1. Introduction
Self-healing materials are artificial materials that can self-heal after being damaged. Recently, self-healing materials have attracted much attention because they can extend material lifetime, reduce maintenance cost, and enhance public safety [
1,
2,
3,
4,
5,
6]. Self-healing materials can be classified broadly into two groups: intrinsic and extrinsic [
5]. Intrinsic-type materials possess a latent self-healing functionality that is triggered by damage or by an external stimulus, so they can heal damage by the materials themselves. In extrinsic-type self-healing materials, the damage is recovered by the release of healing agent from microcontainers embedded in the matrix. The extrinsic materials can be generally categorized into two main groups: capsule based and vascular. For capsule-based systems, when the capsules are ruptured by damage, the healing agent is released from the ruptured capsules, and fills the damaged region which then undergoes a healing reaction. A vascular type works in a similar way, but the capsules are replaced by a vascular network in one, two or three dimensions. Compared to the intrinsic system, the extrinsic system has a major advantage in healing larger damage volume [
6].
Although good self-healing performances have been demonstrated, most reported self-healing materials show their self-healing capability at room temperature or higher [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. However, low-temperature self-healing capability is essential for self-healing materials exposed to cold environments. For example, most self-healing materials for concrete or metals can be exposed to a low-temperature environment in winter. For aerospace applications, self-healing materials must have a self-healing function at temperatures as low as −60 °C. Only a few reports on low-temperature self-healing have been based on intrinsic-type materials. Kalista et al. probed the self-healing of ballistic puncture of poly(ethylene-
co-methacrylic acid) films by the energy transferred to the films from projectiles, and tested the self-healing response in ballistic impact tests at −50 °C [
19]. Self-healing at temperatures as low as −20 °C was reported by Li et al. using a poly(dimethylsiloxane) crosslinked by coordination complexes [
20].
To the best of our knowledge, there has been no report on a capsule-based low-temperature self-healing system wherein the healing ability was demonstrated at low temperature. Yuan et al. reported a low-temperature self-healing system utilizing microencapsulated epoxy and a mercaptan hardener as a two-component healing agent [
21]. In their work, specimens were stored at temperatures as low as −10 °C before and after damage creation, but the self-healing performance of the system was evaluated at room temperature. As they state in the paper, their test method cannot completely rule out the possibility that self-healing might occur as the specimen temperature increased to room temperature. Raimondo et al. studied the polymerization of a 5-ethylidene-2-norbornene/dicyclopentadiene healing agent at −50 °C for aerospace applications, but they conducted self-healing tests at room temperature [
22]. Hillewaere et al. reported self-healing of epoxy thermosets with thiol-isocyanate chemistry at −2 °C, but the self-healing performance was studied at room temperature [
23]. On the other hand, Wang et al. designed and fabricated a fiber-reinforced composite system having high healing efficiency at around −50 °C [
24]. This system was composed of three-dimensional hollow vessels that deliver and release healing agents and a conductive element to provide heat to defrost and promote healing reactions. Therefore, the development of a low-temperature self-healing system that not only can effectively show self-healing capability in low-temperature tests but also can work without an additional heating element is a remaining challenge in the field of extrinsic-type self-healing materials.
In this paper, we report a microcapsule-type self-healing protective coating that can self-heal at temperatures as low as −20 °C. This coating system is the first example of an extrinsic-type low-temperature self-healing system wherein the healing ability was demonstrated at low temperature without the use of a heating element. Silanol-terminated polydimethylsiloxane (STP) and dibutyltin dilaurate (DD) were used as a healing agent and a catalyst, respectively, and they were separately microencapsulated to prepare a dual-capsule self-healing coating. The reaction behavior of STP in the presence of DD and the release of STP and DD from ruptured microcapsules were studied at −20 °C. We evaluated the self-healing performance of the coating at −20 °C using corrosion tests, electrochemical tests, and saline solution permeability tests.
2. Materials and Methods
2.1. Materials
Urea, a formaldehyde solution (37 wt %), poly(ethylene-alt-maleic anhydride) (EMA), resorcinol, 1-octanol, ammonium chloride, tolylene-2,4-diisocyanate (TDI), 1,4-butanediol (BD), ethylene glycol (EG), gum arabic, cyclohexanone, and chlorobenzene were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Tokyo Chemical Industry (Nihonbashi, Japan) (TCI) for use in the microencapsulation process. Gum Arabic and EMA were used as surfactants. Silanol-terminated polydimethylsiloxane (STP) (DMS-S12) and dibutyltin dilaurate (DD) were purchased from Gelest (Morrisville, PA, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively, and were used as core materials (
Figure 1).
n-Hexadecane (melting point = 18 °C) was purchased from Sigma-Aldrich and was used as a comparison core material. A fluorescent fluid (OIL-GLO 44-P) consisting of polyol ester oil, mineral oil, petroleum hydrocarbon, perylene dye and naphthalimide dye was purchased from Spectronics (Westbury, NY, USA). An enamel paint (KCI 7200, white) was purchased from Kunsul Chemical Industrial Co. (Seoul, Korea). Steel panels (20 mm × 50 mm × 1 mm) were used in the corrosion test and the electrochemical test. Mortar specimens (40 mm × 40 mm × 130 mm) were prepared with cement, sand and water with a mass ratio of 2:6:1, respectively, according to a KSF2476 standard method. Mortar paste was first cured in a mold for 48 h at room temperature. Each mortar was further cured for 5 days in water and then finally cured for 7 days under ambient conditions.
