Corrosion Failure Mechanism of Associated Gas Transmission Pipeline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Pipeline
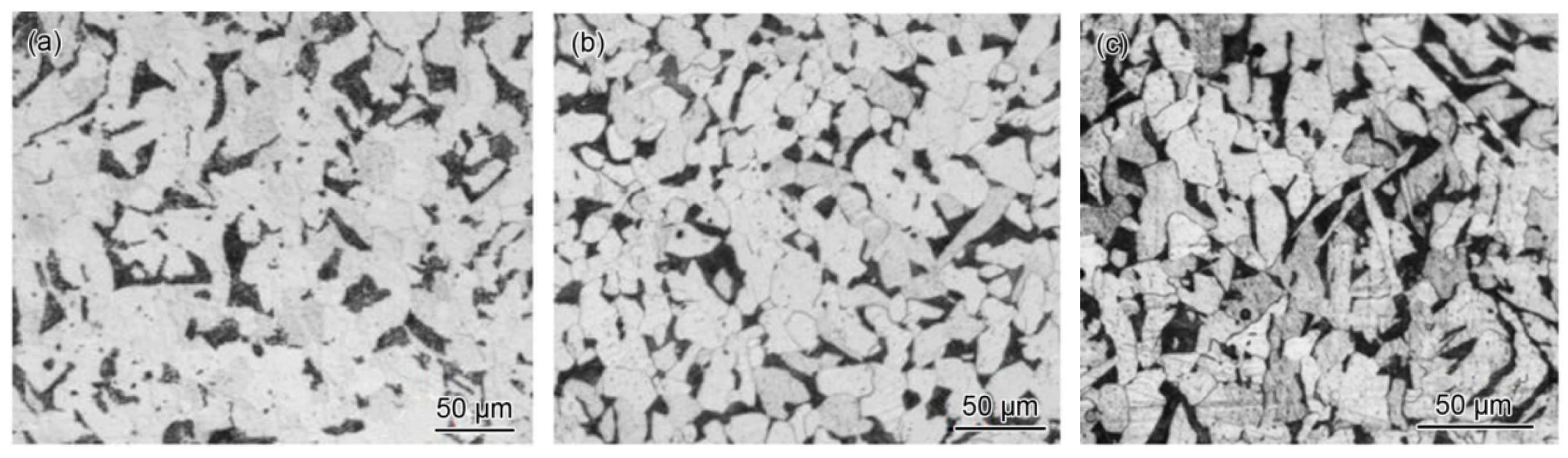
2.2. Laboratory Studies of Corroded Pipe Sections
2.3. Corrosion Simulation Tests
3. Results
3.1. Macroscopic Observation of Corroded Pipe Sections
3.2. Analysis of Corrosion Products
3.3. Corrosion Simulation Test Results
3.3.1. Failure Mode and Corrosion Products
3.3.2. Corrosion Rate
- General corrosion rate
- Local corrosion rate in a gaseous environment
- Uneven general corrosion near the waterline
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, L.; Zhang, F. The study on effects of associated gas generations in oilfield grid. In Environmental Protection and Resources Exploitation, PTS 1–3, Proceedings of International Conference on Advances in Energy and Environmental Science, Guangzhou, China, 30–31 July 2013; Trans Tech Publications Ltd.: Guangzhou, China, 2013; pp. 2589–2594. [Google Scholar]
- Lawal, K.A.; Ovuru, M.I.; Eyitayo, S.I.; Matemilola, S.; Adeniyi, A.T. Underground storage as a solution for stranded associated gas in oil fields. J. Petrol. Sci. Eng. 2017, 150, 366–375. [Google Scholar] [CrossRef]
- Moreno, J.; Badawy, A.; Kartoatmodjo, G.; AlShuraiqi, H.; Zulkhifly, F.; Tan, L.; Friedel, T. Flaring, gas injection and reservoir management optimization: Preserving reservoir energy maximizes recovery and prolong the life of an ageing brown field. In Proceedings of the Asia Pacific Oil and Gas Conference & Exhibition, Jakarta, Indonesia, 4–6 August 2009; Society of Petroleum Engineers: Jakarta, Indonesia, 2009; pp. 262–270. [Google Scholar]
- Kaya, E.; Zarrouk, S.J. Reinjection of greenhouse gases into geothermal reservoirs. Int. J. Greenh. Gas Con. 2017, 67, 111–129. [Google Scholar] [CrossRef]
- Zheng, D.; Xu, H.; Wang, J.; Sun, J.; Zhao, K.; Li, C.; Shi, L.; Tang, L. Key evaluation techniques in the process of gas reservoir being converted into underground gas storage. Petrol. Explor. Dev. 2017, 44, 840–849. [Google Scholar] [CrossRef]
- Blann, J.R.; Laville, G.M. Gas lifting a major oil field in Argentina with high CO2 content associated gas. In Proceedings of the 1995 SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 22–25 October 1995; Society of Petroleum Engineers (SPE): Dallas, TX, USA, 1995; pp. 83–91. [Google Scholar]
- Misener, R.; Gounaris, C.E.; Floudas, C.A. Global optimization of gas lifting operations: A comparative study of piecewise linear formulations. Ind. Eng. Chem. Res. 2009, 48, 6098–6104. [Google Scholar] [CrossRef]
