Magnetic and Mössbauer Spectroscopy Studies of Zinc-Substituted Cobalt Ferrites Prepared by the Sol-Gel Method
Abstract
:1. Introduction
2. Experimental
2.1. Sample Synthesis
2.2. Characterization
3. Results and Discussion
3.1. X-ray Diffraction Analysis
3.2. Structures and Grain Sizes
3.3. Mössbauer Spectroscopy
3.4. Magnetic Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohamed, R.M.; Rashada, M.M.; Haraz, F.A.; Sigmund, W. Structure and magnetic properties of nanocrystalline cobalt ferrite powders synthesized using organic acid precursor method. J. Magn. Magn. Mater. 2010, 322, 2058–2064. [Google Scholar] [CrossRef]
- Amiri, S.; Shokrollah, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. 2013, 33, 1–8. [Google Scholar] [CrossRef]
- Sanpo, N.; Berndt, C.C.; Wen, C.; Wang, J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013, 9, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.H.; Zakaria, S.; Yusoffa, M.; Goh, S.C.; Haw, C.Y.; Ahmadi, Sh.; Huang, N.M.; Lim, H.N. Size and crystallinity-dependent magnetic properties of CoFe2O4 nanocrystals. Ceram. Int. 2010, 36, 605–609. [Google Scholar] [CrossRef]
- Gul, H.; Abbasi, A.Z.; Amin, F.; Anis-ur-Rehman, M.; Maqsood, A. Structural, magnetic and electrical properties of Co1−xZnxFe2O4 synthesized by co-precipitation method. J. Magn. Magn. Mater. 2007, 311, 494–499. [Google Scholar] [CrossRef]
