Ferromagnetic Properties of N-Doped and Undoped TiO2 Rutile Single-Crystal Wafers with Addition of Tungsten Trioxide
Abstract
:1. Introduction
2. Materials and Methods
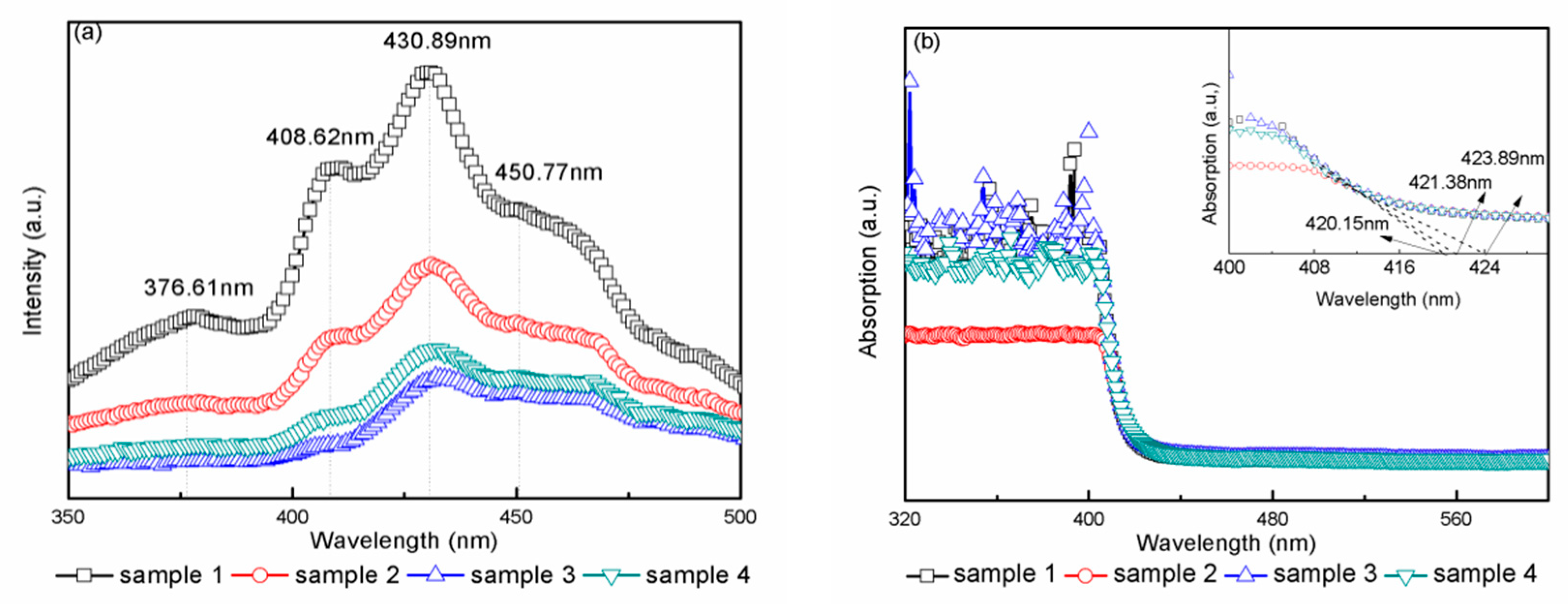
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prinz, G.A. Magnetoelectronics. Science 1998, 282, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Fiederling, R.; Keim, M.; Reuscher, G.; Ossau, W.; Schmidt, G.; Waag, A.; Molenkamp, L.W. Injection and detection of a spin-polarized current in a light-emitting diode. Nature 1999, 402, 787–790. [Google Scholar] [CrossRef]
- Han, H.F.; Wen, Z.C.; Wei, H.X. Nanoring magnetic tunnel junction and its application in magnetic random access memory demo devices with spin-polarized current switching (invited). J. Appl. Phys. 2008, 103, 07E933. [Google Scholar] [CrossRef]
- Manivannan, A.; Seehra, M.S.; Majumder, S.B.; Katiyar, R.S. Magnetism of Co-doped titania thin films prepared by spray pyrolysis. Appl. Phys. Lett. 2003, 83, 111–113. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.H.; Park, B.G.; Noh, H.J.; Oh, S.J.; Yang, J.; Kim, D.H.; Bu, S.; Noh, T.W.; Lin, H.; et al. Ferromagnetism induced by clustered Co in Co-doped anatase TiO2 thin films. Phys. Rev. Lett. 2003, 90, 017401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Y.; Tao, L.L.; Feng, J.F.; Sui, Y.; Tang, J.; Song, B.; Wang, Y.; Wang, Y.; Zhang, Y.; et al. Origin of ferromagnetism in aluminum-doped TiO2 thin films: Theory and experiments. Appl. Phys. Lett. 2014, 105, 262402. [Google Scholar] [CrossRef]
- Xiao, L.; Deng, M.; Zeng, W.; Zhang, B.; Xu, Z.; Yi, C.; Liao, G. Novel robust superhydrophobic coating with self-cleaning properties in air and oil based on rare earth metal oxide. Ind. Eng. Chem. Res. 2017, 56, 12354–12361. [Google Scholar] [CrossRef]
- Cuaila, J.L.S.; Alayo, W.; Avellaneda, C.O. Ferromagnetism in spin-coated cobalt-doped TiO2 thin films and the role of crystalline phases. J. Magn. Magn. Mater. 2017, 442, 212–217. [Google Scholar] [CrossRef]
- Kumar, A.; Kashyap, M.K.; Sabharwal, N.; Kumar, S.; Kumar, P.; Asokan, K. Structural, optical and weak magnetic properties of Co and Mn codoped TiO2 nanoparticles. Solid State Sci. 2017, 73, 19–26. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Z.P.; Wang, H.Y. Origin of ferromagnetism in Ru and N codoped TiO2 nanotubes: Experiments and theory investigations. J. Nanomater. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Elilarassi, R.; Chandrasekaran, G. Influence of nickel doping on the structural, optical and magnetic properties of TiO2 diluted magnetic semiconductor nanoparticles prepared by high energy ball-milling technique. J. Mater. Sci. Mater. Electron. 2017, 28, 14536–14542. [Google Scholar] [CrossRef]