2.2. Instruments
A low-temperature chamber (LTC-27, Lab house, Pocheon, Korea) was used to study the reaction of STP and to evaluate self-healing performance. A mechanical stirrer (NZ-1000, Eyela, Tokyo, Japan) equipped with a propeller-type impeller was used for microencapsulation. An Advanced Rheometric Expansion System (ARES, Rheometric Scientific, Piscataway, NJ, USA) was used to examine the viscoelasticity of the reacted STP. A fluorescence microscope (BX-51, Olympus, Tokyo, Japan) was used to take pictures of microcapsules and scratches on the surfaces of the coatings. Microcapsule size was analyzed using a CCD camera (HK6U3Cool, Koptic, Seoul, Korea) attached to the microscope and image analysis software (HKBasic, Koptic). An electronic balance (ML303, Mettler Toledo, Columbus, OH, USA) was used to weigh materials used in this work. An infrared thermometer (35639-20, Oakion, Vernon Hills, IL, USA) was used to measure the temperature of coating samples and the aqueous electrolyte. Infrared (IR) spectra were recorded on a Fourier transform infrared (FT-IR) spectrophotometer (Spectrum One B, Perkin Elmer Co., Waltham, MA, USA). A field emission scanning electron microscope (FE-SEM) (SU-70, Hitachi, Tokyo, Japan) was used to examine the morphology of the microcapsules. Thermogravimetric analysis (TGA) was conducted using a Shimadzu TA-50 to investigate the core composition of DD microcapsules. A potentiostat/galvanostat (273A, Ametek, Berwyn, PA, USA) was used to examine the conductivity of the coated substrate.
2.3. Reaction Conversion Measurement
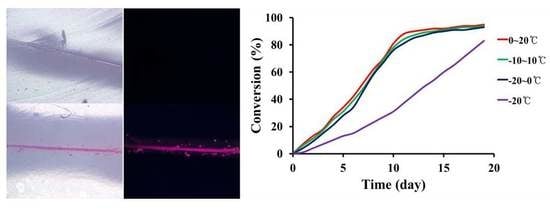
STP and DD were mixed at a mass ratio of 10:1, and the mixture was poured in molds that were 25 mm in diameter and 1 mm in height. The mixture was allowed to react for 19 days at −20 °C (constant temperature), −20–0 °C (cycle), −10–10 °C (cycle), and 0–20 °C (cycle) (
Figure 2a). Samples were taken periodically and analyzed by FT-IR spectroscopy. The mass ratio of 10:1 was selected because viscoelastic products were obtained from STP and DD mixtures with a mass ratio range of 8:1–12:1 in preliminary experiments. The degree of conversion of silanol groups of STP was analyzed by an IR band-ratio method. The absorbance of the O-H stretch band at 3330 cm
−1 was divided by that of a reference C-H stretch band at 2960 cm
−1 (
Figure S1). The degree of conversion was calculated using the following equation,
where A
3330 and A
2960 are the absorbance values of the 3330 and 2960 cm
−1 absorption bands of STP, respectively.
2.4. Viscoelasticity Measurement
STP and DD were mixed at a mass ratio of 10:1, and the mixture was poured into molds that were 25 mm diameter and 1 mm height and allowed to react for 19 days at −20 °C. Disk-like samples of the product were separated from the molds. The viscoelasticity of the reacted STP was examined using an ARES rheometer (Rheometric Scientific, Piscataway, NJ, USA) at −20, 5, and 30 °C. This experiment was conducted under constant strain and temperature, while frequency was varied from 0.1 to 510.0 rad/s. Then, the experimental results were plotted in terms of G’ and G” versus frequency.