- Waje, S.B.; Hashim, M.; Yusoff, W.D.W.; Abbas, Z. Sintering temperature dependence of room temperature magnetic and dielectric properties of Co0.5Zn0.5Fe2O4 prepared using mechanically alloyed nanoparticles. J. Magn. Magn. Mater. 2010, 322, 686–691. [Google Scholar] [CrossRef]
- Veverka, M.; Veverka, P.; Jirák, Z.; Kaman, O.; Knížek, K.; Maryško, M.; Závěta, K. Synthesis and magnetic properties of Co1−xZnxFe2O4+γ nanoparticles as materials for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2010, 322, 2386–2389. [Google Scholar] [CrossRef]
- Hossain, A.A.; Tabata, H.; Kawai, T. Magnetoresistive properties of Zn1−xCoxFe2O4 ferrites. J. Magn. Magn. Mater. 2008, 320, 1157–1162. [Google Scholar] [CrossRef]
- Jadhav, S.S.; Shirsath, S.E.; Patange, S.M.; Jadhav, K.M. Effect of Zn substitution on magnetic properties of nanocrystalline cobalt ferrite. J. Appl. Phys. 2010, 108, 093920. [Google Scholar] [CrossRef]
- Hemeda, O.M.; El-Saadawy, M. Effect of gamma irradiation on the structural properties and diffusion coefficient in Co-Zn ferrite. J. Magn. Magn. Mater. 2003, 256, 63–68. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.G.; Zhang, L.; Min, F.F.; Zhang, M.X. Microstructure and magnetic properties of nanocrystalline Co1−xZnxFe2O4 ferrites. Mater. Res. Bull. 2012, 47, 4174–4180. [Google Scholar] [CrossRef]
- Deraz, N.M.; Alarifi, A. Structural, morphological and magnetic properties of nano-crystalline zinc substituted cobalt ferrite system. J. Anal. Appl. Pyrolysis 2012, 94, 41–47. