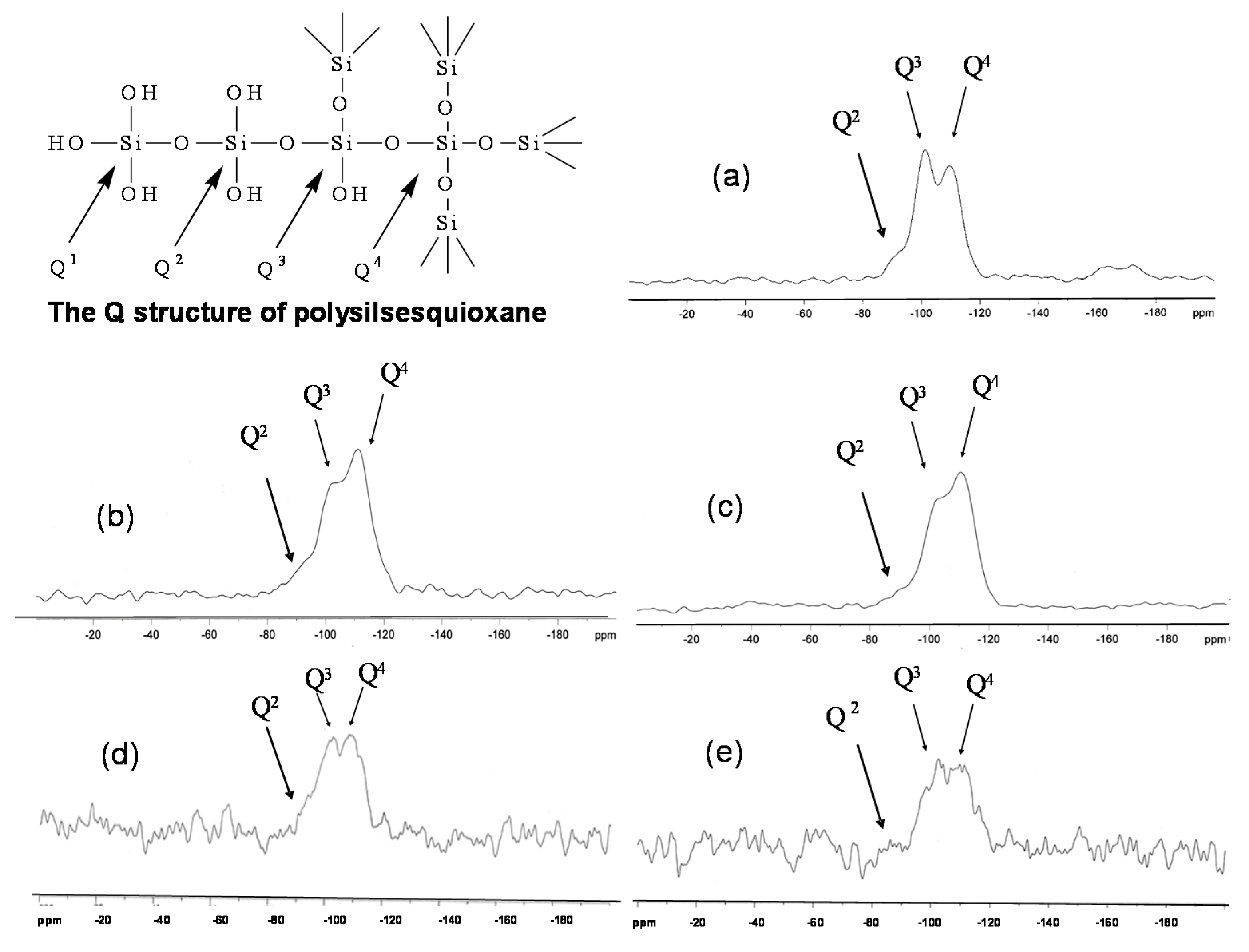
3.2. Characterization of MWCNT-Grafted Silica Nanohybrids
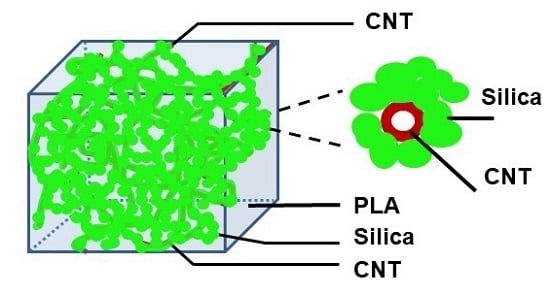
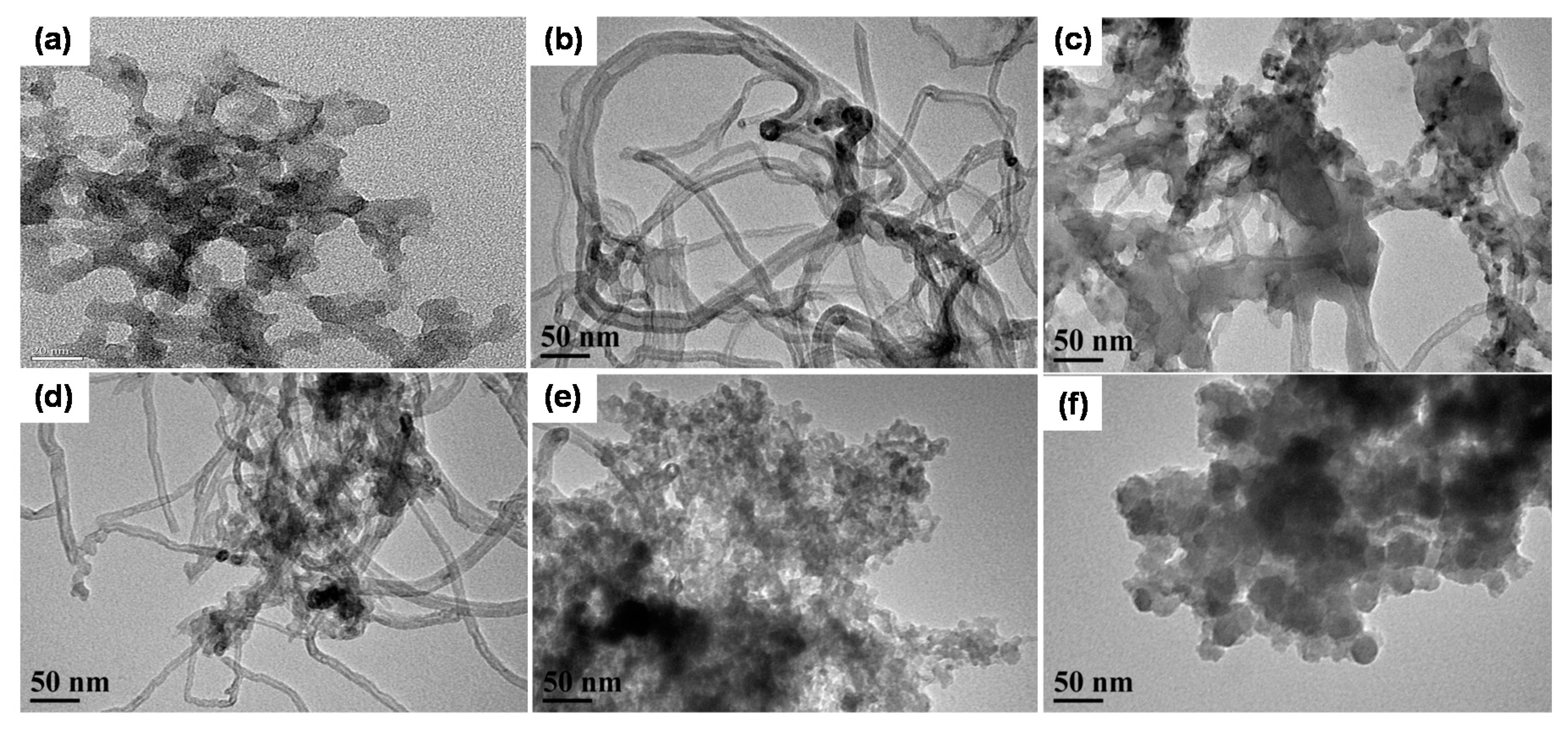
3.3. Morphology of CNT-Grafted Silica Nanohybrids
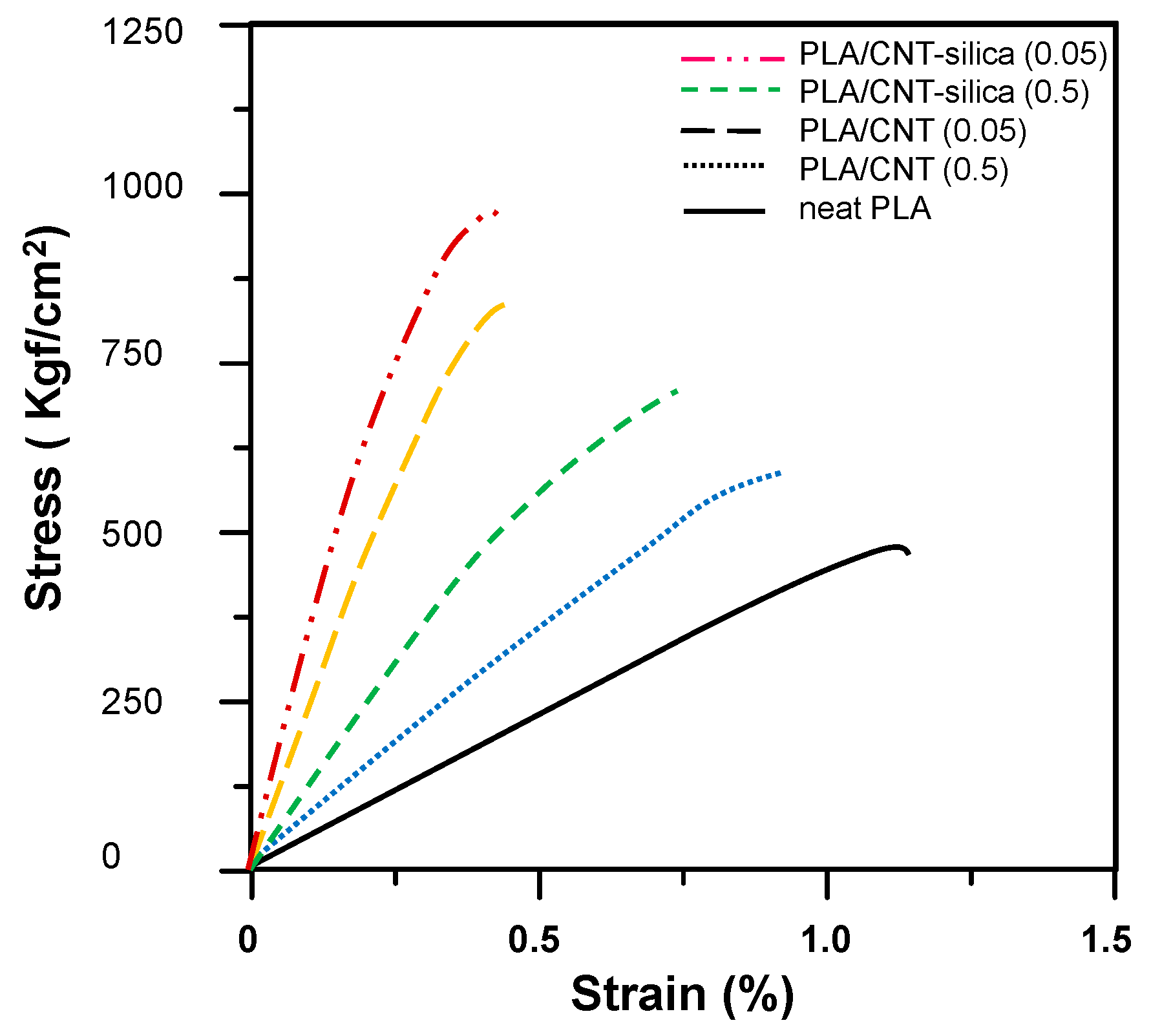
3.4. PLA/MWCNT-Grafted Silica Nanocomposites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaushik, B.K.; Majumder, M.K. Carbon Nanotube Based VLSI Interconnects Analysis and Design; Springer Inc.: New York, NY, USA, 2015; pp. 18–25. [Google Scholar]
- Wang, X. Fabrication of ultralong and electrically uniform single-walled carbon nanotubeson clean substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.K.; Kaushik, B.K.; Manhas, S.K. Performance comparison between single wall carbon nanotube bundle and multiwall carbon nanotube for global interconnects. In Proceedings of the IEEE international conference on networks and computer communications (ETNCC 2011), Udaipur, Rajasthan, India, 22–24 April 2011; pp. 104–109. [Google Scholar]
- Loos, M. Carbon Nanotube Reinforced Composites; Elsevier Inc.: Boston, MA, USA, 2015; Chapter 3; pp. 73–101. [Google Scholar]
- Mintmire, J.W.; White, C.T. Electronic and structural properties of carbon nanotubes. Carbon 1995, 33, 893–902. [Google Scholar] [CrossRef]