2.5. Microencapsulation of STP and n-Hexadecane
Water (20 mL) and a 2.5 wt % aqueous solution of EMA (5 mL) were added to a 100-mL beaker. The beaker was placed in a water bath equipped with a mechanical stirrer. Urea (0.503 g), ammonium chloride (0.050 g), and resorcinol (0.050 g) were added under agitation at 300 rpm. The pH of the resulting mixture was adjusted to 3.5 using a 10 wt % NaOH solution. The mixture was stirred at 1000 rpm, and two drops of 1-octanol were added. Then, 8 mL of STP were added, and the mixture was stirred at 1000 rpm for 15 min to form a stable emulsion. After adding a 37 wt % formaldehyde solution (1.456 g), the temperature of the mixture was increased to 60 °C, which was maintained for 4.5 h. The resulting suspension was cooled to room temperature, and microcapsules were filtered using vacuum filtration. The microcapsules were washed with water and ethyl alcohol and air-dried for 48 h (yield = 91%). n-Hexadecane was microencapsulated in a similar fashion. Measurement of microcapsule payload was conducted using an electronic balance. After weighing, the STP-loaded microcapsules were crushed by pressing them in a 5-mL vial and were washed twice with ethyl alcohol to remove the core (STP). The remaining shell material was dried under ambient conditions and weighed.
2.6. Microencapsulation of DD
TDI (11.50 g, 0.066 mol) was dissolved in cyclohexanone (70 mL) under nitrogen in a 150-mL round-bottom flask. The flask was placed in an oil bath, and BD (2.70 g, 0.030 mol) was added at 55 °C. The resulting solution was heat at 80 °C for 24 h. Then, the reaction mixture was heated under vacuum at 80 °C for 4 h and then at 100 °C for 1 h to remove the solvent and unreacted reagents and to obtain a prepolymer. Gum arabic (3.10 g) was dissolved in distilled water (20 mL) in a 100-mL beaker. The prepolymer (3.00 g) was dissolved in chlorobenzene (32.00 g), and DD (1.00 g) was then added. The prepolymer/DD solution was slowly poured into the gum arabic solution to give an emulsion. EG (3.00 g) as a chain extender was slowly added to the emulsion at 50 °C. The mixture was heated; when the temperature reached 70 °C, the reaction was stopped. After filtration, the microcapsules were washed with distilled water and air-dried for 72 h (yield = 84%). Measurement of the composition of DD-loaded microcapsules was conducted using the TGA instrument and the electronic balance. After weighing, the DD microcapsules were crushed and subjected to isothermal TGA at 25 °C to measure the content of chlorobenzene in the microcapsules (
Figure S2). Chlorobenzene readily evaporated at 25 °C within 10 min, and DD and shell material remained. On the other hand, the DD-containing microcapsules were crushed by pressing them in a 5-mL vial and washing twice with ethyl alcohol to remove the core. The remaining shell material was dried under ambient conditions and weighed.
2.7. Microencapsulation of Fluorescent Core
Microcapsules containing fluorescent core were prepared and used to visualize the release of the core materials from broken microcapsules. Fluorescent cores were prepared by adding the fluorescent fluid into STP, DD or n-hexadecane. The mass ratios of the components of the fluorescent cores were as follows: STP/fluorescent fluid = 9:1, chlorobenzene/DD/fluorescent fluid = 32:0.9:0.1, and n-hexadecane/fluorescent fluid = 9:1. The procedure of microencapsulation of the fluorescent core containing DD was the same as that of DD encapsulation described above. The procedure of microencapsulation of the fluorescent core containing STP or n-hexadecane was the same as that of the STP microencapsulation described above.
2.8. Preparation of the Coating Samples
The STP- and DD-loaded microcapsules were uniformly dispersed into the commercial enamel paint to prepare self-healing coatings. The mass ratio of STP capsules/DD capsules/enamel paint was 18:7:75. The mass ratio of STP capsules/DD capsules of 18:7 corresponds to the 10:1 mass ratio of STP and DD. The n-hexadecane-containing microcapsules were mixed into the enamel paint with a mass ratio of n-hexadecane capsules/enamel paint = 25:75 to prepare comparison coatings. The fluorescent core-containing microcapsules were mixed into the enamel paint with a mass ratio of microcapsules/enamel paint = 25:75. The resulting coating formulation was applied to the surfaces of glass slides for release testing, steel panels for corrosion and electrochemical testing, and mortar specimens for saline solution permeability testing. The steel panels were washed with ethyl alcohol and dried under ambient conditions before coating. The coated samples were dried for 2–3 days at room temperature. Control coating samples were prepared in a similar fashion without adding microcapsules. Coating thicknesses were about 550 μm (on slide glass) and about 90 μm (on the steel panel and mortar). When thick coatings (>500 μm) formed on the steel panel or mortar, the original scratch width was not maintained and tended to decrease to some extent. It was expected that the exact evaluation of self-healing performance would not be possible using the thick coatings on steel panel and mortar, so relatively thin coatings (<100 μm) were prepared.