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H. Nanostructural, magnetic and Mössbauer studies of nanosized Co1−xZnxFe2O4 synthesized by co-precipitation. J. Magn. Magn. Mater. 2012, 324, 2397–2403. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, Z.; Yu, Z. Synthesis of NiZn ferrite nanoparticles by sol-gel method. Mater. Rev. 2006, 20, 49–51. [Google Scholar]
- Gul, H.; Maqsood, A. Structural, magnetic and electrical properties of cobalt ferrites prepared by the sol-gel route. J. Alloys Compd. 2008, 465, 227–231. [Google Scholar] [CrossRef]
- Barpanda, P.; Yamashita, Y.; Yamada, Y.; Yamada, A. High-throughput solution combustion synthesis of high-capacity LiFeBO3 cathode. J. Electrochem. Soc. 2013, 160, 3095–3099. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Wen, D. Structure, Infrared Radiation Properties and Mössbauer Spectroscopic Investigations of Co0.6Zn0.4NixFe2-xO4 Ceramics. J. Mater. Sci. Technol. 2010, 26, 687–692. [Google Scholar] [CrossRef]
- Siddique, M.; Butt, N.M. Effect of particle size on degree of inversion in ferrites investigated by Mössbauer spectroscopy. Phys. Rev. B Condens. Matter 2010, 405, 4211–4215. [Google Scholar] [CrossRef]
- Kumar, S.; Farea, A.M.M.; Batoo, K.M.; Lee, C.G.; Koo, B.H.; Yousef, A. Alimuddin. Mössbauer studies of Co0.5CdxFe2.5-xO4 (0.0–0.5) ferrite. Phys. Rev. B Condens. Matter 2008, 403, 3604–3607. [Google Scholar] [CrossRef]
- He, Y.; Lei, C.; Lin, Q.; Dong, J.; Yu, Y.; Wang, L. Mössbauer and Structural properties of La-substituted Ni0.4Cu0.2Zn0.4Fe2O4 nanocrystalline ferrite. Sci. Adv. Mater. 2015, 7, 1809–1815. [Google Scholar] [CrossRef]