- Su, C.C.; Wu, C.C.; Yang, C.F. Developing the dielectric mechanisms of polyetherimide/multiwalled carbon nanotube/(Ba0.8Sr0.2)(Ti0.9Zr0.1)O3 composites. Nanoscale Res. Lett. 2012, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Hsu, W.C.; Wu, S.M.; Su, C.C. Elucidating How Surface Functionalization of Multiwalled Carbon Nanotube Affects Nanostructured MWCNT/titania Hybrid Materials. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Si, H.Y.; Liu, C.H.; Xu, H.; Wang, T.M.; Zhang, H.L. Shell-Controlled Photoluminescence in CdSe/CNT Nanohybrids. Nanoscale Res. Lett. 2009, 4, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Olin, H. Gold-carbon nanotube nanocomposites: Synthesis and applications. Int. J. Biomed Nanosci. Nanotechnol. 2011, 2, 112–135. [Google Scholar] [CrossRef]
- Kim, M.; Hong, J.; Lee, J.; Hong, C.K.; Shim, S.E. Fabrication of silica nanotubes using silica coated multi-walled carbon nanotubes as the template. J. Colloid Interface Sci. 2008, 322, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hong, J.; Hong, C.K.; Shim, S.E. Preparation of silica-layered multi-walled carbon nanotubes activated by grafting of poly(4-vinylpyridine). Synth. Met. 2009, 159, 62–68. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Feng, X.; He, X.; Chen, L.; Zhang, Y. Fabrication of mesoporous silica-coated CNTs and application in size-selective protein separation. J. Mater. Chem. 2010, 20, 5835–5842. [Google Scholar] [CrossRef]