2.9. Release Test
The coating formulation containing fluorescent-core microcapsules was applied to the surfaces of glass slides and dried. The coated glass slides were stored at −20 °C for 24 h in the low-temperature chamber. Immediately after opening the chamber door, a scratch was generated in each coating with a razor blade. It was confirmed that each cut was deep enough to reach the surface of the glass slide. Optical microscopy showed that the scratch width was about 20 μm. Scratching was performed inside the chamber within 30 s to avoid an increase in sample temperature. The scratched samples were left in the chamber at −20 °C for 10–15 min. The scratched coatings were observed using a fluorescence microscope within 1–2 min immediately after taking the samples out of the chamber. During the observation, the temperature of the coating samples increased from −20 to −17 °C.
2.10. Corrosion Test
The STP/DD-based self-healing coatings and control coatings were applied to one side of the steel panels. The other side of each panel was covered with enamel paint. The coated steel panels were stored at −20 °C for 24 h in the low-temperature chamber. Immediately after opening the chamber door, cross scratches were applied to the self-healing and control coatings with a razor blade inside the chamber. It was confirmed that each cut was deep enough to reach the surface of the steel panel. Optical microscopy showed that the scratch width was about 20 μm. The scratching was performed inside the chamber within 30 s to avoid an increase in sample temperature. After the scratched coatings were left at −20 °C for 12 h in the chamber, they were immediately immersed in a 25 wt % NaCl aqueous solution that was maintained at −20 °C in the chamber as an accelerated corrosion test. After 48 h, the coatings were washed with distilled water and wiped to remove residual water, and the coating surfaces were observed by optical microscopy. For each kind of sample, at least three specimens were used for the corrosion test.
2.11. Electrochemical Test
The STP/DD-based self-healing coatings and control coatings were applied to one side of the steel panels. The coated steel panels were stored at −20 °C for 24 h in the chamber. Immediately after opening the chamber door, cross scratches were applied to the self-healing and control coatings with a razor blade inside the chamber. It was confirmed that each cut was deep enough to reach the surface of the steel panel. Optical microscopy showed that the scratch width was about 20 μm. The scratching was performed inside the chamber within 30 s to avoid an increase in sample temperature. The scratched coatings were left at −20 °C for 12 h in the chamber. Each electrochemical cell was fabricated immediately after taking out a scratched coating sample from the chamber. The fabrication took less than 1 min. Electrochemical tests were conducted using a computer-controlled potentiostat/galvanostat in a conventional three-electrode electrochemical cell equipped with a platinum counter electrode, an Ag/AgCl electrode in a saturated NaCl aqueous solution as the reference electrode, and the coated steel panel as the working electrode (
Figure S3). During the test, dry ice was used to keep the coated steel panel and electrolyte at −20 °C. Steady-state conduction was measured between the coated steel panel and a platinum electrode held at −2 V through an aqueous electrolyte (0.1 M Na
2SO
4). The current passing through the specimen was recorded using Electrochemistry PowerSuite software. At least three specimens were used for each kind of sample, and an average of the measured values was calculated.
2.12. Saline Solution Permeability Test
The self-healing coatings and control coatings were applied to one rectangular side of the square column mortars (
Figure S4). Four side surfaces adjacent to the coated side were covered with epoxy resin. The coated mortar specimens were stored at −20 °C for 24 h in the low-temperature chamber. Immediately after opening the chamber door, scratches were applied to the self-healing and control coatings with a razor blade inside the chamber. It was confirmed that each cut was deep enough to reach the surface of the mortar. Optical microscopy showed that the scratch width was about 25 μm. After the scratched coatings were weighed, they were left at −20 °C for 12 h in the chamber. The scratching and weighing took only about 30 s, and the temperature of the samples was maintained at −20 °C. The scratched surface was immediately immersed in a saline solution (25 wt % NaCl aqueous solution) that was being maintained at −20 °C in the chamber. After 48 h, the increase in the mass of mortar due to the absorbed saline solution was determined by weighing each specimen. For each kind of sample, three replicates were tested, and an average of the measured mass was calculated.
4. Conclusions
STP as a healing agent with DD as a catalyst was used for low-temperature self-healing. The reaction of STP in the presence of DD proceeded at low temperature (−20 °C) to generate a viscoelastic substance, which exhibited viscoelasticity even at −20 °C. STP and DD were separately microencapsulated using urea-formaldehyde resin and polyurethane as shell materials, respectively. When the self-healing coatings containing both microcapsules were damaged, STP and DD were released from the ruptured microcapsules and filled the damaged region at low temperature (−20 °C), which was confirmed by release tests. The applicability of the STP/DD-based self-healing coating system for steel panels and mortar was successfully demonstrated by corrosion tests, electrochemical tests, and saline solution permeability tests that were all conducted at −20 °C. We concluded that the STP/DD-based microcapsule-type self-healing system has good low-temperature self-healing ability. The self-healing coating has advantages because it shows effective self-healing capability under low-temperature conditions, and it can also work without any additional heating element. Our self-healing system can be applied to self-healing protective coatings exposed to cold environments.