- Singhal, S.; Barthwal, S.K.; Chandra, K. XRD, magnetic and Mössbauer spectral studies of nano size aluminum substituted cobalt ferrites (CoAlxFe2-xO4). J. Magn. Magn. Mater. 2006, 306, 233–240. [Google Scholar] [CrossRef]
- Bayoumi, W. Structural and electrical properties of zinc-substituted cobalt ferrite. J. Mater. Sci. 2007, 42, 8254–8261. [Google Scholar] [CrossRef]
- Lee, S.W.; Ryu, Y.G.; Yang, K.J.; Jung, K.D.; An, S.Y.; Kim, C.S. Magnetic properties of Zn2+ substituted ultrafine Co-ferrite grown by a sol-gel method. J. Appl. Phys. 2002, 91, 7610. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3, nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Kamali, S.; Bringas, E.; Hah, H.Y.; Bates, B.; Johnson, J.A.; Johnson, C.E.; Stroeve, P. Magnetism and Mössbauer study of formation of multi-core γ-Fe2O3 nanoparticles. J. Magn. Magn. Mater. 2018, 451, 131–136. [Google Scholar] [CrossRef]
- Chae, K.P.; Kim, W.K.; Lee, S.H.; Lee, Y.B. Crystallographic and magnetic properties of TixCo1+xFe2-2xO4 ferrite powders. J. Magn. Magn. Mater. 2001, 232, 133–138. [Google Scholar] [CrossRef]
- Birajdar, D.S.; Mane, D.R.; More, S.S.; Kawade, V.B.; Jadhav, K.M. Structural and magnetic properties of ZnxCu1.4-xMn0.1Fe1.2O4 ferrites. Mater. Lett. 2005, 59, 2981–2985. [Google Scholar] [CrossRef]