- Fu, H.; Lu, C.G.; Liu, J. Selective Coating of Single Wall Carbon Nanotubes with Thin SiO2 Layer. Nano Lett. 2002, 2, 329–332. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.S.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution properties of single-walled carbon nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Hemraj-Benny, T.; Wong, S.S. Covalent surface chemistry of single-walled carbon nanotubes. Adv. Mater. 2005, 17, 17–29. [Google Scholar] [CrossRef]
- Marsh, D.H.; Rance, G.A.; Whitby, R.J.; Giustiniano, F.; Khlobystov, A.N. Assembly, structure and electrical conductance of carbon nanotube-gold nanoparticle 2D heterostructures. J. Mater. Chem. 2008, 18, 2249–2256. [Google Scholar] [CrossRef]
- Coleman, K.S.; Bailey, S.R.; Fogden, S.; Green, M.L. Functionalization of single-walled carbon nanotubes via the Bingel reaction. J. Am. Chem. Soc. 2003, 125, 8722–8723. [Google Scholar] [CrossRef] [PubMed]
- Zanella, R.; Basiuk, E.; Santiago, P.; Basiuk, V.; Mireles, E.; Puente-Lee, I.; Saniger, J.M. Deposition of gold nanoparticles onto thiol-functionalized multiwalled carbon nanotubes. J. Phys. Chem. B 2005, 109, 16290–16295. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Z.; Li, H. Self-assembly of gold nanoparticles onto the surface of multiwall carbon nanotubes functionalized with mercaptobenzene moieties. J. Nanopart. Res. 2006, 8, 743–747. [Google Scholar] [CrossRef]
