Preparation of Luminescent Metal-Organic Framework Films by Soft-Imprinting for 2,4-Dinitrotoluene Sensing
Abstract
:1. Introduction
2. Results and Discussion
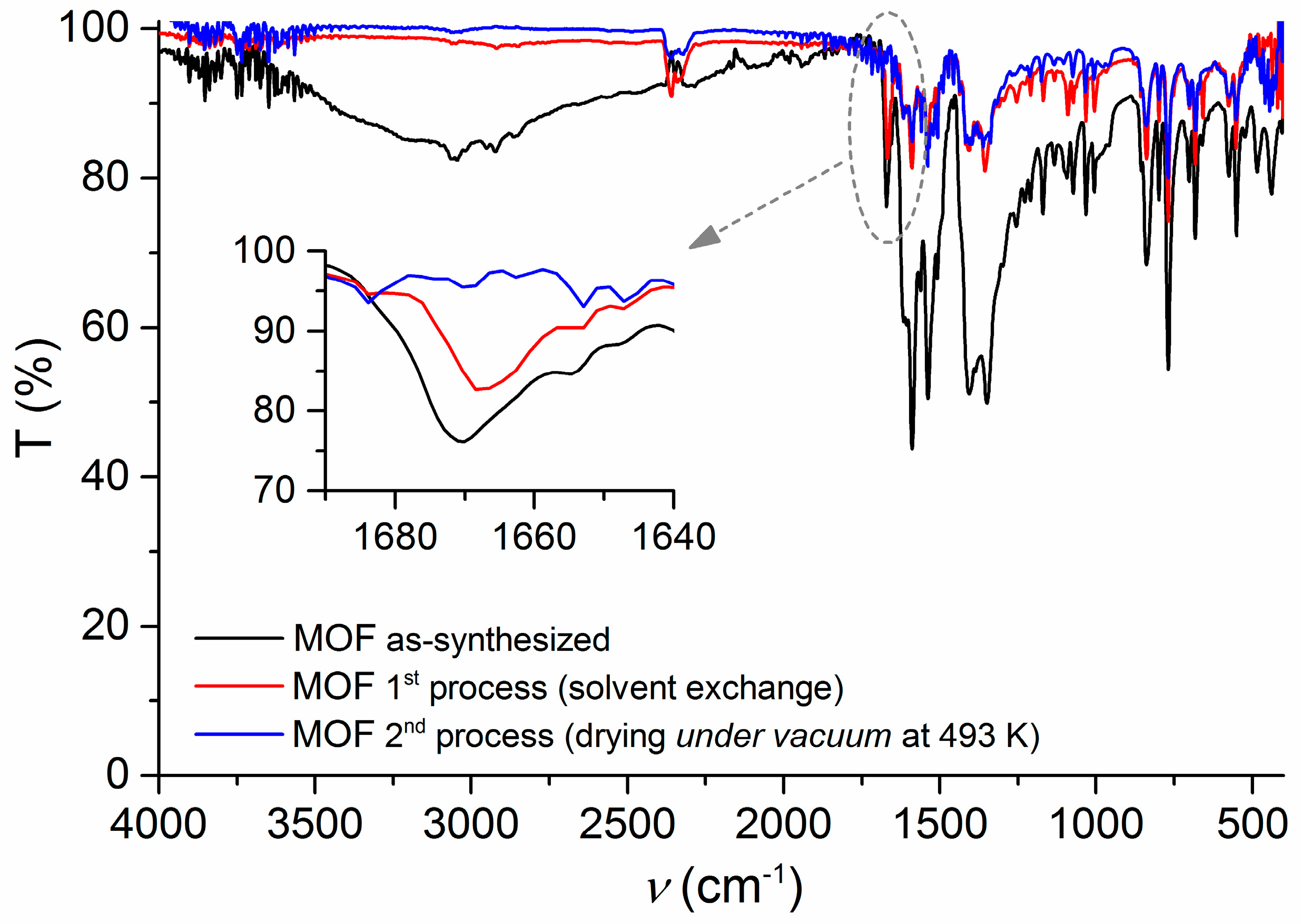
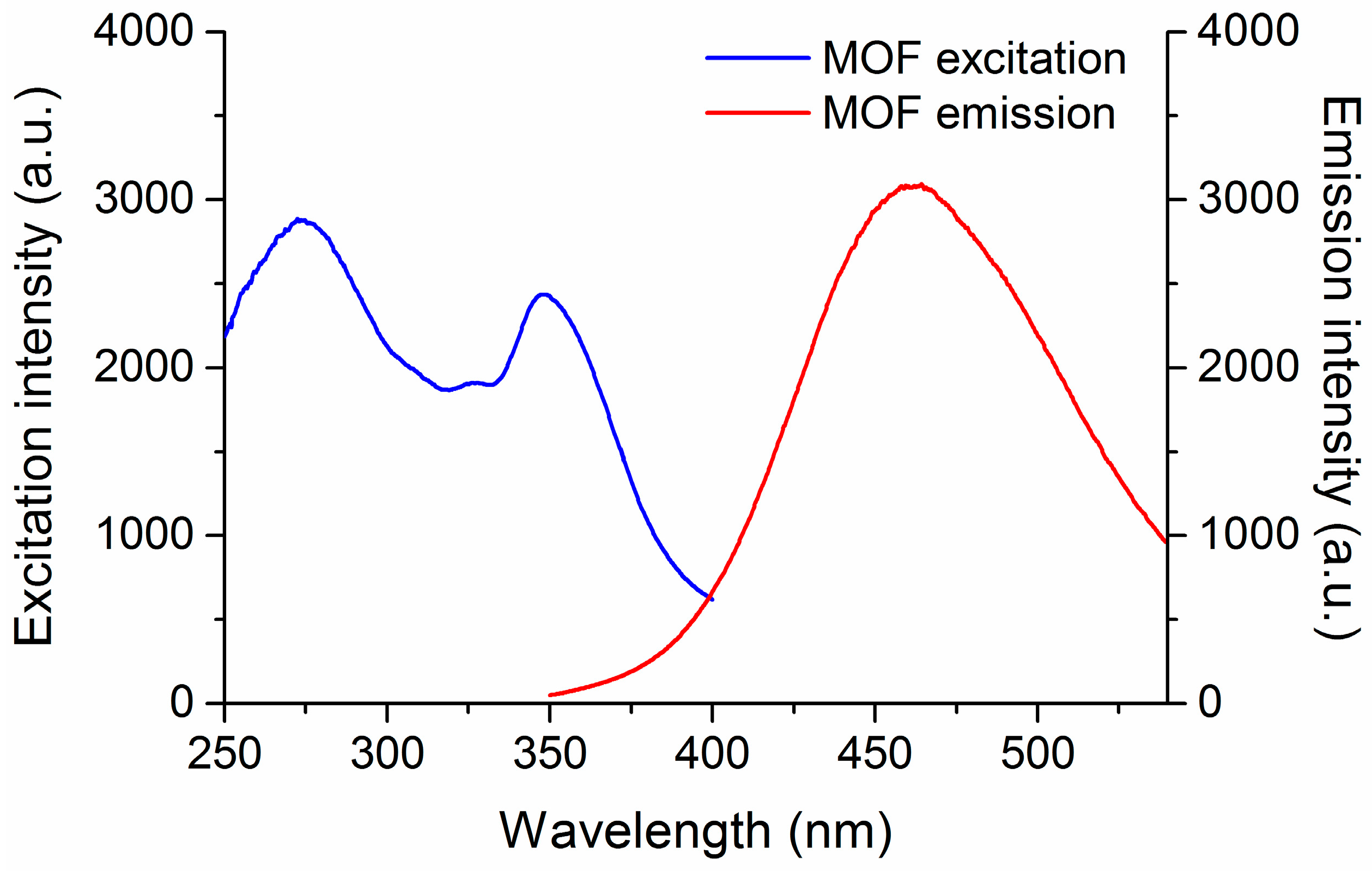
2.1. Synthesis and Characterization
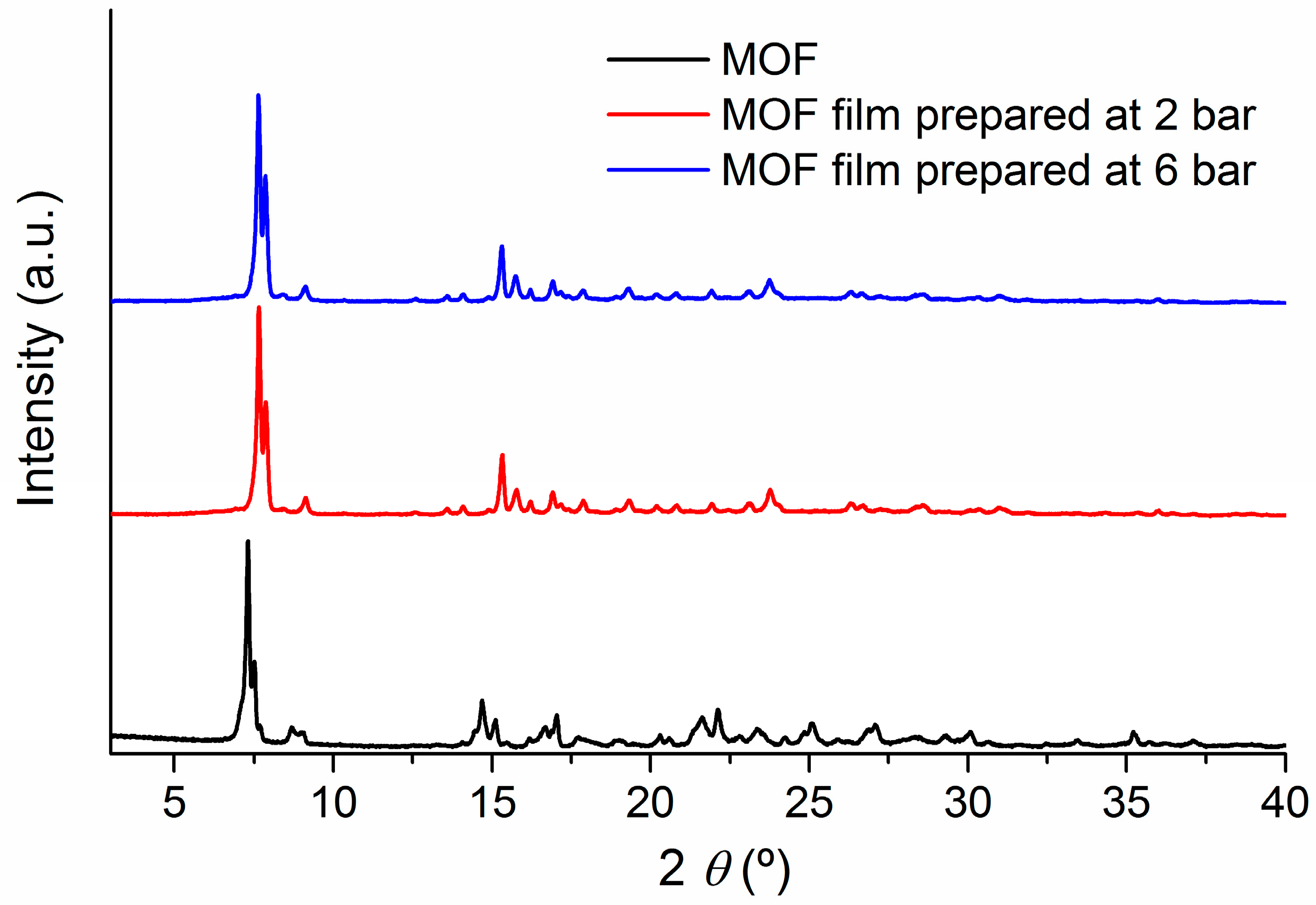
2.2. Soft-Imprinted MOF Films
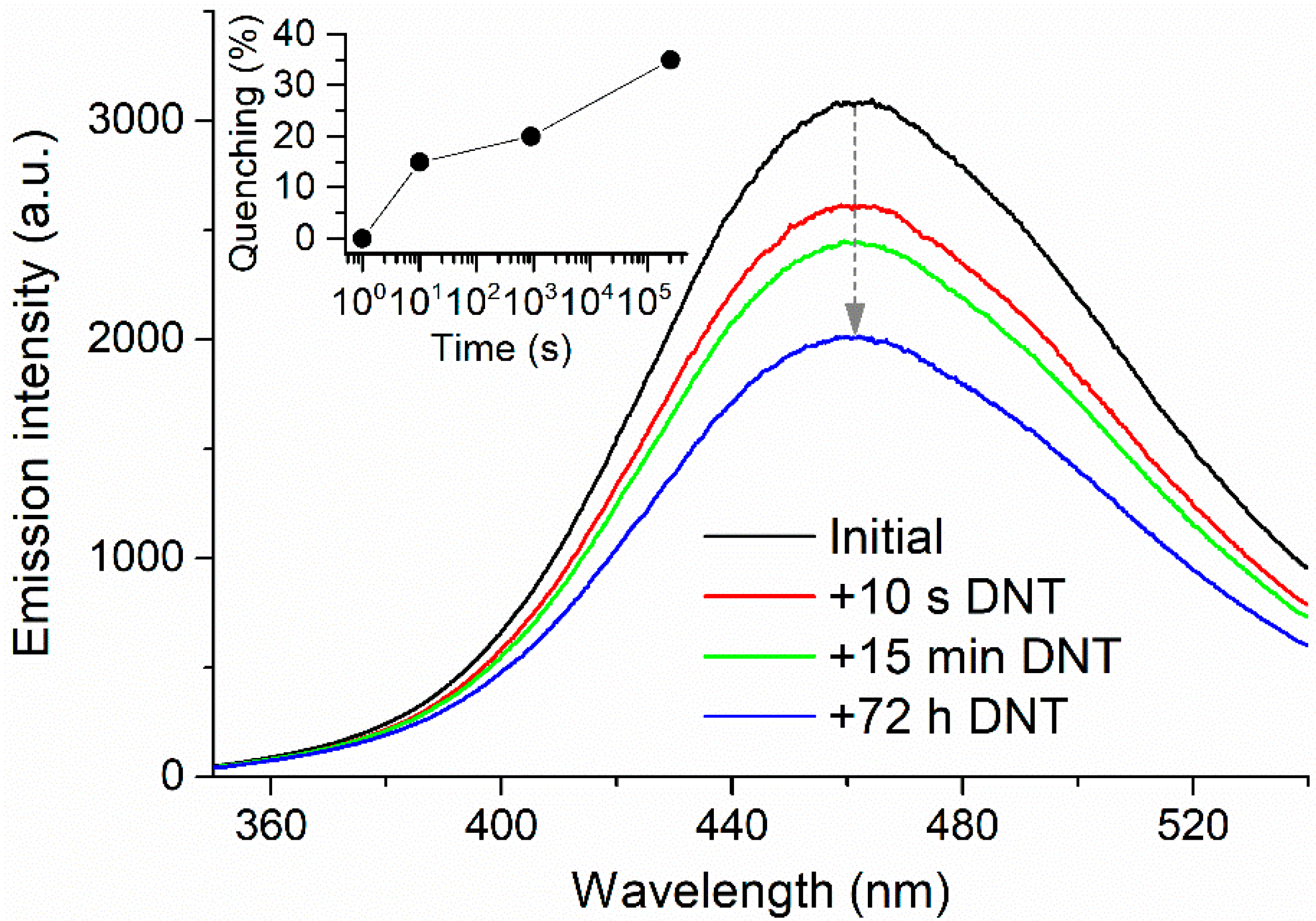
2.3. Explosive Vapor Sensing
3. Materials and Methods
3.1. Chemicals and General Methods
3.2. Synthesis of [Zn2(bpdc)2(bpee)] MOFs
3.3. Fabrication of Adhesive Tape-Based MOF Films
3.4. Fabrication of Soft-Imprinted MOF/CA Films
3.5. Exposure to Explosives
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral Metal-Organic Frameworks for Asymmetric Heterogeneous Catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Li, K.; Wu, H.; Olson, D.H.; Emge, T.J.; Ki, W.; Hong, M.; Li, J. A Luminescent Microporous Metal-Organic Framework for the Fast and Reversible Detection of High Explosives. Angew. Chem. Int. Ed. 2009, 48, 2334–2338. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New Microporous Metal-Organic Framework Demonstrating Unique Selectivity for Detection of High Explosives and Aromatic Compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, M.; Peveler, W.J.; Savory, C.N.; Scanlon, D.O.; Kenyon, A.J.; Parkin, I.P. The vapour phase detection of explosive markers and derivatives using two fluorescent metal–organic frameworks. J. Mater. Chem. A 2015, 3, 6351–6359. [Google Scholar] [CrossRef]
- Lee, T.; Liu, Z.X.; Lee, H.L. A biomimetic nose by microcrystals and oriented films of luminescent porous metal-organic frameworks. Cryst. Growth Des. 2011, 11, 4146–4154. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Y.; Qiu, S.; Lercher, A.J.; Zhang, H. Coordination modulation induced synthesis of nanoscale Eu 1-Tbxmetal-organic frameworks for luminescent thin films. Adv. Mater. 2010, 22, 4190–4192. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; Fenero, M.; Sorribas, S.; Zornoza, B.; Msayib, K.J.; McKeown, N.B.; Téllez, C.; Coronas, J.; Gascón, I. Fabrication of ultrathin films containing the metal organic framework Fe-MIL-88B-NH2 by the Langmuir-Blodgett technique. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 161–170. [Google Scholar] [CrossRef]
- Benito, J.; Sorribas, S.; Lucas, I.; Coronas, J.; Gascon, I. Langmuir-Blodgett Films of the Metal-Organic Framework MIL-101(Cr): Preparation, Characterization, and CO2 Adsorption Study Using a QCM-Based Setup. ACS Appl. Mater. Interfaces 2016, 8, 16486–16492. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, H.; Wöll, C. Epitaxially grown metal-organic frameworks. Mater. Today 2012, 15, 110–116. [Google Scholar] [CrossRef]
- Pramanik, S.; Hu, Z.; Zhang, X.; Zheng, C.; Kelly, S.; Li, J. A systematic study of fluorescence-based detection of nitroexplosives and other aromatics in the vapor phase by microporous metal-organic frameworks. Chem. A Eur. J. 2013, 19, 15964–15971. [Google Scholar] [CrossRef] [PubMed]
