Diffusivities and Atomic Mobilities in bcc Ti-Mo-Zr Alloys
Abstract
:1. Introduction
2. Model
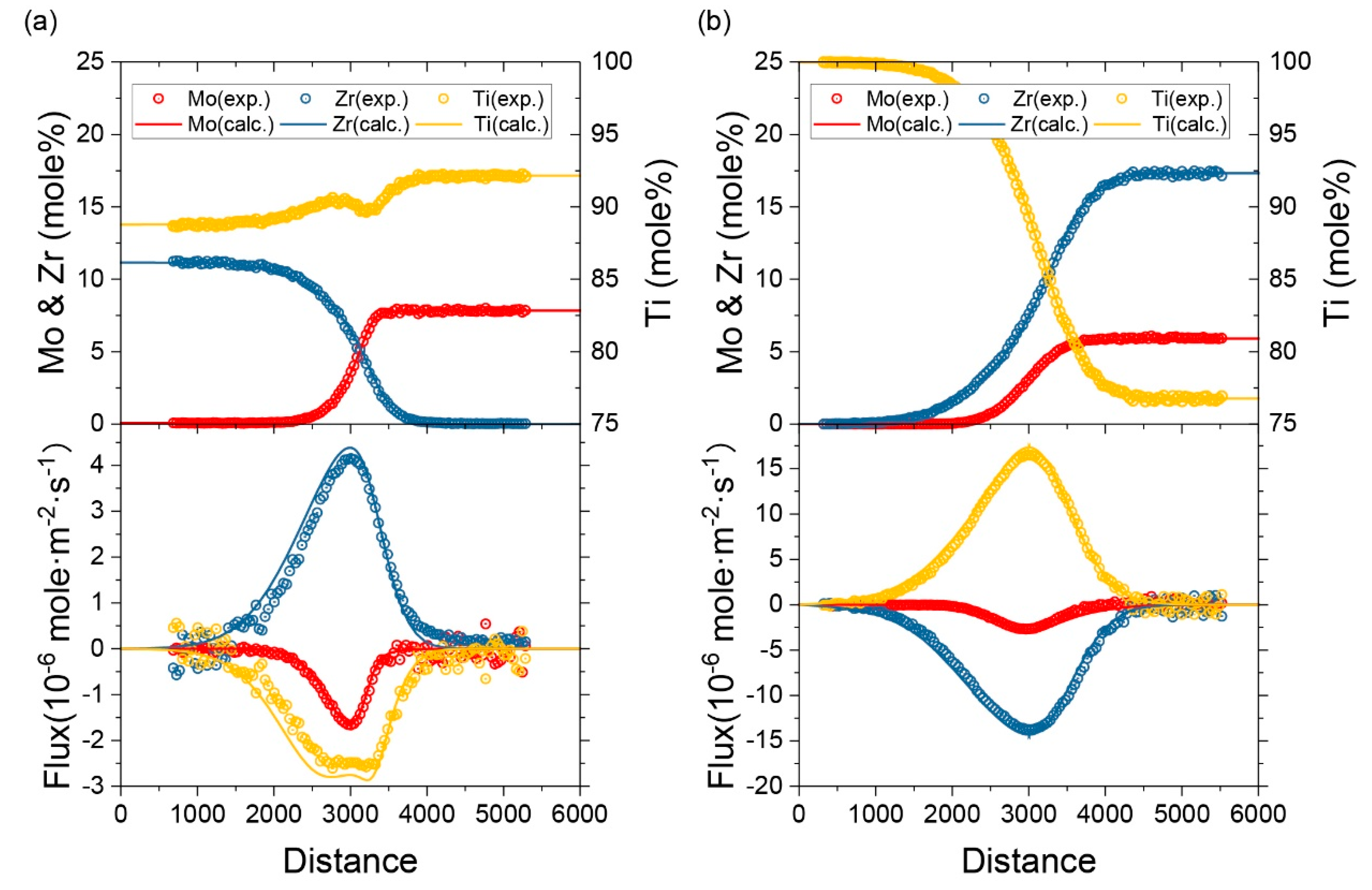
2.1. Extraction of Inter-Diffusion Coefficients and Impurity Diffusion Coefficients
2.2. Atomic Mobility and Diffusivity
3. Experiment
4. Results and Discussions
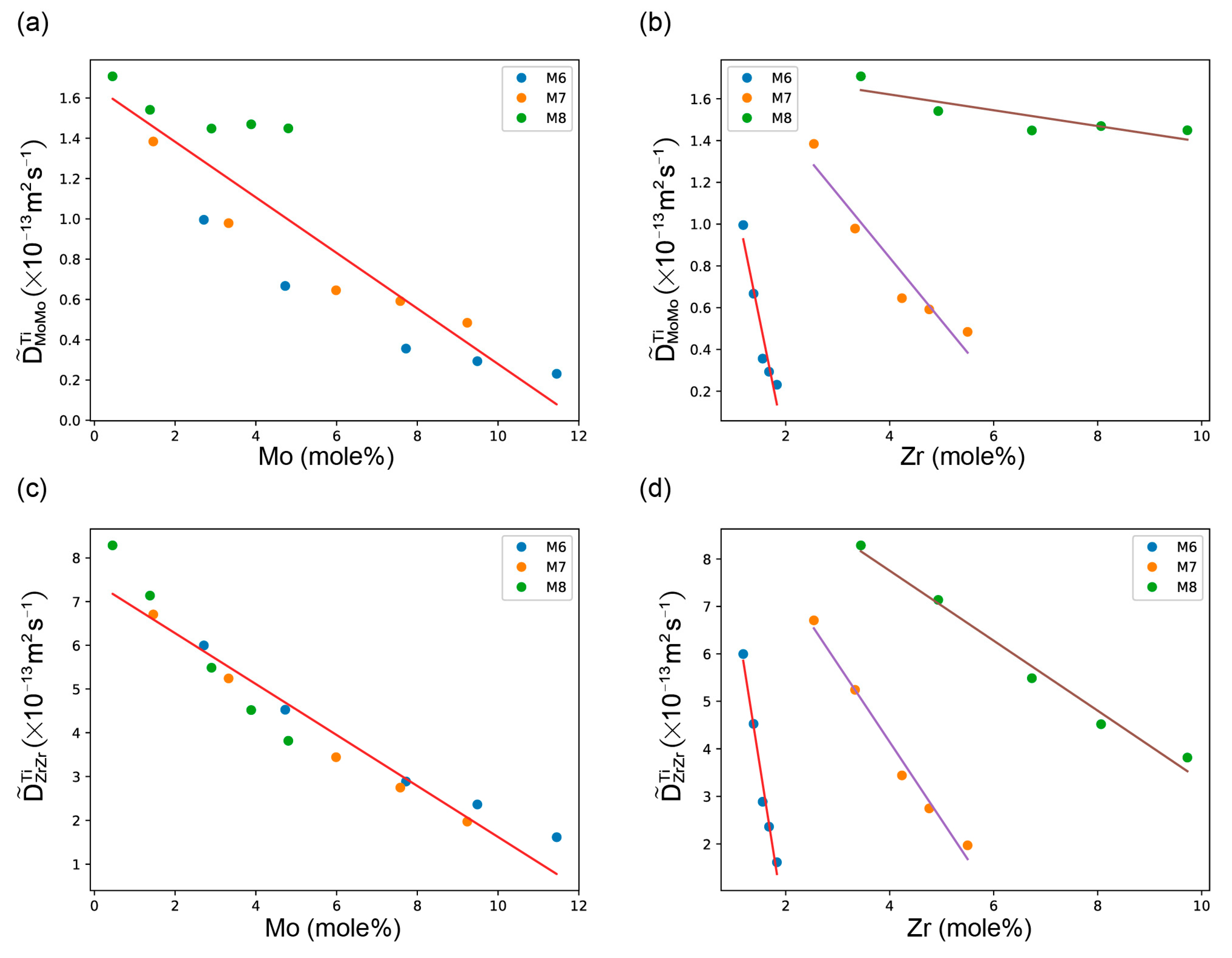
4.1. Inter-Diffusion and Impurity Diffusion Coefficients At 1373 K and 1473 K
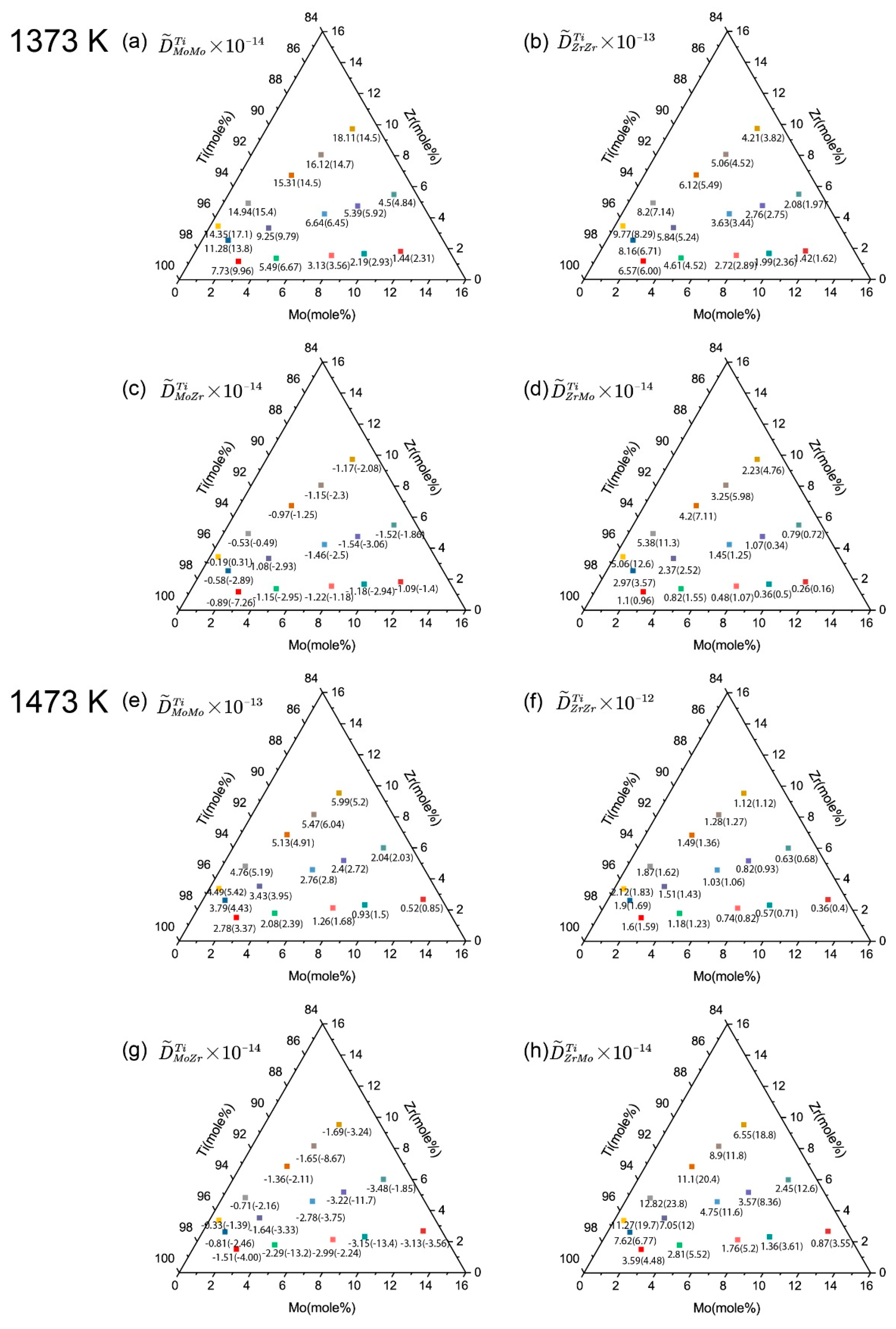
4.2. Atomic Mobilities in bcc Ti-Zr-Mo System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gogia, A.K. High-temperature Titanium Alloys. Defence Sci. J. 2005, 55, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Kuroda, P.A.B.; Grandini, C.R. Structure, Microstructure, and Selected Mechanical Properties of Ti-Zr-Mo Alloys for Biomedical Applications. Adv. Mater. 2014, 922, 75–80. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Wu, W.T.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Nnamchi, P.S.; Obayi, C.S.; Todd, I.; Rainforth, M.W. Mechanical and electrochemical characterisation of new Ti-Mo-Nb-Zr alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2016, 60, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-K.; Hwang, M.-J.; Yang, M.-S.; Yang, H.-S.; Song, H.-J.; Park, Y.-J. Effect of zirconium content on the microstructure, physical properties and corrosion behavior of Ti alloys. Mater. Sci. Eng. A 2014, 616, 268–274. [Google Scholar] [CrossRef]
- Ho, W.F.; Ju, C.P.; Lin, J.C. Structure and properties of cast binary Ti-Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Trentani, L.; Pelillo, F.; Pavesi, F.C.; Ceciliani, L.; Cetta, G.; Forlino, A. Evaluation of the TiMo12Zr6Fe2 alloy for orthopaedic implants: In vitro biocompatibility study by using primary human fibroblasts and osteoblasts. Biomaterials 2002, 23, 2863–2869. [Google Scholar] [CrossRef]
