Feasibility of a Three-Dimensional Porous Uncalcined and Unsintered Hydroxyapatite/poly-d/l-lactide Composite as a Regenerative Biomaterial in Maxillofacial Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
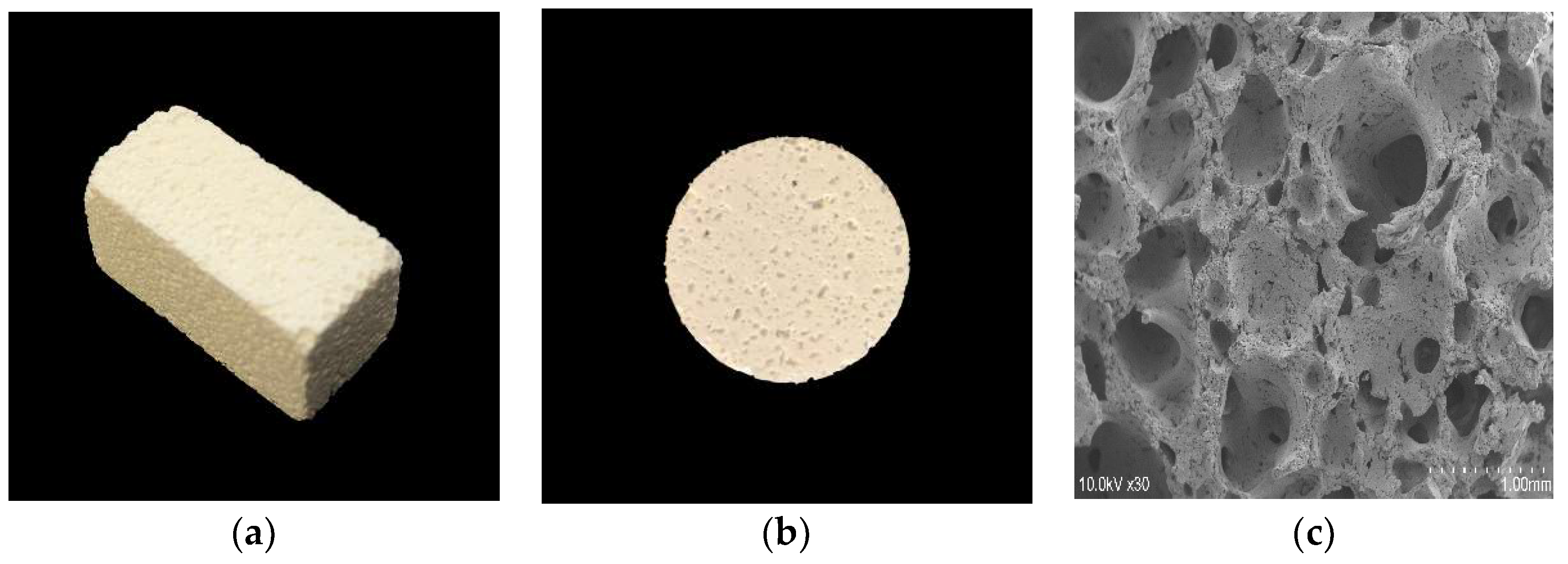
