Construction and Characterization of a Chitosan-Immobilized-Enzyme and β-Cyclodextrin-Included-Ferrocene-Based Electrochemical Biosensor for H2O2 Detection
Abstract
:1. Introduction
2. Results and Discussion
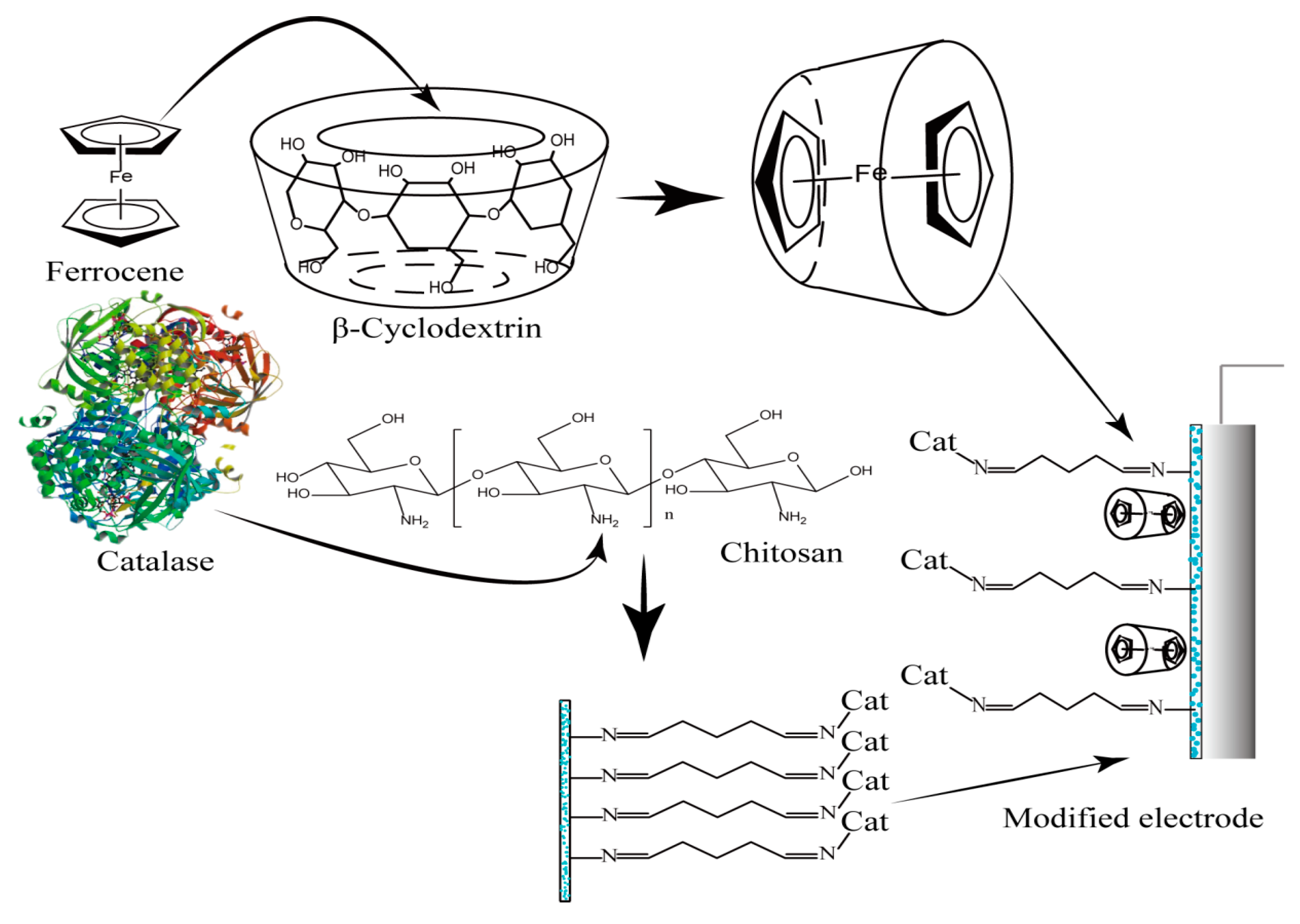
2.1. Characterization of the Ferrocene-Included Complex
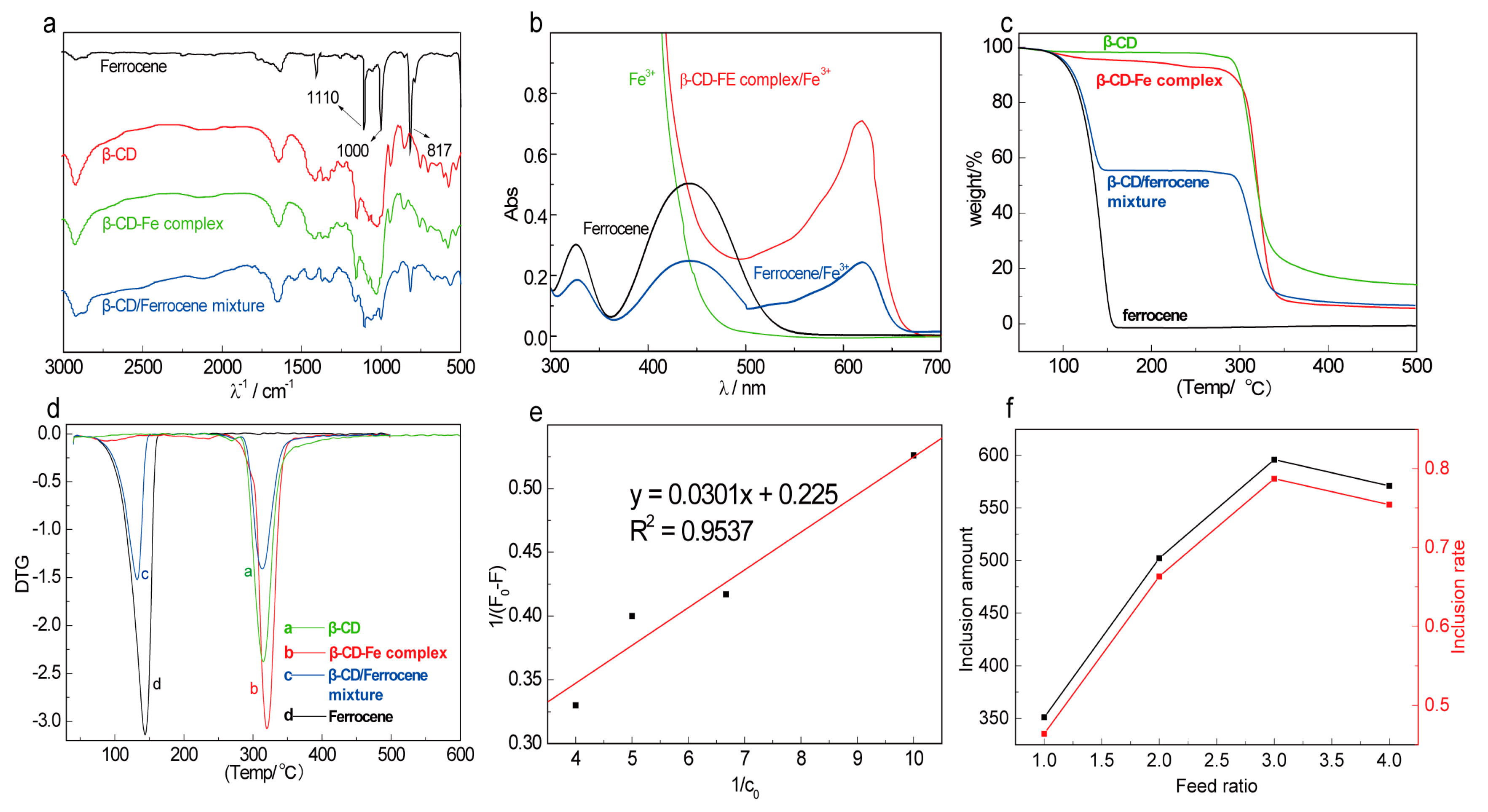
2.1.1. Spectral Characterization of β-CD-FE
2.1.2. Thermal Stability of β-CD-FE
2.1.3. Determination of Inclusion Constant, Inclusion Amount, and Inclusion Rate of β-CD-FE
2.1.4. Electrochemical Properties of β-CD-FE Inclusion Complex
2.2. Optimization of Preparation Conditions for CTS-CAT
2.2.1. Effects of Glutaraldehyde on Enzyme Activity
2.2.2. Effects of the Chitosan Modification Temperature on Enzyme Activity
2.2.3. Effects of the Chitosan Modification Time on Enzyme Activity
2.2.4. Effects of the Enzyme Amount on Enzyme Activity
2.2.5. Effects of the Immobilization Temperature on Enzyme Activity
2.2.6. Effects of the Immobilization Time on Enzyme Activity
2.3. Properties of the CTS-CAT/β-CD-FE Composite Electrode for H2O2 Detection
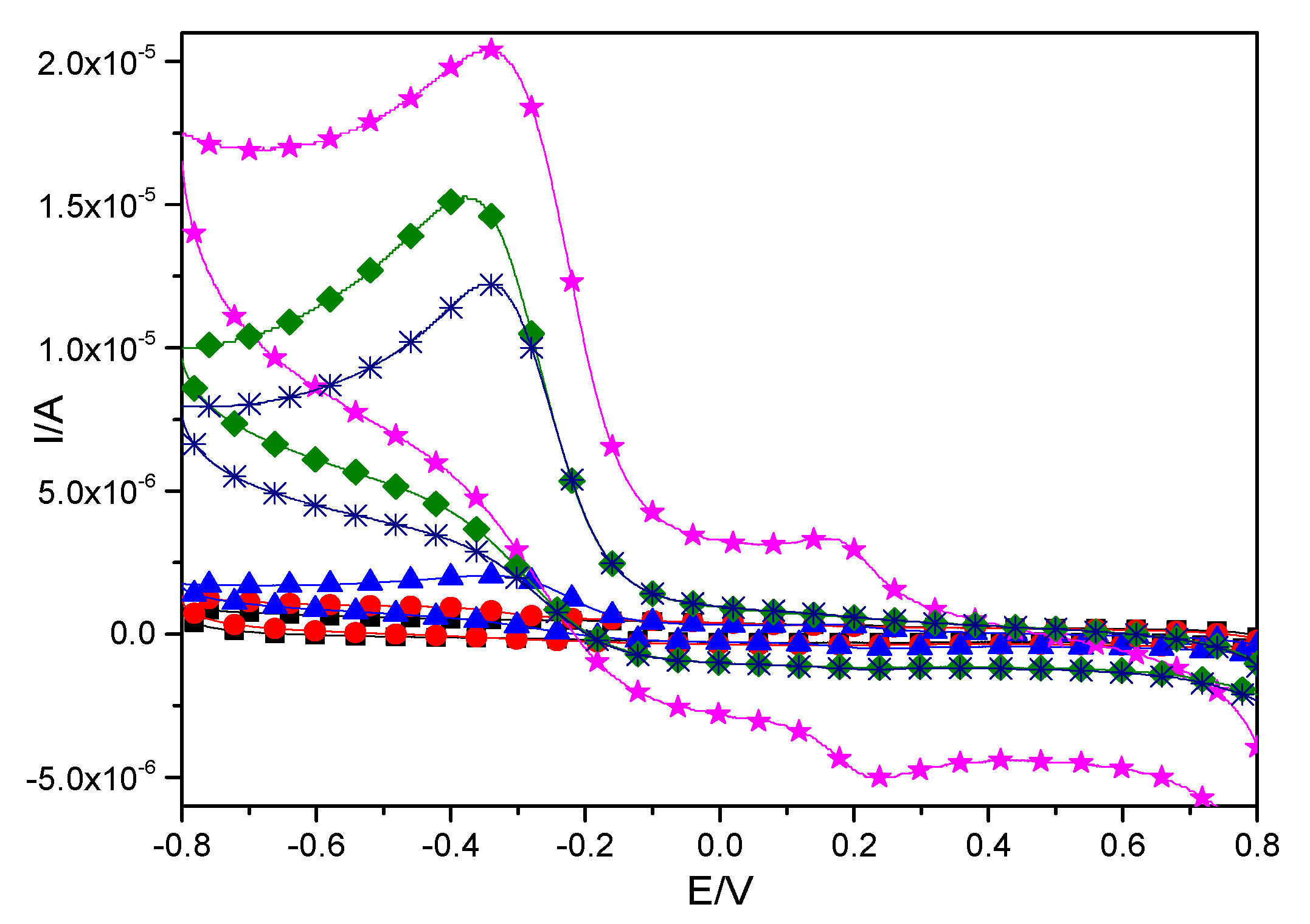
2.3.1. Oxidation-Reduction Properties of CTS-CAT/β-CD-FE Composite Electrodes
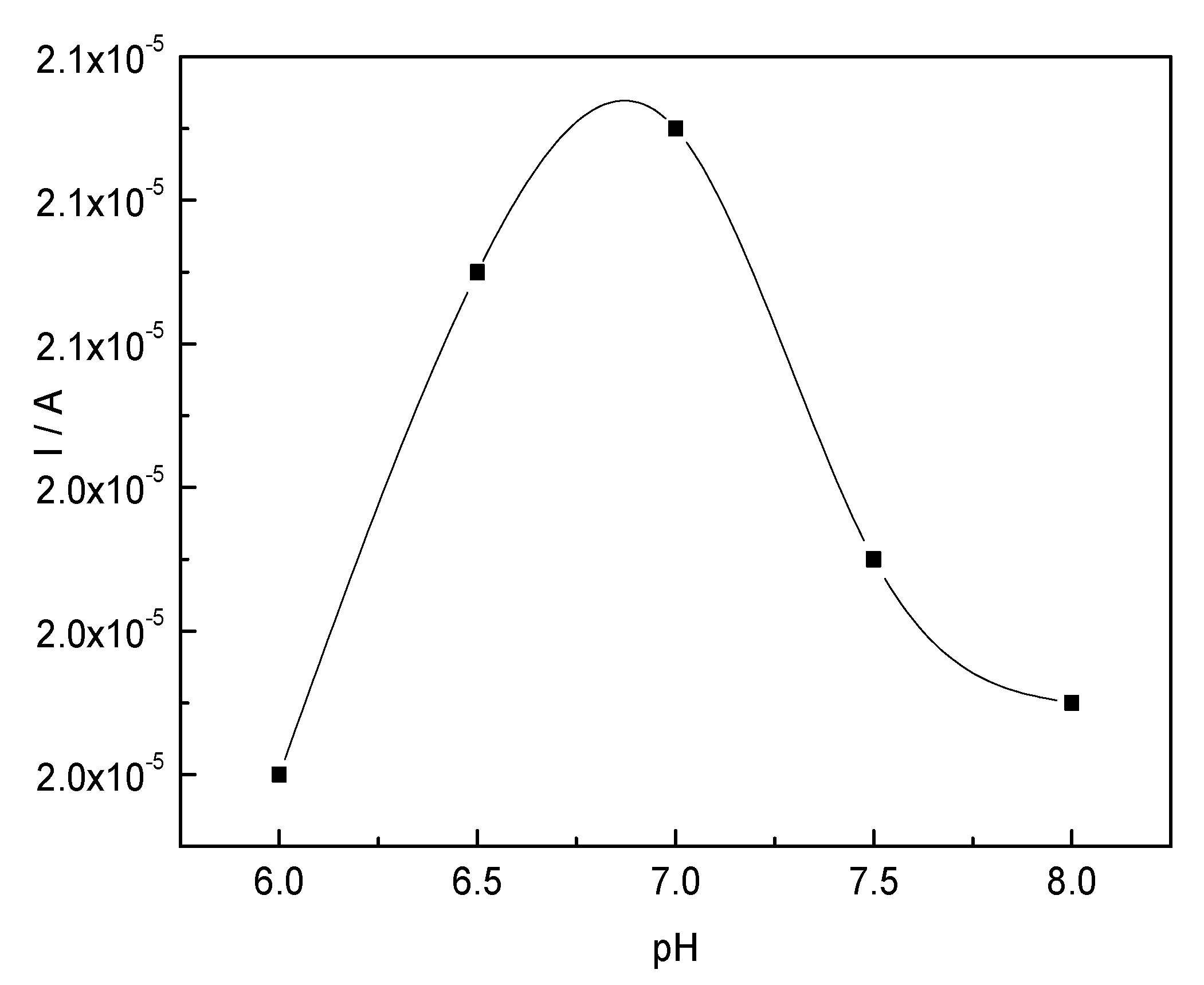
2.3.2. Effect of pH on the CTS-CAT/β-CD-FE Composite Electrode
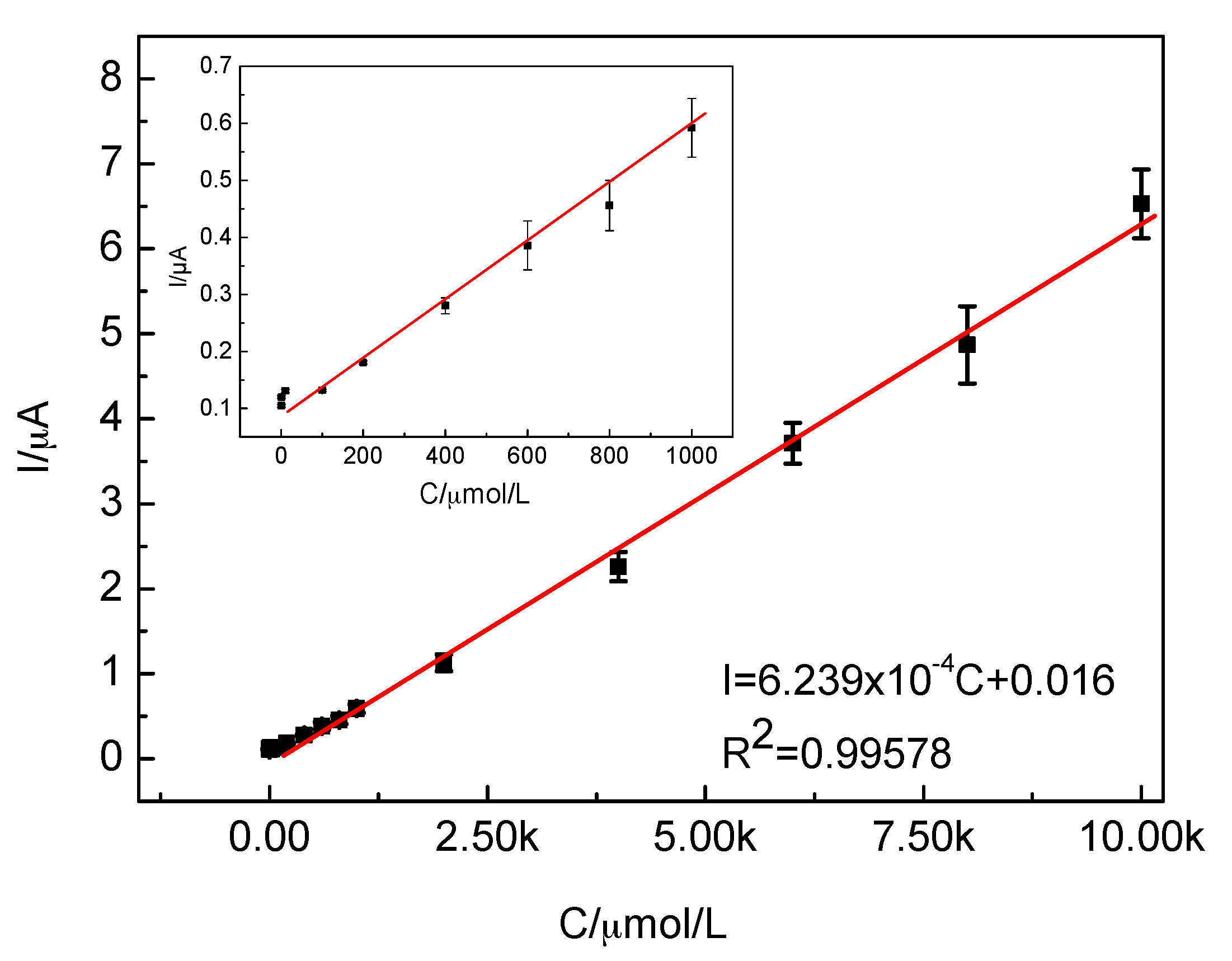
2.3.3. Sensitivity of the CTS-CAT/β-CD-FE Composite Electrodes
3. Materials and Methods
3.1. Materials
3.2. Inclusion of Ferrocene in β-CD