- Ahmed, S.A. Structural, optical, and magnetic properties of Cu-doped TiO2 samples. Cryst. Res. Technol. 2017, 52, 1600335. [Google Scholar] [CrossRef]
- Tseng, L.T.; Luo, X.; Li, S.; Yi, J.B. Magnetic properties of Sm-doped rutile TiO2 nanorods. J. Alloys Compd. 2016, 687, 294–299. [Google Scholar] [CrossRef]
- Shi, B.; Liu, Y.; Song, C.; Han, G. First-principles investigation of the band structure of S-doped TiO2. Rare Met. Mater. Eng. 2008, 37, 638–640. [Google Scholar]
- Vasu, K.; Sreedhara, M.B.; Ghatak, J.; Rao, C.N.R. Atomic Layer Deposition of p-Type Epitaxial Thin Films of Undoped and N-Doped Anatase TiO2. ACS Appl. Mater. Interfaces 2016, 8, 7897–7901. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A. Ferromagnetism in Cr-, Fe-, and Ni-doped TiO2 samples. J. Magn. Magn. Mater. 2017, 442, 152–157. [Google Scholar] [CrossRef]
- Kumar, S.; Park, J.S.; Kim, D.J.; Lee, M.H.; Song, T.K.; Gautam, S.; Chae, K.H.; Kim, S.S.; Kim, M.H. Electronic structure and magnetic properties of Co doped TiO2 thin films using X-ray absorption spectroscopy. Ceram. Int. 2015, 41, S370–S375. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Stamenov, P.; Gunning, R.D.; Venkatesan, M.; Paul, K. Ferromagnetism in defect-ridden oxides and related materials. New. J. Phys. 2010, 12, 053025. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Xiang, H.J.; Wei, S.H.; Li, S.S.; Xia, J.B.; Li, J. Origin and enhancement of hole-induced ferromagnetism in first-row d0 semiconductors. Phys. Rev. Lett. 2009, 102, 017201. [Google Scholar] [CrossRef] [PubMed]
- Bapna, K.; Choudhary, R.J.; Pandey, S.K.; Phase, D.M.; Sharma, S.K.; Knobel, M. Electronic depictions of magnetic origin in undoped and Fe doped TiO2-d epitaxial thin films. Appl. Phys. Lett. 2011, 99, 112502. [Google Scholar] [CrossRef]
- Song, Y.L.; Wang, X.J.; Tao, L.L.; Song, B.Q.; Zhang, L.L.; Zhang, Y.; Sui, Y.; Liu, Z.G.; Tang, J.K.; Han, X.F. Effect of Ga-doping and oxygen vacancies on the ferromagnetism of TiO2 thin films. J. Alloys Compd. 2017, 694, 929–934. [Google Scholar] [CrossRef]
- Wang, J.B.; Wu, K.C.; Mi, J.W.; Luo, C.W.; Wu, K.H.; Uen, T.M.; Lin, J.Y.; Juang, J.Y.; Liu, S.J. Room-temperature ferromagnetism in carbon- and nitrogen-doped rutile TiO2. Appl. Phys. A 2015, 118, 725–731. [Google Scholar] [CrossRef]
- Park, K.S.; Nam, J.H.; Oh, J.H. Magnetic properties of with addition of tungsten trioxide. J. Magn. Magn. Mater. 2001, 226–230, 1415–1417. [Google Scholar] [CrossRef]