- Gordin, D.M.; Gloriant, T.; Texier, G.; Thibon, I.; Ansel, D.; Duval, J.L.; Nagel, M.D. Development of a β-type Ti-12Mo-5Ta alloy for biomedical applications: Cytocompatibility and metallurgical aspects. J. Mater. Sci. Mater. Med. 2004, 15, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Banerjee, R.; Fraser, H.L. Microstructural evolution and strengthening mechanisms in Ti-Nb-Zr-Ta, Ti-Mo-Zr-Fe and Ti-15Mo biocompatible alloys. Mater. Sci. Eng. C 2005, 25, 357–362. [Google Scholar] [CrossRef]
- Yu, Z.T.; Wang, G.; Ma, X.Q.; Dargusch, M.S.; Han, J.Y.; Yu, S. Development of Biomedical Near β Titanium Alloys. Mater. Sci. Forum 2009, 618, 303–306. [Google Scholar] [CrossRef]
- Wang, K.; Gustavson, L.; Dumbleton, J. The characterization of Ti-12Mo-6Zr-2Fe a new biocompatible titanium alloy developed for surgical implants. In Beta Titanium in the 1990’s; The Mineral, Metals and Materials Society: Warrendale, PA, USA, 1993; pp. 2697–2704. [Google Scholar]
- Steinemann, S.G.; Mäusli, P.A.; Szmukler-Moncler, P.; Semlitsch, M.; Pohler, O.; Hintermann, H.E.; Perren, S.M. Beta-titanium alloy for surgical implants. In Beta Titanium in the 1990’; The Mineral, Metals and Materials Society: Warrendale, PA, USA, 1993; pp. 2689–2696. [Google Scholar]
- Helander, T.; Ågren, J. Diffusion in the B2-b.c.c. phase of the Al-Fe-Ni system—Application of a phenomenological model. Acta Mater. 1999, 47, 3291–3300. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, N.; Wu, K.; Wang, Y. Quantitative phase field modeling of diffusion-controlled precipitate growth and dissolution in Ti-Al-V. Scr. Mater. 2004, 50, 471–476. [Google Scholar] [CrossRef]