3.3. Determination of Ferrocene Inclusion Constant, Inclusion Amount, and Inclusion Ratio
3.4. Electrochemical Properties of β-CD-FE
3.5. Preparation of CTS-CAT
3.6. Determination of the Enzyme Activity of CTS-CAT
3.7. Preparation of the CTS-CAT/β-CD-FE Composite Electrode
3.8. Electrochemical Properties of the CTS-CAT/β-CD-FE Composite Electrode
3.9. Sensitivity of the CTS-CAT/β-CD-FE Composite Electrode
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Feng, F.; Wang, X.; Zhou, M.; Zhao, Y.; Ge, C.; Wang, K. The preparation of Fe2O3 nanoparticles by liquid phase-based ultrasonic-assisted method and its application as enzyme-free sensor for the detection of H2O2. RSC Adv. 2015, 5, 21161–21169. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Tan, W.; Gu, G.; Guo, Z. Novel amino-pyridine functionalized chitosan quaternary ammonium derivatives: Design, synthesis, and antioxidant activity. Molecules 2017, 22, 156. [Google Scholar] [CrossRef] [PubMed]
- Villareal, T.A.; Altabet, M.A.; Culverrymsza, K. Nitrogen transport by vertically migrating diatom mats in the north Pacific Ocean. Nature 1993, 363, 709–712. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.S.; Jeong, W.; Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cai, Z.; Gao, Y.; Zhang, H.; Cai, C. Enhancing the electrochemical reduction of hydrogen peroxide based on nitrogen-doped graphene for measurement of its releasing process from living cells. Chem. Commun. 2011, 47, 11327. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cai, H.; Yin, B.; Yao, P. Cholic acid modified n-(2-hydroxy)-propyl-3-trimethylammonium chitosan chloride for superoxide dismutase delivery. Int. J. Pharm. 2013, 454, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wu, S.; Li, Y.; Wan, G. Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim. Acta 2015, 182, 265–272. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Kim, G.; Lee, Y.E.K.; Xu, H.; Philbert, M.A.; Kopelman, R. Nanoencapsulation method for high selectivity sensing of hydrogen peroxide inside live cells. Anal. Chem. 2010, 82, 2165–2169. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, A.J.S. Hydrogen peroxide biosensors based on horseradish peroxidase and hemoglobin. J. Biosens. Bioelectron. 2013. [Google Scholar] [CrossRef]
- Prasad, A.; Kikuchi, H.; Inoue, K.Y.; Suzuki, M.; Sugiura, Y.; Sugai, T.; Tomonori, A.; Tada, M.; Kobayashi, M.; Matsue, T.; et al. Simultaneous real-time monitoring of oxygen consumption and hydrogen peroxide production in cells using our newly developed chip-type biosensor device. Front. Physiol. 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.Y.; Ino, K.; Shiku, H.; Kasai, S.; Yasukawa, T.; Mizutani, F.; Matsuea, T. Electrochemical monitoring of hydrogen peroxide released from leucocytes on horseradish peroxidase redox polymer coated electrode chip. Biosens. Bioelectron. 2010, 25, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, J.; Matharu, Z.; Revzin, A. Electrochemical biosensors for on-chip detection of oxidative stress from cells. Methods Enzymol. 2013, 526, 107–121. [Google Scholar] [PubMed]