- Verma, S.; Chand, J.; Batoo, K.M.; Singh, M. Structural, magnetic and Mössbauer spectral studies of aluminum substituted Mg-Mn-Ni ferrites (Mg0.2Mn0.5Ni0.3AlyFe2-yO4). J. Alloys Compd. 2013, 551, 715–721. [Google Scholar] [CrossRef]
- Hossain, A.A.; Amin, M.R.; Tanaka, H. Increase in initial permeability due to substitution of high spin cations in nanocrystalline Ni-Mg ferrites. J. Magn. Magn. Mater. 2013, 334, 124–129. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Lin, J.; Lin, Q.; Dong, J. Mössbauer spectroscopy, Structural and magnetic studies of Zn2+ substituted magnesium ferrite nanomaterials prepared by sol-gel method. J. Nanomater. 2015, 5, 854840. [Google Scholar] [CrossRef]
- Gözüak, F.; Köseoğlu, Y.; Baykal, A.; Kavas, H. Synthesis and characterization of Co1−xZnxFe2O4magnetic nanoparticles via a PEG-assisted route. J. Magn. Magn. Mater. 2009, 321, 2170–2177. [Google Scholar] [CrossRef]
- Lin, Q.; Lin, J.; He, Y.; Wang, R.; Dong, J. The structural and magnetic properties of gadolinium doped CoFe2O4 nanoferrites. J. Nanomater. 2015, 8, 294239. [Google Scholar]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Shaari, A.H.; Hashim, M. Simple synthesis and characterization of cobalt ferrite nanoparticles by a thermal treatment method. J. Nanomater. 2010, 75, 907686. [Google Scholar]
- Soibam, I.; Phanjoubam, S.; Prakash, C. Mössbauer and magnetic studies of cobalt substituted lithium zinc ferrites prepared by citrate precursor method. J. Alloys Compd. 2009, 475, 328–331. [Google Scholar] [CrossRef]