- Mao, C.; Tan, M.; Zhang, L.; Wu, D.; Bai, W.; Liu, L. Experimental reinvestigation and thermodynamic description of Bi-Te binary system. Calphad 2018, 60, 81–89. [Google Scholar] [CrossRef]
- Chen, F.; Xu, G.; Zhang, X.; Zhou, K. Exploring the Phase Transformation in β-Quenched Ti-55531 Alloy During Continuous Heating via Dilatometric Measurement, Microstructure Characterization, and Diffusion Analysis. Metall. Mater. Trans. A 2016, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Stratmann, M.; Du, Y.; Sundman, B.; Steinbach, I. Incorporating the CALPHAD sublattice approach of ordering into the phase-field model with finite interface dissipation. Acta Mater. 2015, 88, 156–169. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Ågren, J. Models for numerical treatment of multicomponent diffusion in simple phases. J. Appl. Phys. 1992, 72, 1350–1355. [Google Scholar] [CrossRef]
- Borgenstam, A.; Höglund, L.; Ågren, J.; Engström, A. DICTRA, a tool for simulation of diffusional transformations in alloys. J. Phase Equilib. 2000, 21, 269. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Neumann, G.; Tuijn, C. Self-Diffusion and Impurity Diffusion in Pure Metals: Handbook of Experimental Data, 1st ed.; Pergamon: Oxford, UK, 2009. [Google Scholar]
- Heumann, T.; Imm, R. Untersuchungen über den Kirkendall-Effekt in kub. raumzentrierten Titan-Molybdän Legierungen; Forschungsberichte des Landes Nordrhein-Westfalen; VS Verlag für Sozialwissenschaften: Wiesbaden, Germany, 1978. [Google Scholar]
- Thibon, I.; Ansel, D.; Baliveau, M.; Debuigne, J. Interdiffusion in the β Mo-Ti solid solution at high temperatures. Z. Metallkd. 1998, 89, 187–191. [Google Scholar]
- Sprengel, W.; Yamada, T.; Nakajima, H. Interdiffusion in Binary β-Titanium Alloys. Defect Diffusion Forum 1997, 143, 431–436. [Google Scholar] [CrossRef]