- Ding, Y.; Yuan, C.; Wang, Z.; Liu, S.; Shi, J.; Xiong, R.; Yin, D.; Lu, Z. Improving thermostability of Cr2O3 thin films by doping with Sn. Appl. Phys. Lett. 2014, 105, 092401. [Google Scholar] [CrossRef]
- Sajjad, A.K.L.; Shamaila, S.; Tian, B.; Chen, F.; Zhang, J. One step activation of WOx/TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2009, 91, 397–405. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Muilenberg, G.E., Ed.; Perkin-Eimer Corporation: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Jabbari, V.; Hamadanian, M.; Reisi-Vanani, A.; Razi, P.; Hoseinifardc, S.; Villagr´an, D. In, V-codoped TiO2 nanocomposite prepared via a photochemical reduction technique as a novel highefficiency visible-light-driven nanophotocatalyst. RSC Adv. 2015, 5, 78128–78135. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, H.; Zou, Z.; Du, M.; Guo, J.; Yang, Z. Investigations on the origin of ferromagnetism of Cu doped anatase TiO2 nanotubes. Mater. Res. Bull. 2017, 86, 287–294. [Google Scholar] [CrossRef]
- Chan, M.; Lu, F. Preparation of titanium oxynitride thin films by reactive sputtering using air/Ar mixtures. Surf. Coat. Technol. 2008, 203, 614–618. [Google Scholar] [CrossRef]
- Mohanty, P.; Mishra, N.C.; Choudhary, R.J.; Banerjee, A.; Shripathi, T.; Lalla, N.P.; Annapoorni, S.; Rath, C. Oxygen vacancy induced phase formation and room temperature ferromagnetism in un-doped and Co-doped TiO2 thin films. J. Phys. D Appl. Phys. 2012, 45, 1418–1420. [Google Scholar] [CrossRef]
- Sharma, S.; Chaudhary, S.; Kashyap, S.C.; Sharma, S.K. Room temperature ferromagnetism in Mn doped TiO2 thin films: Electronic structure and Raman investigations. J. Appl. Phys. 2011, 109, 083905. [Google Scholar] [CrossRef]
- Chen, L.; Qu, Y.; Yang, X.; Liao, B.; Xue, W.; Cheng, W. Characterization and first-principles calculations of WO3/TiO2 composite films on titanium prepared by microarc oxidation. Mater. Chem. Phys. 2017, 201, 311–322. [Google Scholar] [CrossRef]
- Rajagopal, S.; Nataraj, D.; Mangalaraj, D.; Djaoued, Y.; Robichaud, J.; Khyzhun, O.Y. Controlled growth of WO(3) nanostructures with three different morphologies and their structural, optical, and photodecomposition studies. Nanoscale Res. Lett. 2009, 4, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions inoxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Yoon, M.; Seo, M.; Jeong, C.; Jang, J.H.; Jeon, K.S. Synthesis of Liposome-templated titania nanodisks: optical properties and photocatalytic activities. Chem. Mater. 2005, 17, 6069–6079. [Google Scholar] [CrossRef]
