Microstructure and Mechanical Properties of ZrB2–HfC Ceramics Influenced by HfC Addition
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Effects of HfC Addition on Microstructure of ZrB2–HfC Ceramics
3.2. Effects of HfC Content on Relative Density and Mechanical Properties of ZrB2–HfC Ceramics
4. Conclusions
- (1)
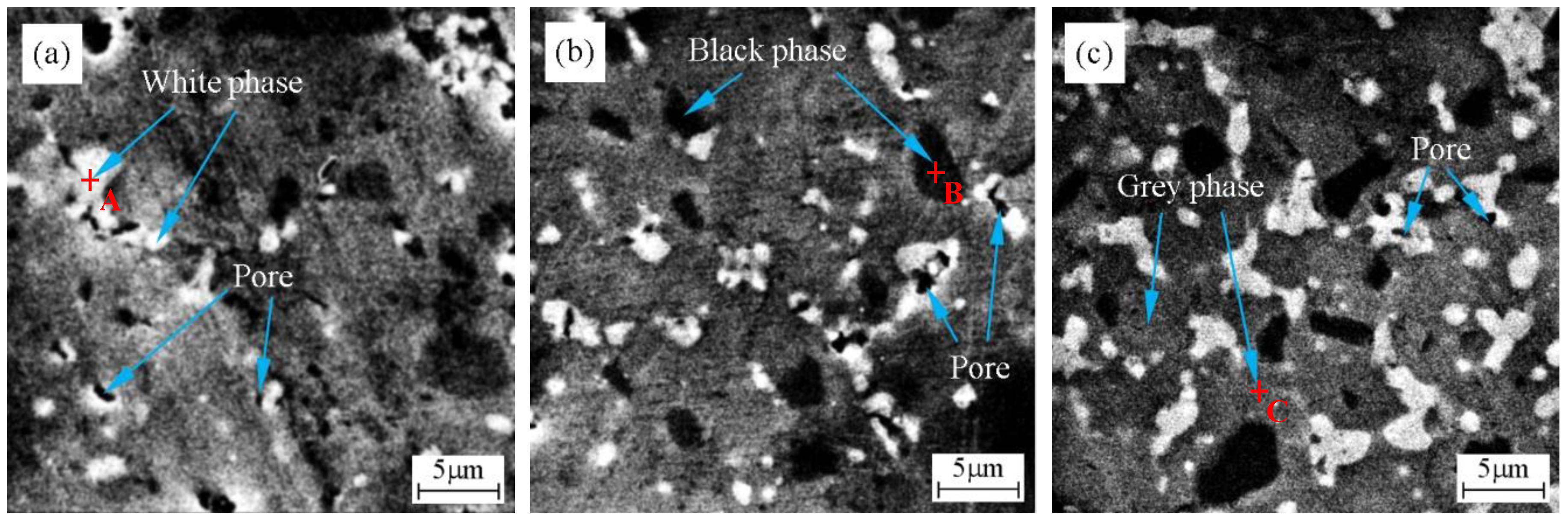
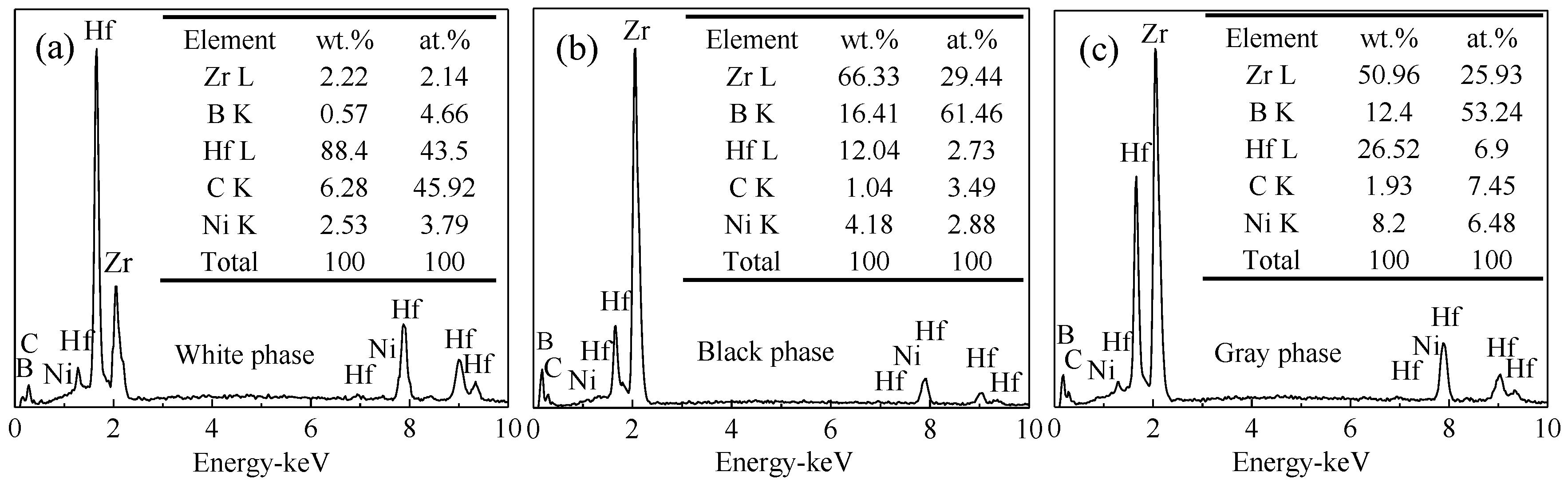
- ZrB2–HfC–Ni ceramics were mainly composed of ZrB2, HfC, and Ni. There were three phases: A white