- Kooy, N.; Mohamed, K.; Pin, L.T.; Guan, O.S. A review of roll-to-roll nanoimprint lithography. Nanoscale Res. Lett. 2014, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.M.; Grenci, G.; Riccò, R.; Mardel, J.I.; Reboul, J.; Furukawa, S.; Kitagawa, S.; Hill, A.J.; Falcaro, P. Combining UV lithography and an imprinting technique for patterning metal-organic frameworks. Adv. Mater. 2013, 25, 4701–4705. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.N.; Hou, L.; Wei, L.; Jing, X.J.; Liu, B.; Wang, Y.Y.; Shi, Q.Z. Five sra topological Ln(III)-MOFs based on novel metal-carboxylate/Cl chain: Structure, near-infrared luminescence and magnetic properties. Cryst. Growth Des. 2013, 13, 1570–1576. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roales, J.; Moscoso, F.G.; Gámez, F.; Lopes-Costa, T.; Sousaraei, A.; Casado, S.; Castro-Smirnov, J.R.; Cabanillas-Gonzalez, J.; Almeida, J.; Queirós, C.; et al. Preparation of Luminescent Metal-Organic Framework Films by Soft-Imprinting for 2,4-Dinitrotoluene Sensing. Materials 2017, 10, 992. https://doi.org/10.3390/ma10090992
Roales J, Moscoso FG, Gámez F, Lopes-Costa T, Sousaraei A, Casado S, Castro-Smirnov JR, Cabanillas-Gonzalez J, Almeida J, Queirós C, et al. Preparation of Luminescent Metal-Organic Framework Films by Soft-Imprinting for 2,4-Dinitrotoluene Sensing. Materials. 2017; 10(9):992. https://doi.org/10.3390/ma10090992
Chicago/Turabian StyleRoales, Javier, Francisco G. Moscoso, Francisco Gámez, Tânia Lopes-Costa, Ahmad Sousaraei, Santiago Casado, Jose R. Castro-Smirnov, Juan Cabanillas-Gonzalez, José Almeida, Carla Queirós, and et al. 2017. "Preparation of Luminescent Metal-Organic Framework Films by Soft-Imprinting for 2,4-Dinitrotoluene Sensing" Materials 10, no. 9: 992. https://doi.org/10.3390/ma10090992