- Lin, J.; He, Y.; Lin, Q.; Wang, R.; Chen, H. Microstructural and Mössbauer spectroscopy Studies of Mg1−xZnxFe2O4 (x = 0.5, 0.7) nanoparticles. J. Spectrosc. 2014, 2014, 540319. [Google Scholar] [CrossRef]
- Motavallian, P.; Abasht, B.; Abdollah-Pour, H. Zr doping dependence of structural and magnetic properties of cobalt ferrite synthesized by sol-gel based Pechini method. J. Magn. Magn. Mater. 2018, 451, 577–586. [Google Scholar] [CrossRef]
- Kamali, S.; Shih, K.; Barbiellini, B.; Wang, Y.J.; Kaprzyk, S.; Itou, M.; Sakurai, Y. Extracting cation distributions in NiFe2-xAlxO4 Solid Solutions using magnetic Compton scattering. J. Phys. Condens. Matter 2015, 27, 456003. [Google Scholar] [CrossRef] [PubMed]
- Kamali, S. Spin structure, magnetism, and cation distributions of NiFe2-xAlxO4 solid solutions. J. Magn. Magn. Mater. 2017, 433, 155–161. [Google Scholar] [CrossRef]
- Hu, Z.P.; Xu, Y.B.; Tan, X.D.; Peng, F.; Gu, X.L.; Zou, Y.; Yu, S.C. Effect of rapid heating on a cold-rolled Mn–Al transformation-induced plasticity steel with coarse delta-ferrite. Sci. Adv. Mater. 2017, 9, 1953–1959. [Google Scholar]

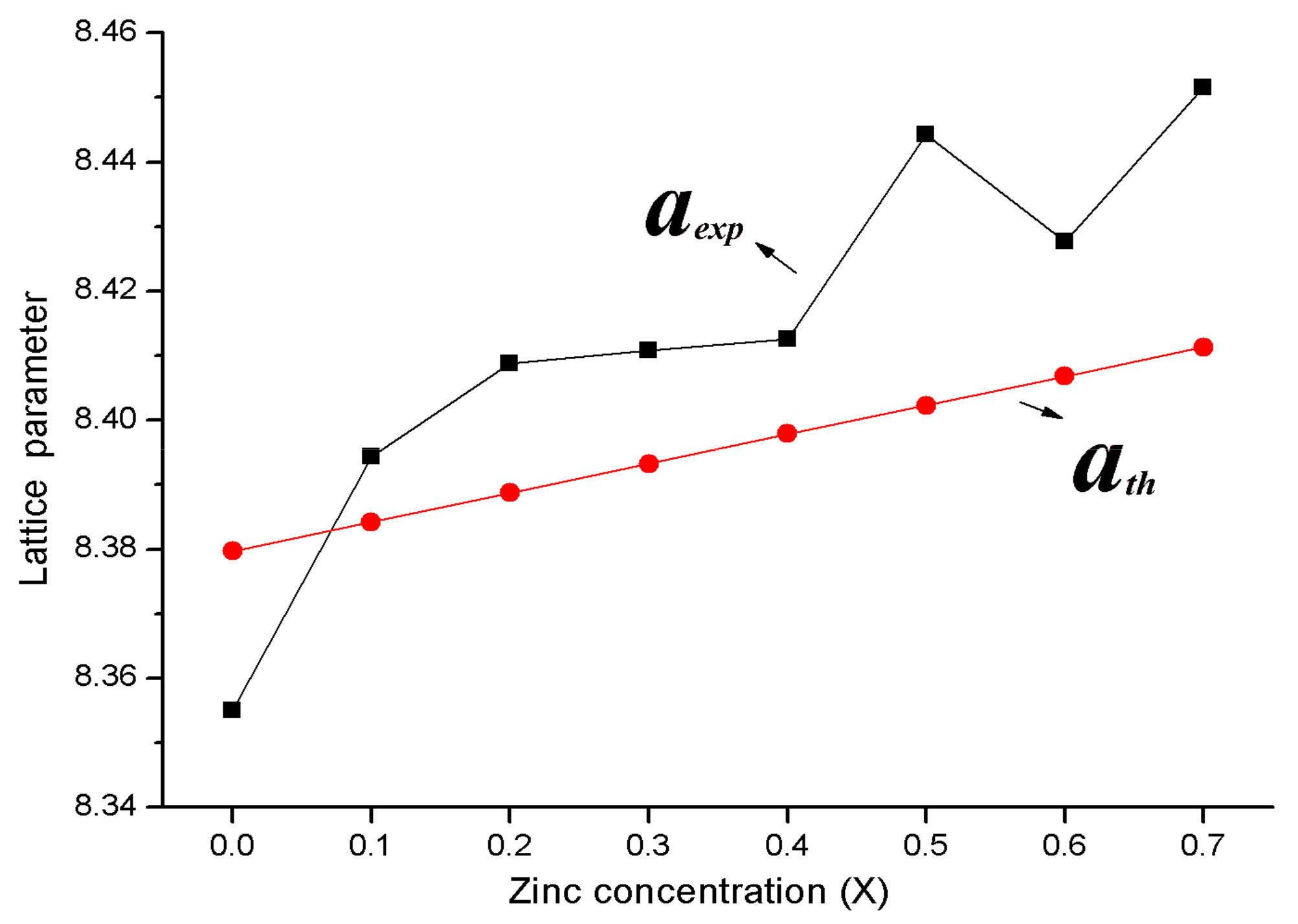

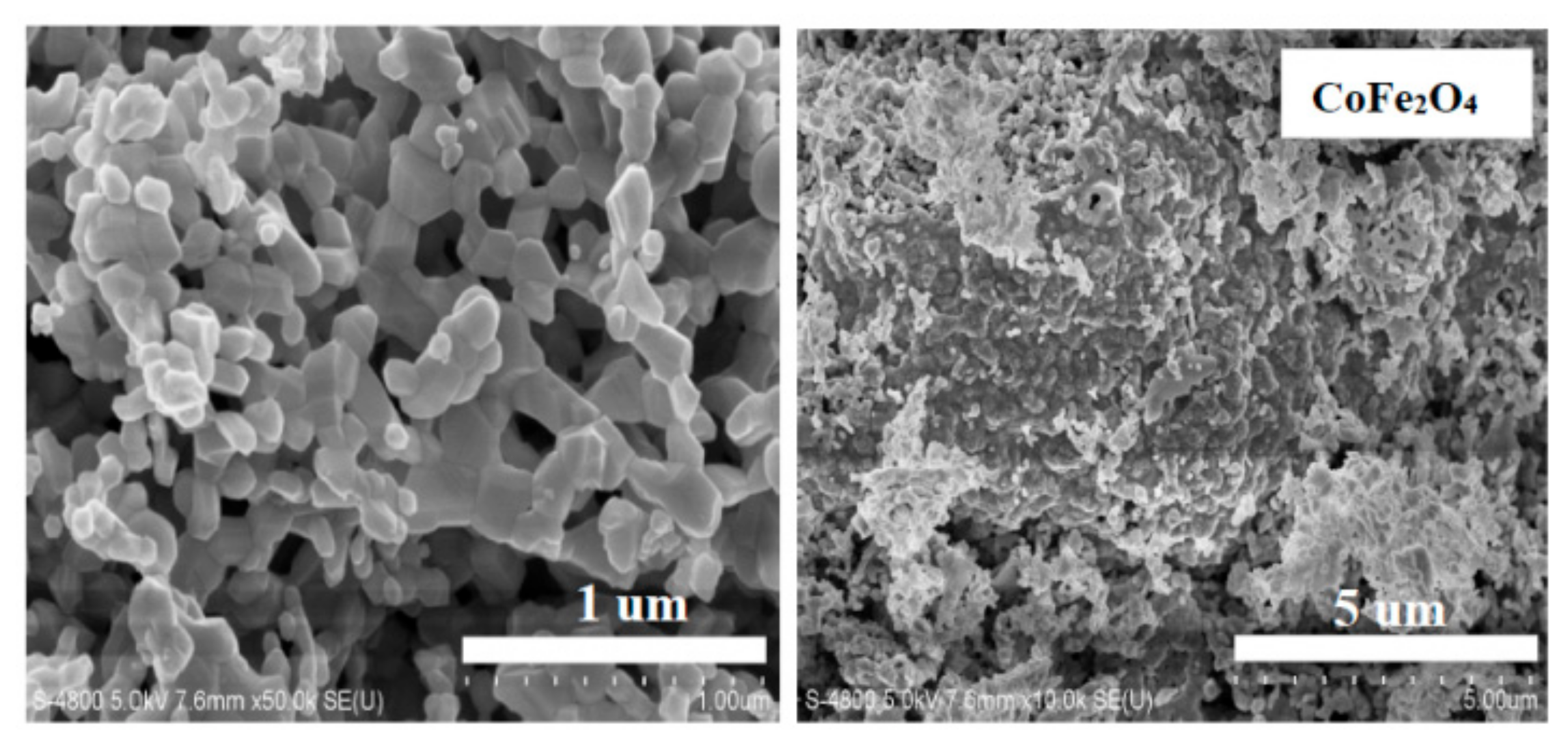
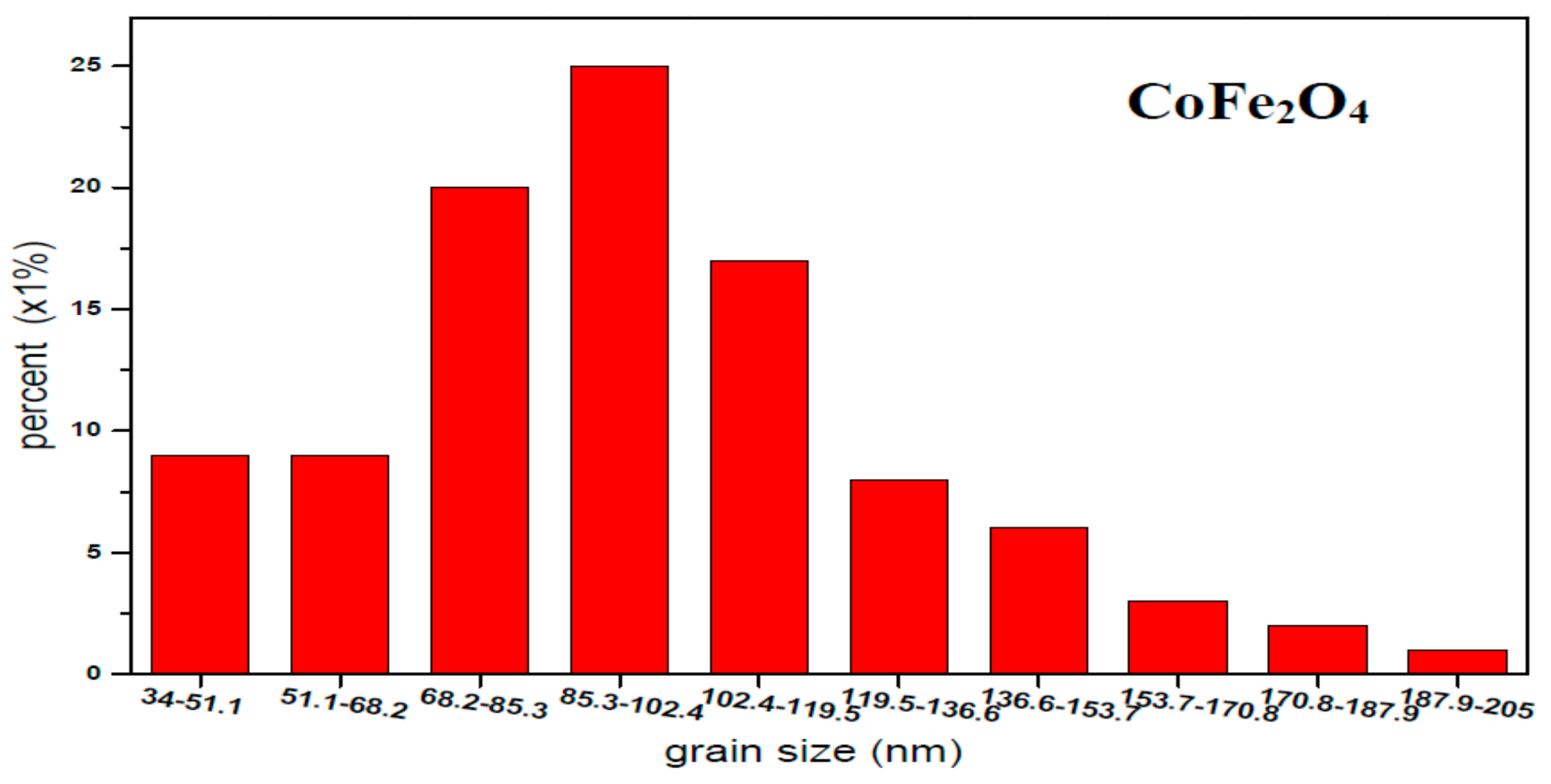

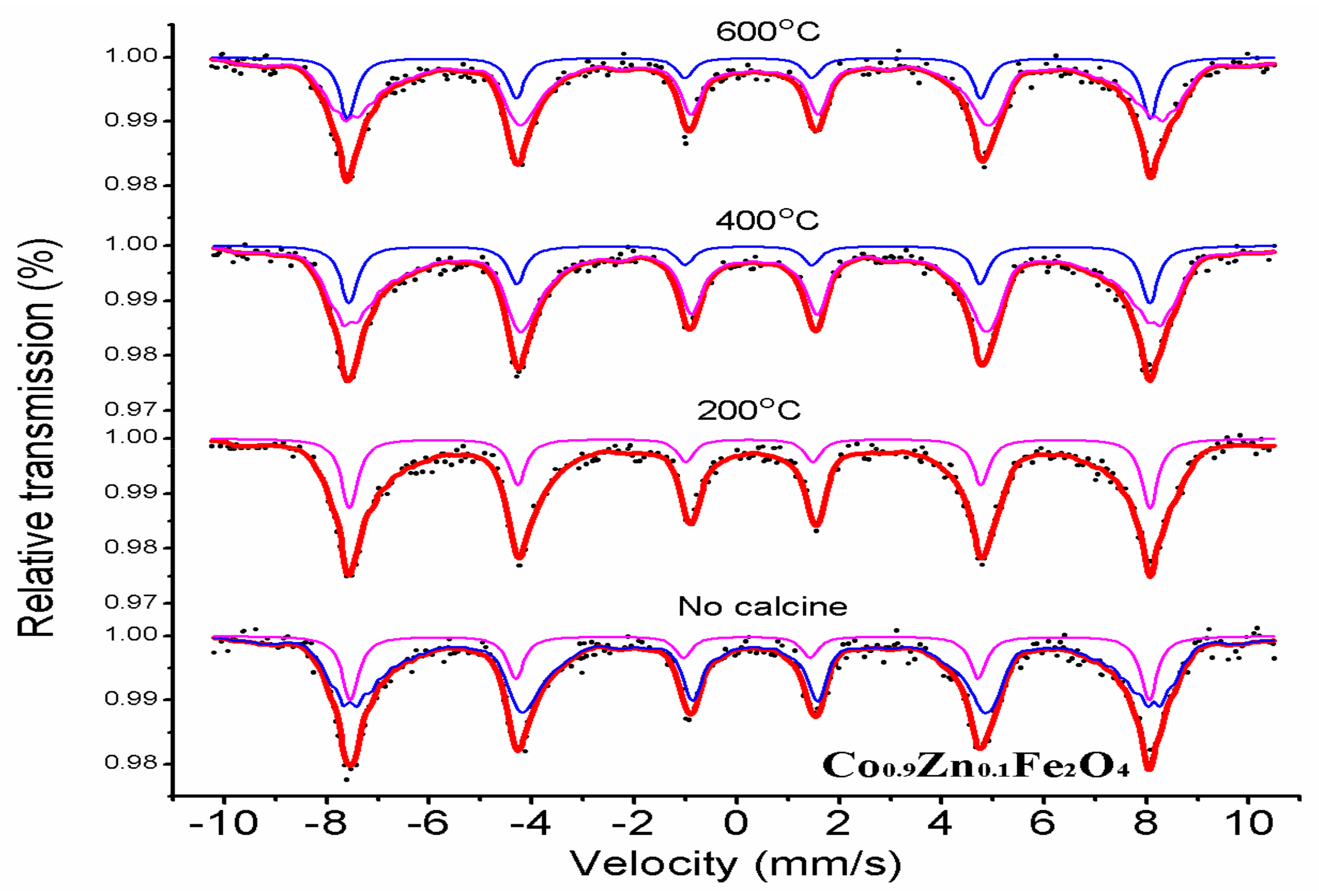


| Content (x) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| 0 | 8.35497 | 556 | 5.3468 |
| 0.1 | 8.39435 | 508 | 5.2693 |
| 0.2 | 8.40884 | 498 | 5.2421 |
| 0.3 | 8.41082 | 455 | 5.2384 |