- Thibon, I.; Ansel, D.; Gloriant, T. Interdiffusion in β-Ti-Zr binary alloys. J. Alloys Compd. 2009, 470, 127–133. [Google Scholar] [CrossRef]
- Brunsch, A.; Steeb, S. Diffusionsuntersuchungen im System Ti-Zr mittels Mikrosonde / Interdiffusion in the System Ti-Zr. Z. Naturforsch. A 1974, 29, 1319–1324. [Google Scholar] [CrossRef]
- Raghunathan, V.S.; Tiwari, G.P.; Sharma, B.D. Chemical diffusion in the β phase of the Zr-Ti alloy system. Metallogr. Trans. 1972, 3, 783–788. [Google Scholar] [CrossRef]
- Bhanumurthy, K.; Laik, A.; Kale, G.B. Novel Method of Evaluation of Diffusion Coefficients in Ti-Zr System. In Phase Transformation and Diffusion; Defect and Diffusion Forum; Trans Tech Publications: Zurich, Switzerland, 2008; pp. 53–62. [Google Scholar]
- Bhatt, Y.J.; Kumar, L.; Patil, R.V.; Kale, G.B.; Garg, S.P. Diffusion studies in Hf-Mo, Zr-Mo, Cr-Nb, Cr-Ta and Th-Re systems above 1900 K. J. Alloys Compd. 2000, 302, 177–186. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Yu, D. Computational Study of Mobilities and Diffusivities in bcc Ti-Zr and bcc Ti-Mo Alloys. J. Phase Equilib. Diffus. 2009, 30, 334–344. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Wang, J.; Kang, Z. Mobilities and diffusivities for bcc Nb-W, Nb-Ta, Zr-Mo and Zr-Hf alloys. J. Alloys Compd. 2013, 555, 381–389. [Google Scholar] [CrossRef]
- Kirkaldy, J.S. Diffusion in Multicomponent Metallic Systems. Can. J. Phys. 1957, 35, 435–440. [Google Scholar] [CrossRef]
- Whittle, D.P.; Green, A. The measurement of diffusion coefficients in ternary systems. Scr. Metall. 1974, 8, 883–884. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, B.; Xu, G.; Wang, C.; Kou, H.; Li, J.; Cui, Y. Diffusion Research in BCC Ti-Al-Mo Ternary Alloys. Metall. Mater. Trans. A 2014, 45, 1647–1652. [Google Scholar] [CrossRef]