phase, a black phase, and a grey phase. The white phase was HfC, the black phase was ZrB2, and the grey phase was a mixture of ZrB2, HfC, and Ni with a small amount of possible (Zr, Hf)B2 solid solution.
- (2)
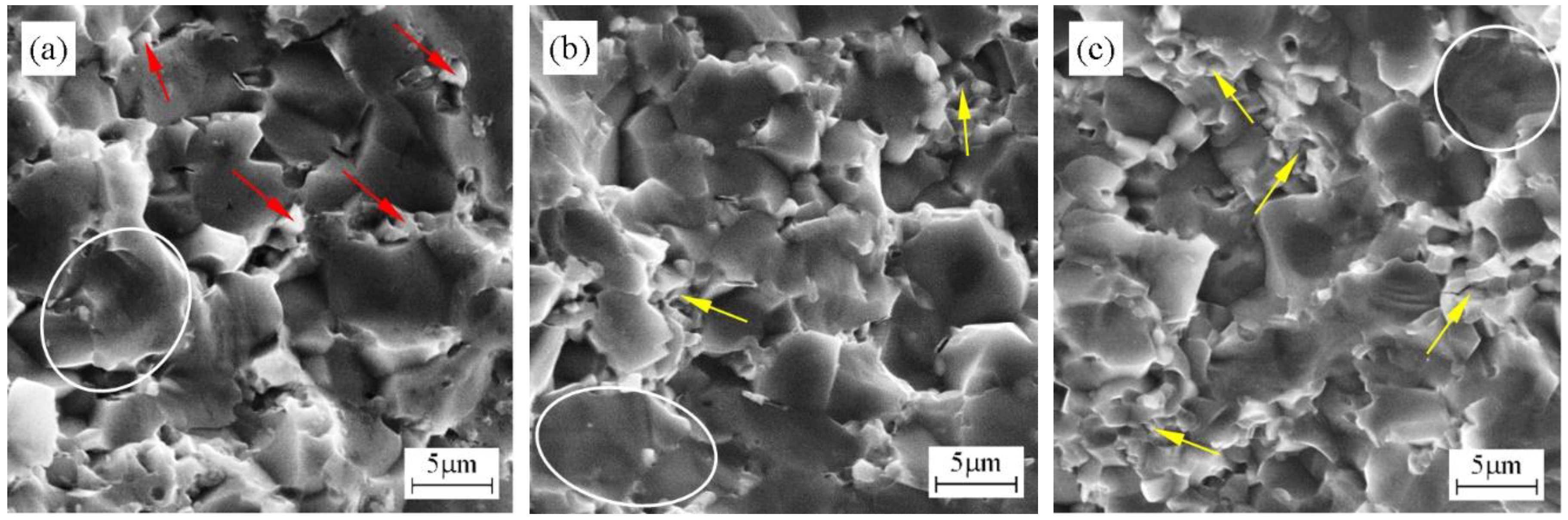
- Small HfC grains were distributed among the ZrB2 grain boundaries. These small grains could improve the density of ZrB2–based ceramics and play the pinning role in these ceramics. ZrB2 grain growth influenced by HfC addition was not significant.