- Javidi, M.; Bekhrad, S. Failure analysis of a wet gas pipeline due to localised CO2 corrosion. Eng. Fail. Anal. 2018, 89, 46–56. [Google Scholar] [CrossRef]
- Mansoori, H.; Mirzaee, R.; Esmaeilzadeh, F.; Vojood, A.; Dowrani, A.S. Pitting corrosion failure analysis of a wet gas pipeline. Eng. Fail. Anal. 2017, 82, 16–25. [Google Scholar] [CrossRef]
- Laumb, J.D.; Glazewski, K.A.; Hamling, J.A.; Azenkeng, A.; Kalenze, N.; Watson, T.L. Corrosion and failure assessment for CO2 EOR and associated storage in the Weyburn Field. Eng. Procedia 2017, 114, 5173–5181. [Google Scholar] [CrossRef]
- Ilman, M.N. Analysis of internal corrosion in subsea oil pipeline. Case Stud. Eng. Fail. Anal. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Pouraria, H.; Seo, J.K.; Paik, J.K. A numerical study on water wetting associated with the internal corrosion of oil pipelines. Ocean Eng. 2016, 122, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Ryder, J.; Tilekar, J.; Srinivasan, S.; Yap, K.M. Advanced internal corrosion direct assessment methodology for wet gas/dry gas pipelines. In Proceedings of the Corrosion 2014: Collaborate Educate Innovate Mitigate, San Antonio, TX, USA, 9–13 March 2014; National Assoc. of Corrosion Engineers International: San Antonio, TX, USA, 2014. [Google Scholar]
- Ren, L.; Jiang, T.; Jia, Z.G.; Li, D.S.; Yuan, C.L.; Li, H.N. Pipeline corrosion and leakage monitoring based on the distributed optical fiber sensing technology. Measurement 2018, 122, 57–65. [Google Scholar] [CrossRef]
- Elhoud, A.; Jewilli, F.; Abouswa, K.; Rageai, O. Failure analysis: Internal corrosion rupture of a 6-in gas line. Mater. Perform. 2012, 51, 70–73. [Google Scholar]
- Zhang, H.; Lan, H.Q. A review of internal corrosion mechanism and experimental study for pipelines based on multiphase flow. Corros. Rev. 2017, 35, 425–444. [Google Scholar] [CrossRef]
- Belarbi, Z.; Farelas, F.; Singer, M.; Nei, S. Role of amines in the mitigation of CO2 top of the line corrosion. Corrosion 2016, 72, 1300–1310. [Google Scholar] [CrossRef]
- Ifezue, D. Root cause failure analysis of corrosion in wet gas piping. J. Fail. Anal. Prev. 2017, 17, 971–978. [Google Scholar] [CrossRef]
- Eckert, R.B. Internal corrosion health check advised for liquid and gas pipeline operators. Mater. Perform. 2012, 51, 48–53. [Google Scholar]
- Wang, B.; Liu, X.L.; Xiong, Z.X.; Cheng, J.C.; Yang, B.; Yu, C. Corrosion reasons and control measures of a natural gas pipeline. Surf. Technol. 2018, 47, 89–94. [Google Scholar] [CrossRef]