- Hall, L.D. An Analytical Method of Calculating Variable Diffusion Coefficients. J. Chem. Phys. 1953, 21, 87–89. [Google Scholar] [CrossRef]
- Jönsson, B. Assessment of the mobility of carbon in fcc C-Cr-Fe-Ni alloys. Z. Metallkd. 1994, 85, 502–509. [Google Scholar]
- Shim, J.-H.; Oh, C.-S.; Lee, D.N. A thermodynamic evaluation of the Ti-Mo-C system. Metall. Mater. Trans. B 1996, 27, 955–966. [Google Scholar] [CrossRef]
- Turchanin, M.A.; Agraval, P.G.; Abdulov, A.R. Thermodynamic assessment of the Cu-Ti-Zr system. II. Cu-Zr and Ti-Zr systems. Powder. Metall. Metal Cream. 2008, 47, 428. [Google Scholar] [CrossRef]
- Jerlerud Pérez, R.; Sundman, B. Thermodynamic assessment of the Mo–Zr binary phase diagram. Calphad 2003, 27, 253–262. [Google Scholar] [CrossRef]
- Watson, A. Molybdenum-Titanium-Zirconium. In Refractory metal systems; Springer: Berlin/Heidelberg, Germany, 2010; pp. 441–454. [Google Scholar]
- Wang, C.; Xu, G.; Cui, Y. Mapping of Diffusion and Nanohardness Properties of Fcc Co-Al-V Alloys Using Ternary Diffusion Couples. Metall. Mater. Trans. A 2017, 48, 4286–4296. [Google Scholar] [CrossRef]
- Kirkaldy, J.S.; Weichert, D.; Haq, Z.U. Diffusion in Multicomponent Metallic Systems: Vi. Some Thermodynamic Properties of the D Matrix and the Corresponding Solutions of the Diffusion Equations. Can. J. Phys. 1963, 41, 2166–2173. [Google Scholar] [CrossRef]






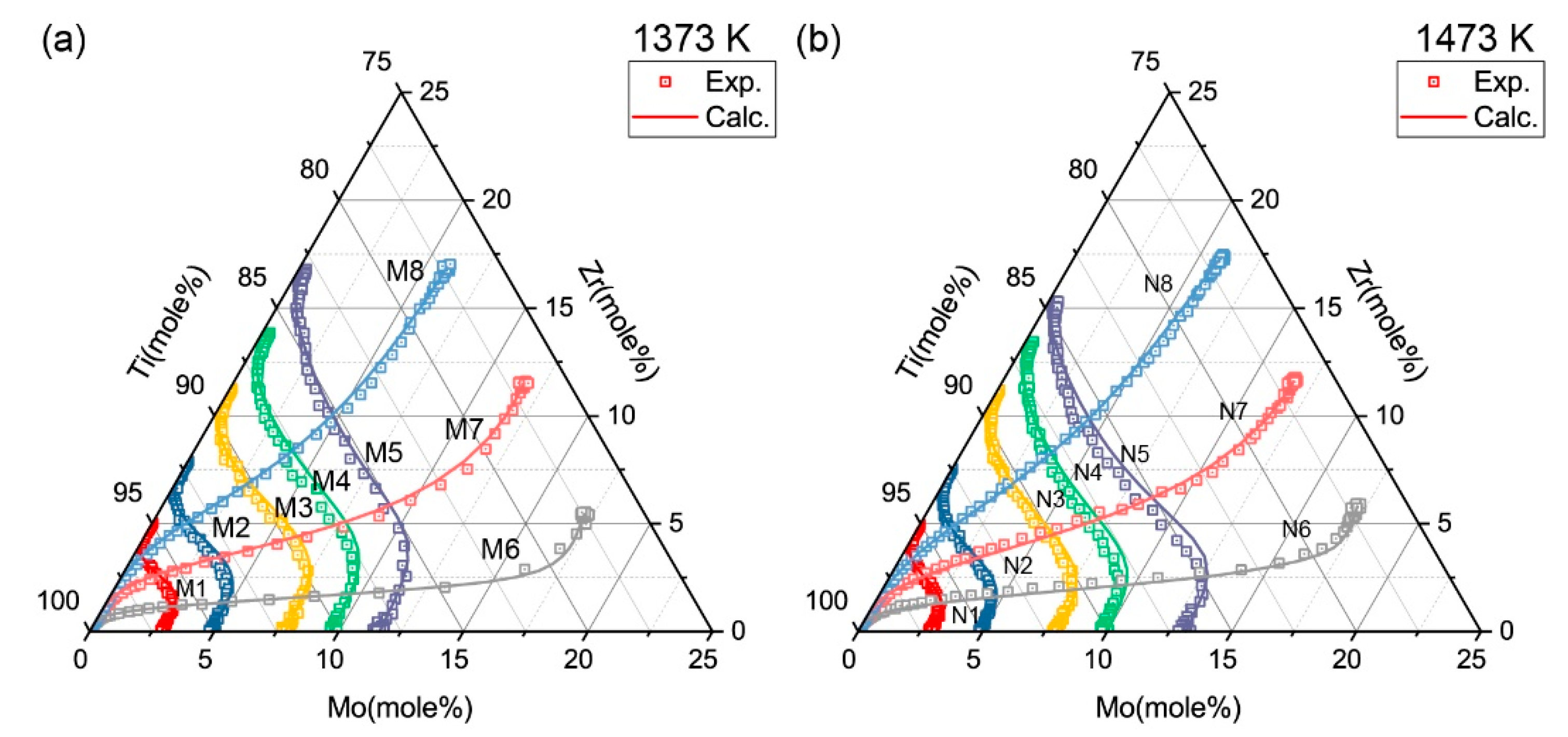
| Temperature (K) | Diffusion Couples | Composition (mole %) |
|---|---|---|
| 1373 | M1 | Ti-2.95Mo/Ti-4.95Zr |
| M2 | Ti-4.90Mo/Ti-7.81Zr | |
| M3 | Ti-7.83Mo/Ti-11.17Zr | |
| M4 | Ti-9.74Mo/Ti-13.64Zr | |
| M5 | Ti-11.61Mo/Ti-16.57Zr | |
| M6 | Pure Ti/Ti-17.18Mo-5.48Zr | |
| M7 | Pure Ti/Ti-11.63Mo-11.51Zr | |
| M8 | Pure Ti/Ti-5.91Mo-16.90Zr | |
| 1473 | N1 | Ti-2.85Mo/Ti-4.85Zr |