- Yang, C.F.; Wang, L.F.; Wu, S.M.; Su, C.C. Characterization and Curing Kinetics of Epoxy/Silica Nano-Hybrids. Materials 2015, 8, 7032–7040. [Google Scholar] [CrossRef]
- Wen, Z.; Ci, S.; Mao, S.; Cui, S.; He, Z.; Chen, J. CNT@TiO2 nanohybrids for high-performance anode of lithium-ion batteries. Nanoscale Res. Lett. 2013, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, X.; Lu, S. Shape-controlled synthesis of TiO2 hollow structures and their application in lithium batteries. J. Mater. Chem. 2012, 22, 1969–1976. [Google Scholar] [CrossRef]
- Vakili, H.; Ramezanzadeh, B.; Amini, R. The corrosion performance and adhesion properties of the epoxy coating applied on the steel substrates treated by cerium-based conversion coatings. Corros. Sci. 2015, 94, 466–475. [Google Scholar] [CrossRef]
- Su, C.C.; Wei, C.H.; Li, B.C. Thermal and cure kinetics of epoxy molding compounds cured with thermal latency accelerators. Adv. Mater. Sci. Eng. 2013, 2016, 391267. [Google Scholar] [CrossRef]
- Subasri, R.; Madhav, C.S.; Somaraju, K.R.C.; Padmanabham, G. Decorative, hydrophobic sol-gel coatings densified using near-infrared radiation. Surf. Coat. Technol. 2012, 206, 2417–2421. [Google Scholar] [CrossRef]