| 0.4 | 8.41256 | 489 | 5.2351 |
| 0.5 | 8.44421 | 408 | 5.3187 |
| 0.6 | 8.42766 | 482 | 5.3501 |
| 0.7 | 8.45158 | 360 | 5.3048 |
| Temperature (°C) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| unsintered | 8.38224 | 270 | 5.2921 |
| 200 | 8.40569 | 312 | 5.2480 |
| 400 | 8.38644 | 314 | 5.2842 |
| 600 | 8.38464 | 332 | 5.2876 |
| 800 | 8.39435 | 508 | 5.2693 |
| Content (x) | Component | I.S. (mm/s) | Q.S. (mm/s) | H (T) | Γ (mm/s) | A0 (mm/s) |
|---|---|---|---|---|---|---|
| 0 | Sextet (A) | 0.237 | −0.004 | 48.946 | 0.360 | 33 |
| Sextet (B) | 0.375 | −0.024 | 45.695 | 0.322 | 67 | |
| 0.1 | Sextet (A) | 0.247 | 0.019 | 48.459 | 0.344 | 26 |
| Sextet (B) | 0.343 | −0.027 | 45.227 | 0.378 | 74 | |
| 0.2 | Sextet (A) | 0.248 | 0.038 | 47.735 | 0.386 | 21 |
| Sextet (B) | 0.340 | −0.016 | 42.532 | 0.352 | 79 | |
| 0.3 | Sextet (A) | 0.235 | 0.055 | 47.508 | 0.429 | 11 |
| Sextet (B) | 0.306 | −0.050 | 38.946 | 0.338 | 89 | |
| 0.4 | Sextet (B) | 0.288 | −0.022 | 34.911 | 0.375 | 100 |
| 0.5 | Sextet (B) | 0.309 | 0.0002 | 28.6 | 0.375 | 100 |
| 0.6 | Sextet (B) | 0.346 | −0.005 | 18.8 | 0.291 | 100 |
| 0.7 | Double | 0.347 | 0.4305 | 0 | 0.357 | 100 |
| Temperature (°C) | Component | I.S. (mm/s) | Q.S. (mm/s) | H (T) | Γ (mm/s) | A0 (mm/s) |
|---|---|---|---|---|---|---|
| unsintered | Sextet (A) | 0.225 | 0.056 | 48.356 | 0.394 | 22.5 |
| Sextet (B) | 0.328 | −0.050 | 43.905 | 0.336 | 77.5 | |
| 200 | Sextet (A) | 0.249 | 0.007 | 48.449 | 0.391 | 22.5 |