| N2 | Ti-4.92Mo/Ti-7.39Zr | |
| N3 | Ti-7.90Mo/Ti-11.02Zr | |
| N4 | Ti-9.90Mo/Ti-13.27Zr | |
| N5 | Ti-13.11Mo/Ti-15.15Zr | |
| N6 | Pure Ti/Ti-17.14Mo-5.81Zr | |
| N7 | Pure Ti/Ti-11.67Mo-11.61Zr | |
| N8 | Pure Ti/Ti-5.91Mo-17.32Zr |
| Temp. (K) | Diffusion Couple | Intersection Composition (mole %) | Inter-Diffusion Coefficients (m2·s−1) | ||||
|---|---|---|---|---|---|---|---|
| Mo | Zr | ||||||
| ×10−14 | ×10−14 | ×10−14 | ×10−13 | ||||
| 1373 | M1-M6 | 2.71 | 1.18 | 9.96 ± 1.12 | −7.26 ± 5.24 | 0.96 ± 0.38 | 6.00 ± 0.42 |
| M1-M7 | 1.46 | 2.54 | 13.84 ± 0.56 | −2.89 ± 1.72 | 3.57 ± 2.24 | 6.71 ± 0.37 | |
| M1-M8 | 0.45 | 3.45 | 17.08 ± 1.81 | 0.31 ± 0.25 | 12.63 ± 5.54 | 8.29 ± 0.31 | |
| M2-M6 | 4.73 | 1.38 | 6.67 ± 0.60 | −2.95 ± 1.93 | 1.55 ± 0.20 | 4.52 ± 0.11 | |
| M2-M7 | 3.32 | 3.33 | 9.79 ± 0.25 | −2.93 ± 0.27 | 2.52 ± 1.47 | 5.24 ± 0.10 | |
| M2-M8 | 1.38 | 4.93 | 15.41 ± 0.56 | −0.49 ± 0.33 | 11.30 ± 1.09 | 7.14 ± 0.10 | |
| M3-M6 | 7.72 | 1.56 | 3.56 ± 0.41 | −1.18 ± 0.41 | 1.07 ± 0.13 | 2.89 ± 0.10 | |
| M3-M7 | 5.99 | 4.24 | 6.45 ± 0.11 | −2.50 ± 0.36 | 1.25 ± 0.60 | 3.44 ± 0.15 | |
| M3-M8 | 2.90 | 6.74 | 14.48 ± 0.28 | −1.25 ± 0.48 | 7.11 ± 0.99 | 5.49 ± 0.15 | |
| M4-M6 | 9.49 | 1.68 | 2.93 ± 0.17 | −2.94 ± 1.04 | 0.50 ± 0.15 | 2.36 ± 0.06 | |
| M4-M7 | 7.58 | 4.76 | 5.92 ± 0.28 | −3.06 ± 0.58 | 0.34 ± 0.71 | 2.75 ± 0.14 | |
| M4-M8 | 3.88 | 8.07 | 14.69 ± 0.93 | −2.30 ± 0.84 | 5.98 ± 1.07 | 4.52 ± 0.04 | |
| M5-M6 | 11.45 | 1.83 | 2.31 ± 0.29 | −1.40 ± 1.31 | 0.16 ± 0.46 | 1.62 ± 0.06 | |
| M5-M7 | 9.24 | 5.50 | 4.84 ± 0.60 | −1.86 ± 0.68 | 0.72 ± 0.78 | 1.97 ± 0.02 | |
| M5-M8 | 4.80 | 9.73 | 14.49 ± 1.04 | −2.08 ± 0.50 | 4.76 ± 3.54 | 3.82 ± 0.16 | |
| ×10−13 | ×10−14 | ×10−13 | ×10−12 | ||||