- Swain, S.; Sharma, R.A.; Patil, S.; Bhattacharya, S.; Gadiyaram, S.P.; Chaudhari, L. Effect of Allyl Modified/Silane Modified Multiwalled Carbon Nano Tubes on the Electrical Properties of Unsaturated Polyester Resin Composites. Trans. Electr. Electron. Mater. 2012, 13, 267–272. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M. Philippe Dubois, Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and processes for a sustainable chemical industry: A review of achievements and prospects. Green Chem. 2004, 6, 544–556. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.G.; Kim, S.H. Isothermal crystallization behavior and mechanical propertiesof polylactide/carbon nanotube nanocomposites. Compos. Part A Appl. Sci. Manuf. 2013, 46, 11–18. [Google Scholar] [CrossRef]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversionalapproach. Polym. Degrad. Stabil. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Lv, P.; Almeida, G.; Perré, P. TGA-FTIR Analysis of Torrefaction of Lignocellulosic Components (cellulose, xylan, lignin) in Isothermal Conditions over a Wide Range of Time Durations. Bioresources 2015, 10, 4239–4251. [Google Scholar] [CrossRef]
- Jianhua, L.; Joseph, A.D.; Gary, E.M. Chemistry of the Silica Surface: Liquid-Solid Reactions of Silica Gel with Trimethylaluminum. J. Am. Chem. Soc. 2006, 128, 17093–17101. [Google Scholar]
- Puurunen, R.L.; Root, A.; Haukka, S.; Iiskola, E.I.; Lindblad, M.; Krause, A.O.I. IR and NMR Study of the Chemisorption of Ammonia on Trimethylaluminum-Modified Silica. J. Phys. Chem. B 2000, 104, 6599–6609. [Google Scholar] [CrossRef]