- (3)
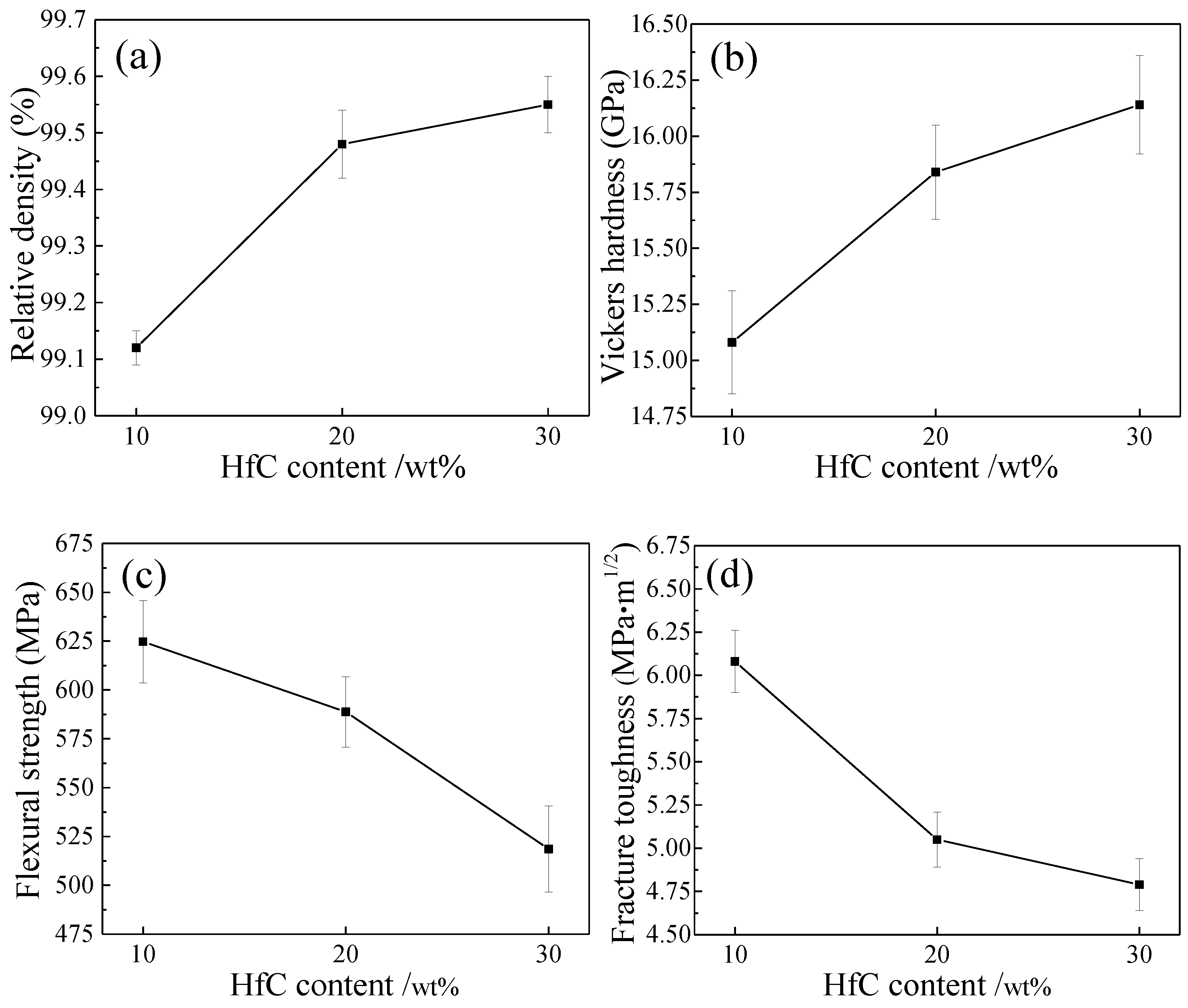
- With HfC content increasing from 10 wt % to 30 wt %, more HfC grains were distributed among ZrB2 grain boundaries, leading to weak interface bonding among HfC grains; the relative density and Vickers hardness increased and flexural strength and fracture toughness decreased. The weak interface bonding in ZH20N and ZH30N ceramics accounted for lowering the flexural strength and fracture toughness of these ceramics.