- Tian, F.M.; Xu, B.; Zhu, L.D.; Zhu, G.Y. Hydrogen peroxide biosensor with enzyme entrapped within electrodeposited polypyrrole based on mediated sol–gel derived composite carbon electrode. Anal. Chim. Acta 2001, 443, 9–16. [Google Scholar] [CrossRef]
- Dhyani, H.; Ali, A.; Pal, S.P.; Srivastava, S.; Solanki, P.R.; Malhotra, B.D.; Sen, P. Mediator-free biosensor using chitosan capped cds quantum dots for detection of total cholesterol. RSC Adv. 2015, 5, 45928–45934. [Google Scholar] [CrossRef]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]
- Wang, D.L.; Jia, M.C.; Wang, L.Y.; Song, S.; Feng, J.T.; Zhang, X. Chitosan and β-cyclodextrin-epichlorohydrin polymer composite film as a plant healthcare material for carbendazim-controlled release to protect rape against Sclerotinia sclerotiorum (Lib.) de Bary. Materials 2017, 10, 343. [Google Scholar] [CrossRef]
- Sajomsang, W.; Nuchuchua, O.; Gonil, P.; Saesoo, S.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Water-soluble β-cyclodextrin grafted with chitosan and its inclusion complex as a mucoadhesive eugenol carrier. Carbohydr. Polym. 2012, 89, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Mi, J.; Wu, X.; Luo, C.; Li, J.; Tang, Y.; Li, J. Structural features and bioactivities of the chitosan. Int. J. Biol. Macromol. 2011, 49, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Pattar, V.P. Electroanalytical method for the determination of 5-fluorouracil using reduced graphene oxide/chitosan modified sensor. RSC Adv. 2015, 5, 34292–34301. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Wang, S.; Li, J.; Tang, J.; Wang, S.; Wang, Y. Sensitive and selective detection of Hg2+ based on an electrochemical platform of PDDA functionalized rGO and glutaraldehyde cross-linked chitosan composite film. RSC Adv. 2016, 6, 69815–69821. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Sakuragawa, A.; Taniai, T.; Okutani, T. Fluorometric determination of microamounts of hydrogen peroxide with an immobilized enzyme prepared by coupling horseradish peroxidase to chitosan beads. Anal. Chim. Acta 1998, 374, 191–200. [Google Scholar] [CrossRef]
- Sajomsang, W.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Chaleawlertumpon, S.; Pimpha, N.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. A comparison of spacer on water-soluble cyclodextrin grafted chitosan inclusion complex as carrier of eugenol to mucosae. Carbohydr. Polym. 2013, 92, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ye, Y.; Gao, F.; Yuan, H.; Lan, M.; Lou, K.; Wang, W. Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. Int. J. Pharm. 2013, 446, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, Ö.; Demirkol, D.O.; Gülcemal, S.; Kılınç, A.; Timur, S.; Çetinkaya, B. Chitosan–ferrocene film as a platform for flow injection analysis applications of glucose oxidase and gluconobacter oxydans biosensors. Colloid Surf. B 2012, 100, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pepi, F.; Tata, A.; Garzoli, S.; Giacomello, P.; Ragno, R.; Patsilinakos, A.; Fusco, M.D.; D’Annibale, A.; Cannistraro, S.; Baldacchini, C. Chemically