- Zhang, K.; Lim, J.Y.; Choi, H.J. Amino functionalization and characteristics of multi-walled carbon nanotube/poly(methyl methacrylate) nanocomposite. Diam. Relat. Mater. 2009, 18, 316–318. [Google Scholar] [CrossRef]
- Sakka, S.; Kamiya, K. Glasses from metal alcoholates. J. Non-Cryst. Solids 1980, 42, 403–421. [Google Scholar] [CrossRef]
- Jiang, Q.; Qu, M.Z.; Zhou, G.M.; Zhang, B.L.; Yu, Z.L. A study of activated carbon nanotubes as electrochemical supercapacitors electrode materials. Mater. Lett. 2002, 57, 988–991. [Google Scholar] [CrossRef]
- Tam, N.T.T.; Nghia, N.X.; Quynh, N.T.; Khoi, P.H.; Minh, P.N. Analyzing the purity of carbon nanotubes by using different methods. J. Korean Phys. Soc. 2008, 52, 1382–1385. [Google Scholar] [CrossRef]
- Chow, W.S.; Mohd Ishak, Z.A. Polyamide blend-based nanocomposites: A review. Exp. Polym. Lett. 2015, 9, 211–232. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Mittal, V. Manufacturing of Nanocomposites with Engineering Plastics; Elsevier: New York, NY, USA, 2015; pp. 234–236. [Google Scholar]
- Wootthikanokkhan, J.; Cheachun, T.; Sombatsompop, N.; Thumsorn, S.; Kaabbuathong, N.; Wongta, N.; Wong-On, J.; Isarankura Na Ayutthaya, S.; Kositchaiyong, A. Crystallization and thermomechanical properties of PLA composites: Effects of additive types and heat treatment. J. Appl. Polym. Sci. 2013, 129, 215–223. [Google Scholar] [CrossRef]