Author Contributions
Funding
Conflicts of Interest
References
- Sun, X.; Han, W.B.; Liu, Q.; Hu, P.; Hong, C.Q. ZrB2–ceramic toughened by refractory metal Nb prepared by hot-pressing. Mater. Des. 2010, 31, 4427–4431. [Google Scholar] [CrossRef]
- Wang, S.B.; Xu, C.; Ding, Y.B.; Zhang, X.H. Thermal shock behavior of ZrB2–SiC composite ceramics with added TaSi2. Int. J. Refract. Met. Hard Mater. 2013, 41, 507–516. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Jayaseelan, D.D.; Brown, P.M.; Lee, W.E. Effect of oxidation on room temperature strength of ZrB2– and HfB2–based ultrahigh temperature ceramics. Adv. Appl. Ceram. 2015, 114, 407–417. [Google Scholar] [CrossRef]
- Han, J.C.; Hu, P.; Zhang, X.H.; Meng, S.H.; Han, W.B. Oxidation-resistant ZrB2–SiC composites at 2200 °C. Compos. Sci. Technol. 2008, 68, 799–806. [Google Scholar] [CrossRef]
- Wang, M.F.; Wang, C.A.; Zhang, X.H. Effects of SiC platelet and ZrSi2 additive on sintering and mechanical properties of ZrB2–based ceramics by hot-pressing. Mater. Des. 2012, 34, 293–297. [Google Scholar] [CrossRef]
- Li, B.; Wang, H. Prediction and analysis of microstructural effects on fabrication of ZrB2/(Ti,W)C composites. Int. J. Refract. Met. Hard Mater. 2013, 36, 167–173. [Google Scholar] [CrossRef]
- Choi, S.K.; Ui, S.W.; Choi, I.S.; Choi, S.C. Densification behavior of ZrB2 with Co–WC as additives. J. Ceram. Soc. Jpn. 2014, 122, 198–203. [Google Scholar] [CrossRef]
- Sonber, J.K.; Murthy, T.S.R.C.; Subramanian, C.; Hubli, R.C.; Fotedar, R.K.; Suri, A.K. Effect of WSi2 addition on densification and properties of ZrB2. Adv. Appl. Ceram. 2014, 113, 114–119. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Zhang, H.A.; Yang, Y.H.; Zhao, T.Y. Densification and properties of ZrO2 fiber toughed ZrB2–SiC ceramics via spark plasma sintering. Mat. Sci. Eng. A 2015, 644, 204–209. [Google Scholar] [CrossRef]
- Diletta, S.; Stefano, G. Densification and mechanical behavior of HfC and HfB2 fabricated by spark plasma sintering. J. Am. Ceram. Soc. 2008, 91, 1433–1440. [Google Scholar]
- Xiang, L.Y.; Cheng, L.F.; Shi, L.; Yin, X.W.; Zhang, L.T. Laminated HfC–SiC ceramics produced by aqueous tape casting and hot pressing. Ceram. Int. 2015, 41, 14406–14411. [Google Scholar] [CrossRef]
- Ludovic, C.; Marianne, B.P.; Sans, J.L.; Diletta, S.; Laura, S. Effect of high temperature oxidation on the radiative properties of HfC–based ceramics. Corros. Sci. 2017, 126, 255–264. [Google Scholar]
- Pienti, L.; Sciti, D.; Silvestroni, L.; Cecere, A.; Savino, R. Ablation tests on HfC– and TaC–based ceramics for aeropropulsive applications. J. Eur. Ceram. Soc. 2015, 35, 1401–1411. [Google Scholar] [CrossRef]
- Omar, C.B.; Salvatore, G.; Nasrin, A.N.; Daniel, D.J.; Michael, J.R.; William, E.L. Sintering behaviour, solid solution formation and characterisation of TaC, HfC and TaC–HfC fabricated by spark plasma sintering. J. Eur. Ceram. Soc. 2016, 36, 1539–1548. [Google Scholar]
- Cai, T.; Liu, D.; Qiu, W.F.; Han, W.J.; Zhao, T. Polymer precursor-derived HfC–SiC ultrahigh-temperature ceramic nanocomposites. J. Am. Ceram. Soc. 2018, 101, 20–24. [Google Scholar] [CrossRef]