- Qiao, Q.; Cheng, G.; Wu, W.; Li, Y.; Huang, H.; Wei, Z. Failure analysis of corrosion at an inhomogeneous welded joint in a natural gas gathering pipeline considering the combined action of multiple factors. Eng. Fail. Anal. 2016, 64, 126–143. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nesic, S.; Young, D. Effect of impurities on the corrosion behavior of CO2 transmission pipeline steel in supercritical CO2-water environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Al-Khamis, J.; Nesic, S. Experimental study of sour top-of-the-line corrosion using a novel experimental setup. Corrosion 2013, 69, 624–638. [Google Scholar] [CrossRef]
- Singer, M.; Camacho, A.; Brown, B.; Neic, S. Sour top-of-The-line corrosion in the presence of acetic acid. Corrosion 2011, 67, 0850031–08500316. [Google Scholar] [CrossRef]
- Kovalenko, S.Y.; Rybakov, A.O.; Klymenko, A.V.; Shytova, L.H. Corrosion of the internal wall of a field gas pipeline. Mater. Sci. 2012, 48, 225–230. [Google Scholar] [CrossRef]
- Wan, H.X.; Yang, X.J.; Liu, Z.Y.; Song, D.D.; Du, C.W.; Li, X.G. Pitting behavior of L415 pipeline steel in simulated leaching liquid environment. J. Mater. Eng. Perform. 2017, 26, 715–722. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Wang, Y.; Sui, P.; Lin, X.; Liu, H.; Cheng, X.; Zhou, M. Effect of impurity interaction on the corrosion film characteristics and corrosion morphology evolution of X65 steel in water-saturated supercritical CO2 system. Int. J. Greenh. Gas Con. 2017, 65, 117–127. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Sun, J.; Lin, X.; Li, X.; Liu, H.; Cheng, X. Effect of impurity on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. J. Supercrit. Fluid 2016, 116, 70–82. [Google Scholar] [CrossRef]
- Zhao, W.-M.; Wang, Y.; Dong, L.-X.; Wu, K.-Y.; Xue, J. Corrosion mechanism of NiCrBSi coatings deposited by HVOF. Surf. Coat. Technol. 2005, 190, 293–298. [Google Scholar] [CrossRef]
- De Oliveira, L.A.; Correa, O.V.; dos Santos, D.J.; Páez, A.A.Z.; de Oliveira, M.C.L.; Antunes, R.A. Effect of silicate-based films on the corrosion behavior of the API 5L X80 pipeline steel. Corros. Sci. 2018, 139, 21–34. [Google Scholar] [CrossRef]
- Wang, S.; Du, C.; Li, X.; Liu, Z.; Zhu, M.; Zhang, D. Field corrosion characterization of soil corrosion of X70 pipeline steel in a red clay soil. Prog. Nat. Sci. Mater. Int. 2015, 25, 242–250. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Dao, A.Q.; Zhang, G.; Lv, Y.; Liu, H. Study of corrosion behavior and mechanism of carbon steel in the presence of Chlorella vulgaris. Corros. Sci. 2015, 101, 84–93. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Yu, Y.; Luo, Z.; Liu, W.; Wang, C. Corrosion behavior of X65 pipeline steel: Comparison of wet–Dry cycle and full immersion. Corros. Sci. 2018, 133, 276–287. [Google Scholar] [CrossRef]