- Ahmed, S.A. Annealing effects on structure and magnetic properties of Mn-doped TiO2. J. Magn. Magn. Mater. 2016, 402, 178–183. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gajbhiye, N.S. Room temperature magnetic properties of Cu-doped titanate, TiO2 (B) and anatasenanorods synthesized by hydrothermal method. Mater. Chem. Phys. 2012, 132, 175–179. [Google Scholar] [CrossRef]
- Huang, C.; Guo, Y.; Liu, X.; Wang, Y. Structural and optical properties of Ti1−xCoxO2 films prepared by sol–gel spin coating. Thin Solid Films 2006, 505, 141–144. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2−xNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Larumbe, S.; Monge, M. Room temperature ferromagnetism and absorption red-shift in nitrogen-doped TiO2 nanoparticles. J. Alloys Compd. 2014, 612, 450–455. [Google Scholar] [CrossRef]
- Zhou, S.; Lv, J.; Guo, L.K.; Xu, G.Q.; Wang, D.M.; Zheng, Z.X.; Wu, Y.C. Preparation and photocatalytic properties and N-doped nano-TiO2/muscovite composites. Appl. Surf. Sci. 2012, 258, 6136–6141. [Google Scholar] [CrossRef]
- Kim, D.; Hong, J.; Park, Y.R.; Kim, K.J. The origin of oxygen vacancy induced ferromagnetism in undoped TiO2. J. Phys. Condens. Matter 2009, 21, 195405. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gajbhiye, N.S. Oxygen deficiency induced ferromagnetism in Cr-doped TiO2 nanorods. J. Magn. Magn. Mater. 2013, 330, 21–24. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gajbhiye, N.S. Intrinsic room-temperature ferromagnetism of V-doped TiO2 (B) nanotubes synthesized by the hydrothermal method. Solid State Commun. 2011, 151, 1500–1503. [Google Scholar] [CrossRef]
- Tolea, F.; Grecu, M.N.; Kuncser, V.; Constantinescu, S.G.; Ghica, D. On the role of Fe ions on magnetic properties of doped TiO2 nanoparticles. Appl. Phys. Lett. 2015, 106, 142404. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakitab, K.; Fujii, M. Evidence of oxygen vacancy induced room temperature ferromagnetism in solvothermally synthesized undoped TiO2 nanoribbons. Nanoscale 2013, 5, 5476–5488. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, H.; Zhou, Z.; Zou, Z. Ferromagnetic Properties of N-Doped and Undoped TiO2 Rutile Single-Crystal Wafers with Addition of Tungsten Trioxide. Materials 2018, 11, 1934. https://doi.org/10.3390/ma11101934
Xu J, Wang H, Zhou Z, Zou Z. Ferromagnetic Properties of N-Doped and Undoped TiO2 Rutile Single-Crystal Wafers with Addition of Tungsten Trioxide. Materials. 2018; 11(10):1934. https://doi.org/10.3390/ma11101934
Chicago/Turabian StyleXu, Jing, Haiying Wang, Zhongpo Zhou, and Zhaorui Zou. 2018. "Ferromagnetic Properties of N-Doped and Undoped TiO2 Rutile Single-Crystal Wafers with Addition of Tungsten Trioxide" Materials 11, no. 10: 1934. https://doi.org/10.3390/ma11101934