- Gao, J.J.; Song, J.P.; Liang, G.X.; An, J.; Cao, L.; Xie, J.C.; Lv, M. Effects of HfC addition on microstructures and mechanical properties of TiC0.7N0.3–based and TiC0.5N0.5–based ceramic tool materials. Ceram. Int. 2017, 43, 14945–14950. [Google Scholar] [CrossRef]
- Song, J.P.; Jiang, L.K.; Liang, G.X.; Gao, J.J.; An, J.; Cao, L.; Xie, J.C.; Wang, S.Y.; Lv, M. Strengthening and toughening of TiN–based and TiB2–based ceramic tool materials with HfC additive. Ceram. Int. 2017, 43, 8202–8207. [Google Scholar] [CrossRef]
- Song, J.P.; Cao, L.; Jiang, L.K.; Liang, G.X.; Gao, J.J.; Li, D.X.; Wang, S.Y.; Lv, M. Effect of HfN, HfC and HfB2 additives on phase transformation, microstructure and mechanical properties of ZrO2–based ceramics. Ceram. Int. 2018, 44, 5371–5377. [Google Scholar] [CrossRef]
- Wang, R.Z.; Li, W.G. Effects of microstructures and flaw evolution on the fracture strength of ZrB2–MoSi2 composites under high temperatures. J. Alloy Compd. 2015, 644, 582–588. [Google Scholar] [CrossRef]
- Chakraborty, S.; Debnath, D.; Mallick, A.R.; Das, P.K. Mechanical, tribological, and thermal properties of hot-pressed ZrB2–B4C composite. Int. J. Appl. Ceram. Technol. 2015, 12, 568–576. [Google Scholar] [CrossRef]
- He, J.B.; Cao, Y.J.; Zhang, Y.X.; Wang, Y.G. Mechanical properties of ZrB2–SiC ceramics prepared by polymeric precursor route. Ceram. Int. 2018, 44, 6520–6526. [Google Scholar] [CrossRef]
- Chakraborty, S.; Debnath, D.; Mallick, A.R.; Das, P.K. Mechanical and thermal properties of hot pressed ZrB2 system with TiB2. Int. J. Refract. Met. Hard Mater. 2014, 46, 35–42. [Google Scholar] [CrossRef]
- Ma, H.B.; Liu, H.L.; Zhao, J.; Xu, F.F.; Zhang, G.J. Pressureless sintering, mechanical properties and oxidation behavior of ZrB2 ceramics doped with B4C. J. Eur. Ceram. Soc. 2015, 35, 2699–2705. [Google Scholar] [CrossRef]
- Jin, X.X.; Dong, L.M.; Xu, H.Y.; Liu, L.Z.; Li, N.; Zhang, X.H.; Han, J.C. Effects of porosity and pore size on mechanical and thermal properties as well as thermal shock fracture resistance of porous ZrB2–SiC ceramics. Ceram. Int. 2016, 42, 9051–9057. [Google Scholar] [CrossRef]
- Alireza, R.; William, G.F.; Gregory, E.H. Effect of hot pressing time and temperature on the microstructure and mechanical properties of ZrB2–SiC. J. Mater. Sci. 2007, 42, 2735–2744. [Google Scholar]
- Zhang, X.; Liu, R.T.; Zhang, X.Y.; Zhu, Y.Y.; Sun, W.; Xiong, X. Densification and ablation behavior of ZrB2 ceramic with SiC and / or Fe additives fabricated at 1600 and 1800 °C. Ceram. Int. 2016, 42, 17074–17080. [Google Scholar] [CrossRef]
- Debnath, D.; Chakraborty, S.; Mallick, A.R.; Gupta, R.K.; Ranjan, A.; Das, P.K. Mechanical, tribological and thermal properties of hot pressed ZrB2–SiC composite with SiC of different morphology. Adv. Appl. Ceram. 2015, 114, 45–54. [Google Scholar] [CrossRef]
- Monteverde, F.; Fabbriche, D.D.; Bellosi, A. Zirconium diboride–based composites. Key Eng. Mater. 2002, 206–213, 961–964. [Google Scholar] [CrossRef]
- Mousavi, M.J.; Zakeri, M.; Rahimipour, M.R.; Amini, E. Effect of Ni and C additives on pressureless sintering and mechanical properties of ZrB2. Adv. Appl. Ceram. 2015, 114, 261–266. [Google Scholar] [CrossRef]
- Zhao, G.L.; Huang, C.Z.; Liu, H.L.; Zou, B.; Zhu, H.T.; Wang, J. Microstructure and mechanical properties of TiB2–SiC ceramic composites by Reactive Hot Pressing. Int. J. Refract. Met. Hard Mater. 2014, 42, 36–41. [Google Scholar] [CrossRef]