modified multiwalled carbon nanotubes electrodes with ferrocene derivatives through reactive landing. J. Phys. Chem. C 2011, 115, 4863–4871. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, X.; Zhao, H.; Xu, J.; Sun, Y. Reagentless amperometric glucose biosensor based on the immobilization of glucose oxidase on a ferrocene@NaY zeolite composite. Microchim. Acta 2011, 174, 281–288. [Google Scholar] [CrossRef]
- Singh, A.; Chowdhury, D.R.; Paul, A. A kinetic study of ferrocenium cation decomposition utilizing an integrated electrochemical methodology composed of cyclic voltammetry and amperometry. Analyst 2014, 139, 5747. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.J.; Chen, F.; Hou, X.Z.; Ma, X.Y. Advances in the Application of Ferrocene and Its Derivatives in Sensors. Appl. Chem. 2013, 30, 1393–1398. [Google Scholar]
- Şenel, M.; Çevik, E.; Abasıyanık, M.F. Amperometric hydrogen peroxide biosensor based on covalent immobilization of horseradish peroxidase on ferrocene containing polymeric mediator. Sens. Actuators B 2010, 145, 444–450. [Google Scholar] [CrossRef]
- Jiao, S.F.; Shen, R.X.; Fang, B. Preparation of ferrocene-containing chitosan derivative and its application to enzyme-based electrochemical biosensors. Chin. J. Anal. Lab. 2009, 28, 27–30. [Google Scholar]