| 1473 | N1-N6 | 1.524 | 3.605 | 3.37 ± 0.18 | −4.00 ± 2.19 | 0.45 ± 0.22 | 1.59 ± 0.06 |
| N1-N7 | 2.597 | 1.898 | 4.43 ± 0.01 | −2.46 ± 1.08 | 0.68 ± 0.40 | 1.69 ± 0.02 | |
| N1-N8 | 3.365 | 0.81 | 5.42 ± 0.13 | −1.39 ± 1.30 | 1.97 ± 1.16 | 1.83 ± 0.05 | |
| N2-N6 | 1.707 | 4.399 | 2.39 ± 0.19 | −13.24 ± 4.86 | 0.55 ± 0.06 | 1.23 ± 0.01 | |
| N2-N7 | 3.589 | 3.386 | 3.95 ± 0.12 | −3.33 ± 2.17 | 1.20 ± 0.23 | 1.43 ± 0.03 | |
| N2-N8 | 5.701 | 1.951 | 5.19 ± 0.12 | −2.16 ± 1.40 | 2.38 ± 0.21 | 1.62 ± 0.02 | |
| N3-N6 | 2.43 | 8.144 | 1.68 ± 0.04 | −2.24 ± 2.12 | 0.52 ± 0.09 | 0.82 ± 0.02 | |
| N3-N7 | 4.724 | 5.243 | 2.80 ± 0.05 | −3.75 ± 0.51 | 1.16 ± 0.15 | 1.06 ± 0.02 | |
| N3-N8 | 6.881 | 2.508 | 4.91 ± 0.08 | −2.11 ± 0.76 | 2.04 ± 0.51 | 1.36 ± 0.03 | |
| N4-N6 | 2.552 | 8.725 | 1.50 ± 0.09 | −13.40 ± 4.90 | 0.36 ± 0.10 | 0.71 ± 0.01 | |
| N4-N7 | 5.489 | 6.461 | 2.72 ± 0.17 | −11.73 ± 2.86 | 0.84 ± 0.06 | 0.93 ± 0.01 | |
| N4-N8 | 8.913 | 3.466 | 6.04 ± 0.46 | −8.67 ± 2.82 | 1.18 ± 0.19 | 1.27 ± 0.02 | |
| N5-N6 | 3.438 | 12.795 | 0.85 ± 0.03 | −3.56 ± 1.06 | 0.36 ± 0.06 | 0.40 ± 0.01 | |
| N5-N7 | 6.723 | 8.091 | 2.03 ± 0.07 | −1.85 ± 0.97 | 1.26 ± 0.26 | 0.68 ± 0.01 | |
| N5-N8 | 9.993 | 3.932 | 5.20 ± 0.44 | −3.24 ± 2.66 | 1.88 ± 0.66 | 1.12 ± 0.03 | |
| Temperature/K | Composition | Impurity Diffusion Coefficients (×10−13 m2·s−1) | Composition | Impurity Diffusion Coefficients (×10−13 m2·s−1) |
|---|---|---|---|---|
| 1373 | 5.85 ± 0.58 | 1.54 ± 0.09 | ||
| 4.51 ± 0.88 | 1.96 ± 0.62 | |||
| 2.55 ± 0.05 | 2.86 ± 0.71 | |||
| 2.13 ± 0.14 | 4.37 ± 1.68 | |||
| 1.39 ± 0.15 | 5.07 ± 1.45 | |||
| 1473 | 15.45 ± 1.22 | 5.04 ± 1.24 | ||
| 11.31 ± 0.74 | 6.42 ± 2.38 | |||
| 7.24 ± 0.07 | 9.78 ± 1.82 | |||
| 6.08 ± 0.26 | 12.61 ± 3.46 | |||
| 3.04 ± 0.17 | 14.66 ± 2.39 |
| Mobility | Parameter, J/mole | Reference |