- De Motte, R.A.; Barker, R.; Burkle, D.; Vargas, S.M.; Neville, A. The early stages of FeCO3 scale formation kinetics in CO2 corrosion. Mater. Chem. Phys. 2018, 216, 102–111. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Brown, B.; Nesic, S.; Singer, M. Investigation of precipitation kinetics of FeCO3 by EQCM. Corros. Sci. 2018, 141, 195–202. [Google Scholar] [CrossRef]
- Pfennig, A.; Wolthusen, H.; Kranzmann, A. Unusual corrosion behavior of 1.4542 exposed a laboratory saline aquifer water CCS-environment. Eng. Procedia 2017, 114, 5229–5240. [Google Scholar] [CrossRef]
- Speight, J.G. Oil and Gas Corrosion Prevention: From Surface Facilities to Refineries; Elsevier Inc.: Waltham, MA, USA, 2014; pp. 1–151. [Google Scholar]
- Wu, J.; Wang, P.; Gao, J.; Tan, F.; Zhang, D.; Cheng, Y.; Chen, S. Comparison of water-line corrosion processes in natural and artificial seawater: The role of microbes. Electrochem. Commun. 2017, 80, 9–15. [Google Scholar] [CrossRef]
- Jeffrey, R.; Melchers, R.E. Corrosion of vertical mild steel strips in seawater. Corros. Sci. 2009, 51, 2291–2297. [Google Scholar] [CrossRef]
- Li, W.; Pots, B.F.M.; Brown, B.; Kee, K.E.; Nesic, S. A direct measurement of wall shear stress in multiphase flow-Is it an important parameter in CO2 corrosion of carbon steel pipelines? Corros. Sci. 2016, 110, 35–45. [Google Scholar] [CrossRef]
- Hatami, S.; Ghaderi-Ardakani, A.; Niknejad-Khomami, M.; Karimi-Malekabadi, F.; Rasaei, M.R.; Mohammadi, A.H. On the prediction of CO2 corrosion in petroleum industry. J. Supercrit. Fluid 2016, 117, 108–112. [Google Scholar] [CrossRef]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gas pipelines–A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Mahmoud, A.; Liu, S.; Sunarso, J. Revamping existing glycol technologies in natural gas dehydration to improve the purity and absorption efficiency: Available methods and recent developments. J. Nat. Gas Sci. Eng. 2018, 56, 486–503. [Google Scholar] [CrossRef]
- Sakheta, A.; Zahid, U. Process simulation of dehydration unit for the comparative analysis of natural gas processing and carbon capture application. Chem. Eng. Res. Des. 2018, 137, 75–88. [Google Scholar] [CrossRef]












| C | Si | Mn | P | S | V | Nb | Ti | Cu | Ni | Cr | Mo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2″ | 0.167 | 0.245 | 0.408 | 0.009 | 0.025 | 0.000 | 0.000 | 0.000 | 0.018 | 0.000 | 0.027 | 0.000 |
| 4″ | 0.199 | 0.207 | 0.360 | 0.017 | 0.009 | 0.000 | 0.000 | 0.001 | 0.106 | 0.007 | 0.032 | 0.000 |
| 6″ | 0.175 | 0.251 | 0.499 | 0.010 | 0.010 | 0.000 | 0.000 | 0.000 | 0.100 | 0.000 | 0.036 | 0.000 |