- Ramírezsilva, M.T.; Palomarpardavé, M.; Coronaavendaño, S.; Romeroromo, M.; Alarcónangeles, G. Guest-host complex formed between ascorbic acid and β-cyclodextrin immobilized on the surface of an electrode. Molecules 2014, 19, 5952–5964. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. 2010, 19, 344–362. [Google Scholar] [CrossRef]
- Frasconi, M.; D’Annibale, A.; Favero, G.; Mazzei, F.; Santucci, R.; Ferri, T. Ferrocenyl alkanethiols−thio β-cyclodextrin mixed self-assembled monolayers: Evidence of ferrocene electron shuttling through the β-cyclodextrin cavity. Lungmuir 2009, 25, 12937–12944. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Jing, L.; Shuai, Q. Mn(ii) and Co(ii) complexes functionalized by imidazolyl-ferrocene: Electrochemical property and different supramolecular architectures driven by intriguing hydrogen-bonded system. Polyhedron 2017, 126, 252–261. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Ma, X.W.; Liang, W.T.; Dong, C.; Shan, S.M. Properties of β-Cyclodextrin and Derivatives with β-Carotene Inclusion Complexes. Appl. Chem. 2016, 33, 827–833. [Google Scholar]
- Matencio, A.; Garcíacarmona, F.; Lópeznicolás, J.M. The inclusion complex of oxyresveratrol in modified cyclodextrins: A thermodynamic, structural, physicochemical, fluorescent and computational study. Food Chem. 2017, 232, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.J.; Zhou, J.P. Determination and prediction of inclusion characteristics and inclusion constants of cyclodextrin. Pharm. Prog. 2005, 29, 491–497. [Google Scholar]