- Yue, X.Y.; Cai, Z.X.; Lv, X.H.; Wang, J.J.; Ru, H.Q. Effect of Ni content on microstructures and mechanical properties of hot-pressed TiC–TiB2–Ni composite. Mat. Sci. Eng. A 2016, 668, 208–214. [Google Scholar] [CrossRef]
- GB/T 6569-2006/ISO 14704: 2000. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Flexural Strength of Monolithic Ceramics at Room Temperature; Chinese Standard Publishing House: Beijing, China, 2006; Available online: www.spc.org.cn (accessed on 22 February 2006). (In Chinese)
- GB/T 16534-2009. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Hardness of Monolithic Ceramics at Room Temperature; Chinese Standard Publishing House: Beijing, China, 2009; Available online: www.spc.org.cn (accessed on 13 May 2009). (In Chinese)
- Balak, Z.; Zakeri, M. Effect of HfB2 on microstructure and mechanical properties of ZrB2–SiC–based composites. Int. J. Refract. Met. Hard Mater. 2016, 54, 127–137. [Google Scholar] [CrossRef]
- An, J.; Song, J.P.; Liang, G.X.; Gao, J.J.; Xie, J.X.; Cao, L.; Wang, S.Y.; Lv, M. Effects of HfB2 and HfN additions on the microstructures and mechanical properties of TiB2–based ceramic tool materials. Materials 2017, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Song, J.P.; Cao, L.; Gao, J.J.; Liang, G.X.; Wang, S.Y.; Lv, M. Effects of HfN content and metallic additives on the microstructure and mechanical properties of TiC0.7N0.3–based ceramic tool materials. J. Alloy Compd. 2018, 753, 85–92. [Google Scholar] [CrossRef]
- Liu, J.X.; Huang, X.; Zhang, G.J. Pressureless Sintering of Hafnium Carbide–Silicon Carbide Ceramics. J. Am. Ceram. Soc. 2013, 96, 1751–1756. [Google Scholar] [CrossRef]
- Li, R.J. Ceramic—Metal Composite Materials, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Yin, Z.B.; Yan, S.Y.; Xu, W.W.; Yuan, J.T. Microwave sintering of Ti(C, N)–based cermet cutting tool material. Ceram. Int. 2018, 44, 1034–1040. [Google Scholar] [CrossRef]
- Zhao, G.L.; Huang, C.Z.; He, N.; Liu, H.L.; Zou, B. Microstructural development and mechanical properties of reactive hot pressed nickel-aided TiB2–SiC ceramics. Int. J. Refract. Met. Hard Mater. 2016, 61, 13–21. [Google Scholar] [CrossRef]
- Andrea, B.; Diletta, S. Spark plasma sintering and hot pressing of ZrB2–MoSi2 ultra-high-temperature ceramics. Mat. Sci. Eng. A 2008, 475, 108–112. [Google Scholar]
- Diletta, S.; Stefano, G.; Alida, B. Properties of a pressureless-sintered ZrB2–MoSi2 ceramic composite. J. Am. Ceram. Soc. 2006, 89, 2320–2322. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Yuan, H.; Lian, Z. Microstructure and Mechanical Properties of ZrB2–HfC Ceramics Influenced by HfC Addition. Materials 2018, 11, 2046. https://doi.org/10.3390/ma11102046
Jing Y, Yuan H, Lian Z. Microstructure and Mechanical Properties of ZrB2–HfC Ceramics Influenced by HfC Addition. Materials. 2018; 11(10):2046. https://doi.org/10.3390/ma11102046
Chicago/Turabian StyleJing, Yi, Hongbing Yuan, and Zisheng Lian. 2018. "Microstructure and Mechanical Properties of ZrB2–HfC Ceramics Influenced by HfC Addition" Materials 11, no. 10: 2046. https://doi.org/10.3390/ma11102046