- Chen, G.G.Q. Plastics from Bacteria: Natural Functions and Applications; Springer: New York, NY, USA, 2010; pp. 336–337. [Google Scholar]
- Grady, B.P.; Pompeo, F.; Shambaugh, R.L.; Resasco, D.E. Nucleation of Polypropylene Crystallization by Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2002, 106, 5852–5858. [Google Scholar] [CrossRef]
- Lu, K.; Grossiord, N.; Koning, C.E.; Miltner, H.E.; Mele, B.; Loos, J. Carbon Nanotube/Isotactic Polypropylene Composites Prepared by Latex Technology: Morphology Analysis of CNT-Induced Nucleation. Macromolecules 2008, 41, 8081–8085. [Google Scholar] [CrossRef]
- Avalos-Belmontes, F.; Ramos-deValle, L.F.; Espinoza-Martínez, A.B.; Martínez-Colunga, J.G.; Ramírez-Vargas, E.; Sánchez-Valdés, S.; Ortíz-Cisneros, J.C.; Martínez-Segovia, E.E.; Beltrán-Ramírez, F.I. Effect of Different Nucleating Agents on the Crystallization of Ziegler-Natta Isotactic Polypropylene. Int. J. Polym. Sci. 2016, 2016, 9839201. [Google Scholar] [CrossRef]
- Vaaben, S.R.; Aguilar, A.; Avalos, F.; Valle, L.F.R. Carbon nanoparticles as effective nucleating agents for polypropylene. J. Therm. Anal. Calorim. 2008, 93, 1–10. [Google Scholar]
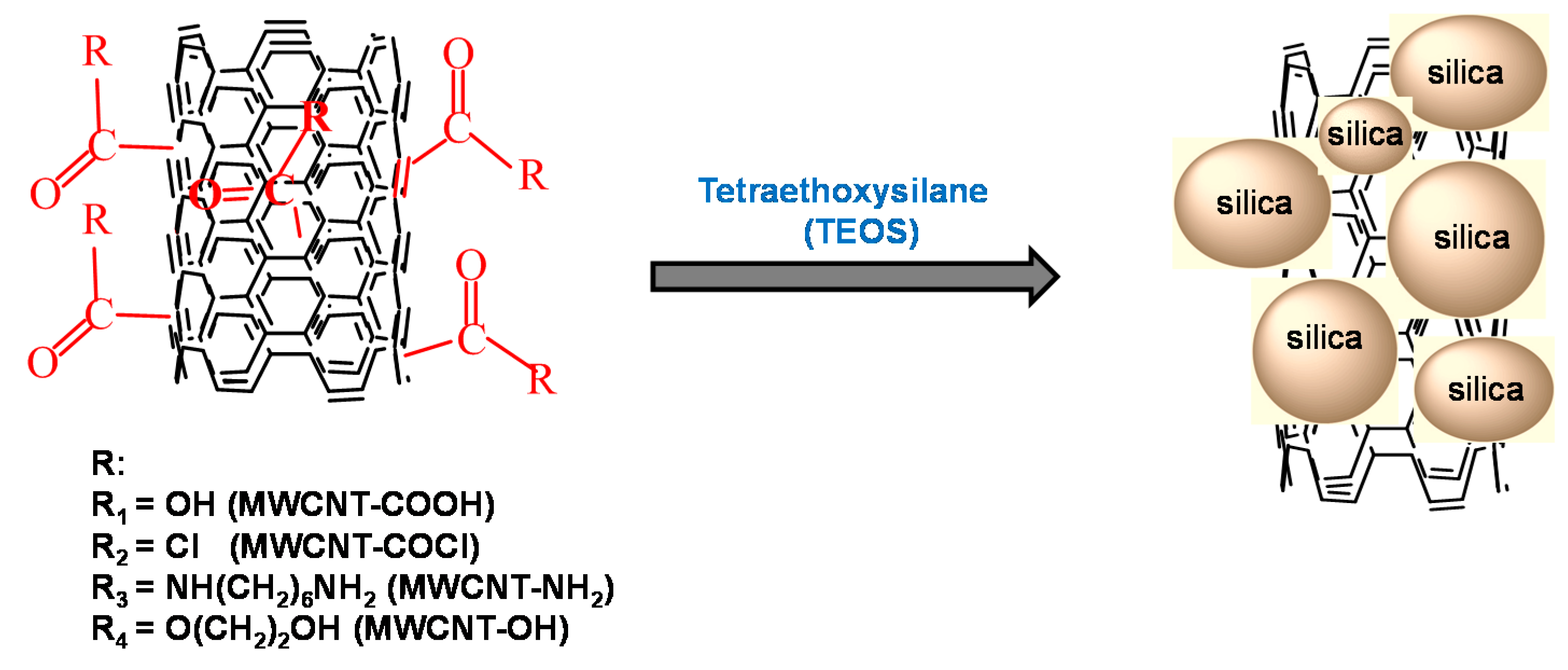
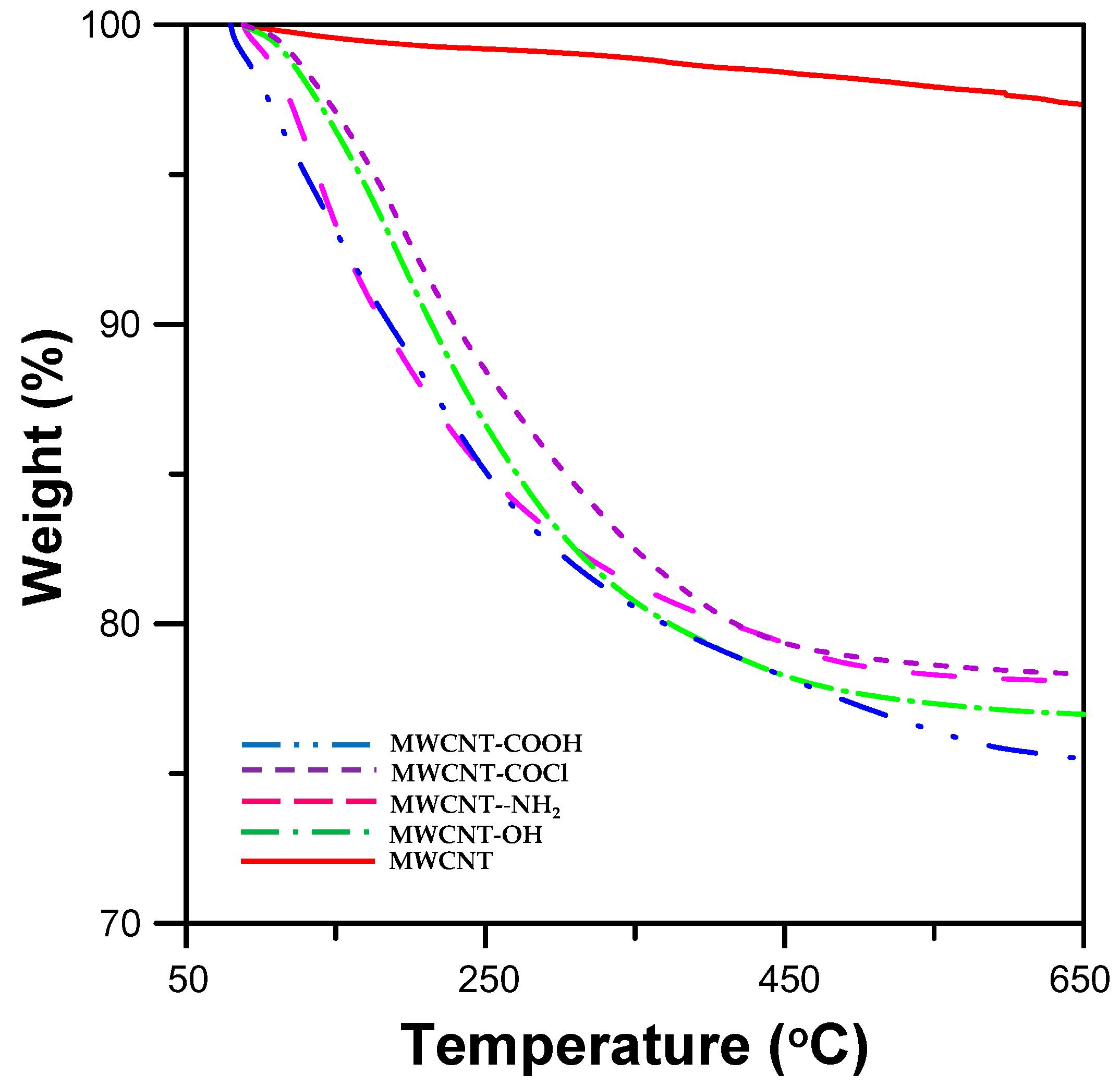