| Spec | ≤0.24 | ≤0.40 | ≤1.20 | ≤0.025 | ≤0.015 | ≤0.06 | ≤0.05 | ≤0.04 | ≤0.50 | ≤0.30 | ≤0.30 | ≤0.15 |
| Steel Sample | Rt0.5 (MPa) | Rm (MPa) | Rt0.5/Rm |
|---|---|---|---|
| 2″ | 435 | 500 | 0.87 |
| 4″ | 350 | 498 | 0.70 |
| 6″ | 375 | 515 | 0.73 |
| Spec | 290–495 | 415–655 | ≤0.93 |
| Composition | Percent | Composition | Percent | Composition | Percent | Composition | Percent |
|---|---|---|---|---|---|---|---|
| Methane | 72.72 | Nitrogen | 19.89 | Ethane | 3.66 | CO2 | 1.30 |
| Propane | 0.87 | Oxygen | 0.47 | n-Butane | 0.36 | i-Butane | 0.25 |
| iso-Pentane | 0.19 | N-Pentane | 0.14 | >C5 | 0.10 | CO | 0.05 |
| Composition | [C] | Composition | [C] | Composition | [C] | Composition | [C] |
|---|---|---|---|---|---|---|---|
| Li+ | 0.45 | Na+ | 24.18 | NH4+ | 0.43 | K+ | 2.3 |
| Mg2+ | 0.14 | Ca2+ | 1.62 | Cl- | 0.88 | SO42- | 72.21 |
| Specimen | No. | Surface Area (mm2) | Weight Loss (g) | Corrosion Rate (mm/y) | |
|---|---|---|---|---|---|
| 2″ | 1 | 1207.83 | 0.1637 | 1.12 | 1.15 |
| 2 | 1211.13 | 0.1787 | 1.13 | ||
| 3 | 1208.51 | 0.1745 | 1.19 | ||
| 4″ | 5 | 1332.04 | 0.2210 | 1.37 | 1.35 |
| 6 | 1341.24 | 0.2203 | 1.36 | ||
| 7 | 1335.49 | 0.2117 | 1.31 | ||
| 6″ | 9 | 1298.14 | 0.2198 | 1.40 | 1.34 |
| 10 | 1350.60 | 0.2164 | 1.32 | ||
| 11 | 1333.63 | 0.2075 | 1.29 | ||
| Specimen | Corrosion Pits Depth (μm) | Corrosion Rate (mm/y) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Ave. | Max. | |
| 2″ | 15.46 | 16.11 | 15.46 | 16.08 | 15.88 | 16.12 | 15.65 | 16.09 | 16.22 | 16.12 | 1.0330 | 1.0460 |
| 4″ | 19.36 | 19.64 | 18.98 | 19.17 | 19.55 | 18.87 | 19.86 | 19.52 | 18.64 | 19.06 | 1.2501 | 1.2744 |
| 6″ | 18.69 | 19.38 | 19.93 | 18.76 | 19.46 | 18.66 | 19.86 | 19.84 | 19.37 | 18.99 | 1.2520 | 1.2887 |
| Specimen | Location | ||
|---|---|---|---|
| 1 mm Below Waterline | 5 mm Below Waterline | Near Bottom | |
| 2″ | 2.3661 | 1.2016 | 1.2146 |
| 4″ | 2.7675 | 1.3642 | 1.3578 |
| 6″ | 2.7864 | 1.3489 | 1.3562 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhang, T.; Wang, Y.; Qiao, J.; Wang, Z. Corrosion Failure Mechanism of Associated Gas Transmission Pipeline. Materials 2018, 11, 1935. https://doi.org/10.3390/ma11101935
Zhao W, Zhang T, Wang Y, Qiao J, Wang Z. Corrosion Failure Mechanism of Associated Gas Transmission Pipeline. Materials. 2018; 11(10):1935. https://doi.org/10.3390/ma11101935
Chicago/Turabian StyleZhao, Weimin, Timing Zhang, Yonglin Wang, Jianhua Qiao, and Zerui Wang. 2018. "Corrosion Failure Mechanism of Associated Gas Transmission Pipeline" Materials 11, no. 10: 1935. https://doi.org/10.3390/ma11101935