- Zarzycki, P.K.; Lamparczyk, H. The equilibrium constant of beta-cyclodextrin-phenolphtalein complex; influence of temperature and tetrahydrofuran addition. J. Pharmaceut. Biomed. 1998, 18, 165–170. [Google Scholar] [CrossRef]



| T/s | C/(mol/L) | V/(μL) | T/s | C/(mol/L) | V/(μL) | T/s | C/(mol/L) | V/(μL) |
|---|---|---|---|---|---|---|---|---|
| 150 | 1.0 × 10−4 | 5 | 300 | 1.0 × 10−1 | 10 | 420 | 1.0 × 10−1 | 50 |
| 180 | 1.0 × 10−3 | 5 | 330 | 1.0 × 10−1 | 10 | 450 | 1.0 × 10−1 | 100 |
| 210 | 1.0 × 10−2 | 5 | 360 | 1.0 × 10−1 | 10 | 480 | 1.0 × 10−1 | 100 |
| 240 | 1.0 × 10−1 | 5 | 390 | 1.0 × 10−1 | 10 | 510 | 1.0 × 10−1 | 100 |
| 270 | 1.0 × 10−1 | 5 | 420 | 1.0 × 10−1 | 50 | 540 | 1.0 × 10−1 | 100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Wang, K.; Chen, Y.; Li, W.; Ye, Y.; Jin, S. Construction and Characterization of a Chitosan-Immobilized-Enzyme and β-Cyclodextrin-Included-Ferrocene-Based Electrochemical Biosensor for H2O2 Detection. Materials 2017, 10, 868. https://doi.org/10.3390/ma10080868
Dong W, Wang K, Chen Y, Li W, Ye Y, Jin S. Construction and Characterization of a Chitosan-Immobilized-Enzyme and β-Cyclodextrin-Included-Ferrocene-Based Electrochemical Biosensor for H2O2 Detection. Materials. 2017; 10(8):868. https://doi.org/10.3390/ma10080868
Chicago/Turabian StyleDong, Wenbo, Kaiyin Wang, Yu Chen, Weiping Li, Yanchun Ye, and Shaohua Jin. 2017. "Construction and Characterization of a Chitosan-Immobilized-Enzyme and β-Cyclodextrin-Included-Ferrocene-Based Electrochemical Biosensor for H2O2 Detection" Materials 10, no. 8: 868. https://doi.org/10.3390/ma10080868