| Surface-Functionalized MWCNTs | Mass Loss of Surface-Functionalized MWCNT (wt %) | Degree of Surface-Functionalization of MWCNTs (mol %) |
|---|---|---|
| MWCNT-COOH | 19.23% | 0.37 |
| MWCNT-COCl | 18.16% | 0.25 |
| MWCNT-CONH(CH2)6NH2 | 18.35% | 0.11 |
| MWCNT-COO(CH2)2OH | 20.02% | 0.21 |
| MWCNT | 2.50% | --- |
| Properties | Neat PLA | PLA/MWCNT-Silica | PLA/MWCNT | ||
|---|---|---|---|---|---|
| 0.05 wt % | 0.5 wt % | 0.05 wt % | 0.5 wt % | ||
| Tensile strength (Kgf/cm2) | 470.6 | 710.3 | 975.6 | 615.9 | 856.3 |
| Elongation (%) | 1.2 | 0.8 | 0.5 | 0.9 | 0.5 |
| Surface resistivity (ohms/sq) | 2.0 × 1012 | 2.6 × 107 | 4.7 × 104 | 8.8 × 107 | 4.5 × 104 |
| HDT (°C) | 95.1 | 134.2 | 143.8 | 130.5 | 136.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-W.; Wu, C.-C.; Wu, S.-M.; Su, C.-C. Synthesis and Properties of Carbon Nanotube-Grafted Silica Nanoarchitecture-Reinforced Poly(Lactic Acid). Materials 2017, 10, 829. https://doi.org/10.3390/ma10070829
Hsu Y-W, Wu C-C, Wu S-M, Su C-C. Synthesis and Properties of Carbon Nanotube-Grafted Silica Nanoarchitecture-Reinforced Poly(Lactic Acid). Materials. 2017; 10(7):829. https://doi.org/10.3390/ma10070829
Chicago/Turabian StyleHsu, Yao-Wen, Chia-Ching Wu, Song-Mao Wu, and Chean-Cheng Su. 2017. "Synthesis and Properties of Carbon Nanotube-Grafted Silica Nanoarchitecture-Reinforced Poly(Lactic Acid)" Materials 10, no. 7: 829. https://doi.org/10.3390/ma10070829