|---|---|---|
| Mobility of Mo | ||
| −479740.87 − 63.98·T | [33] | |
| −196255.40 − 105.21·T | [33] | |
| −154895.63 − 140.91·T (T ≤ 1450 K) −214913.77 − 114.17·T (T ≥ 1450 K) | [34] | |
| −24153.22 − 45.32·T | [33] | |
| −61804.04 | [33] | |
| 150325.48 + 10.03·T | [34] | |
| −268357.13 + 283.18·T | This work | |
| 53485.75 | This work | |
| 2707991.87 | This work | |
| −719018.41 | This work | |
| Mobility of Ti | ||
| −435701.23 − 72.67·T | [33] | |
| −151989.95 − 127.37·T | [33] | |
| −140356.54 − 138.12·T | [33] | |
| −91728.48 + 64.56·T | [33] | |
| −96300.05 | [33] | |
| −15826.04 + 62.55·T | [33] | |
| 8243.54 | [33] | |
| −1304851.67 | This work | |
| −6019149.28 | This work | |
| −1128202.89 | This work | |
| Mobility of Zr | ||
| −464587.32 − 64.72·T | [34] | |
| −131670.56 − 133.36·T | [33] | |
| −104624.81 − 163.15·T (T≤1573 K) −161543.53 − 126.10·T (T≥1573 K) | [33] | |
| −81189.65 + 88.51·T | This work | |
| 210325.67 + 15.19·T | [34] | |
| −12581.03 + 33.38·T | [33] | |
| 2898.60 | [33] | |
| 2864755.37 | This work | |
| −340701.76 | This work | |
| −609499.74 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, W.; Xu, G.; Tan, M.; Yang, Z.; Zeng, L.; Wu, D.; Liu, L.; Zhang, L. Diffusivities and Atomic Mobilities in bcc Ti-Mo-Zr Alloys. Materials 2018, 11, 1909. https://doi.org/10.3390/ma11101909
Bai W, Xu G, Tan M, Yang Z, Zeng L, Wu D, Liu L, Zhang L. Diffusivities and Atomic Mobilities in bcc Ti-Mo-Zr Alloys. Materials. 2018; 11(10):1909. https://doi.org/10.3390/ma11101909
Chicago/Turabian StyleBai, Weimin, Guanglong Xu, Mingyue Tan, Zhijie Yang, Lijun Zeng, Di Wu, Libin Liu, and Ligang Zhang. 2018. "Diffusivities and Atomic Mobilities in bcc Ti-Mo-Zr Alloys" Materials 11, no. 10: 1909. https://doi.org/10.3390/ma11101909