| Sextet (B) | 0.348 | −0.011 | 43.712 | 0.351 | 77.5 | |
| 400 | Sextet (A) | 0.237 | 0.016 | 48.458 | 0.406 | 18.9 |
| Sextet (B) | 0.324 | −0.030 | 44.154 | 0.387 | 81.1 | |
| 600 | Sextet (A) | 0.234 | 0.008 | 48.582 | 0.396 | 23.1 |
| Sextet (B) | 0.346 | 0.001 | 44.563 | 0.366 | 76.9 |
| Content (x) | MS (emu/g) | HC (Oe) | Mr1 (emu/g) | nB |
|---|---|---|---|---|
| 0 | 72.58 | 1005.33 | 34.71 | 3.05 |
| 0.1 | 80.46 | 703.91 | 33.14 | 3.39 |
| 0.2 | 82.61 | 402.24 | 26.46 | 3.49 |
| 0.3 | 83.51 | 301.75 | 25.00 | 3.54 |
| 0.4 | 76.97 | 100.73 | 23.64 | 3.27 |
| 0.5 | 63.63 | 75.62 | 15.08 | 2.71 |
| 0.6 | 41.23 | 25.39 | 4.06 | 1.76 |
| 0.7 | 20.09 | 0.24 | 0.13 | 0.86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Q.; Xu, J.; Yang, F.; Lin, J.; Yang, H.; He, Y. Magnetic and Mössbauer Spectroscopy Studies of Zinc-Substituted Cobalt Ferrites Prepared by the Sol-Gel Method. Materials 2018, 11, 1799. https://doi.org/10.3390/ma11101799
Lin Q, Xu J, Yang F, Lin J, Yang H, He Y. Magnetic and Mössbauer Spectroscopy Studies of Zinc-Substituted Cobalt Ferrites Prepared by the Sol-Gel Method. Materials. 2018; 11(10):1799. https://doi.org/10.3390/ma11101799
Chicago/Turabian StyleLin, Qing, Jianmei Xu, Fang Yang, Jinpei Lin, Hu Yang, and Yun He. 2018. "Magnetic and Mössbauer Spectroscopy Studies of Zinc-Substituted Cobalt Ferrites Prepared by the Sol-Gel Method" Materials 11, no. 10: 1799. https://doi.org/10.3390/ma11101799