2.1.1. 3D-HA/PDLLA and Dense-HA/PDLLA Composite Biomaterial
2.1.2. Osteoblastic Cells
2.1.3. Cell Proliferation Assay
2.1.4. Reverse Transcription (RT) Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.2. In Vivo Experiments
2.2.1. Critical Bone Defection and Intraosseous Implantation
2.2.2. Micro-Computer Tomography (CT) Evaluation
2.2.3. Histomorphological Examination
2.3. Statistical Analysis
3. Results
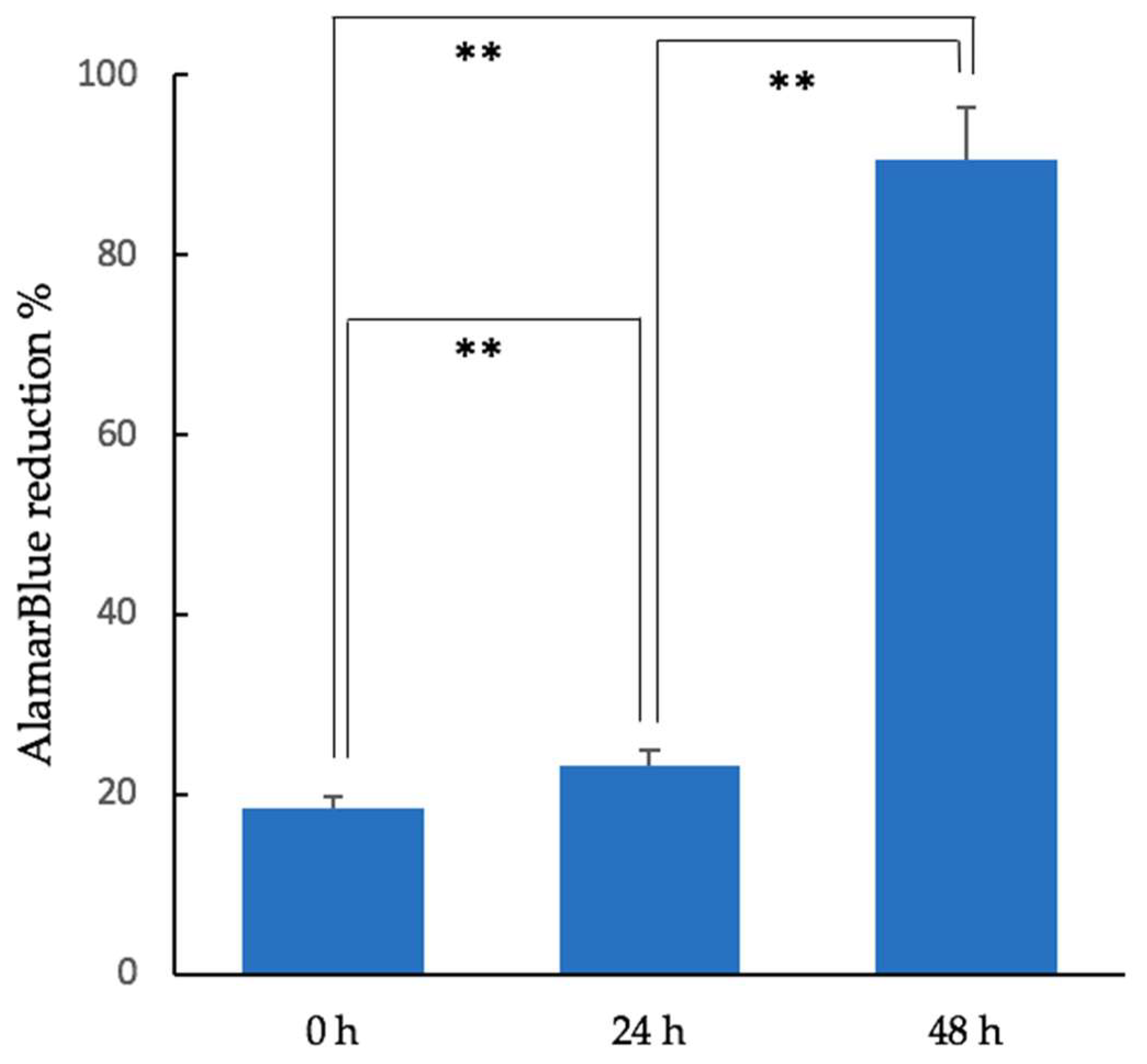
3.1. Cell Proliferation of Cubic Composites
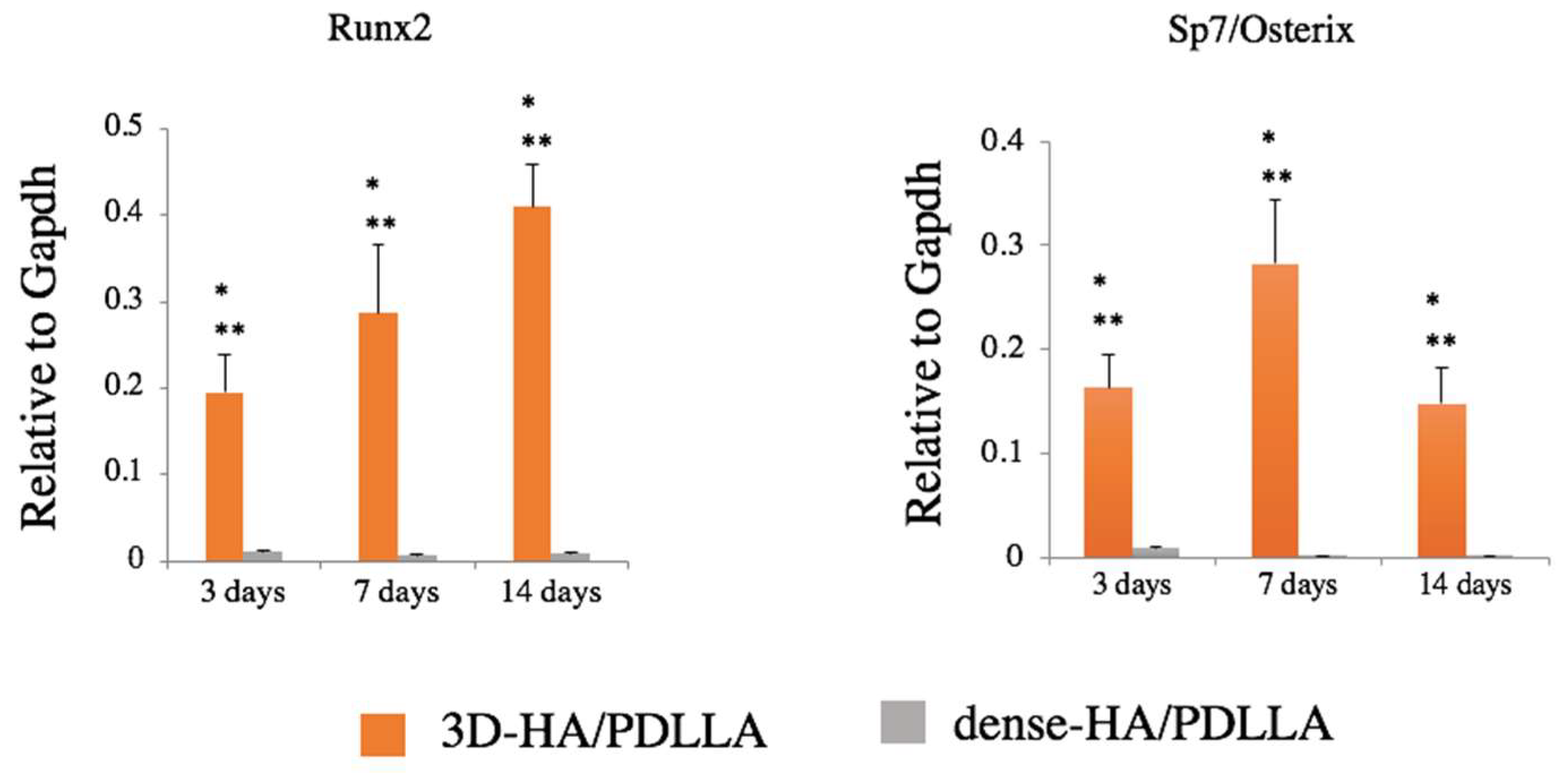
3.2. Gene Expression of Osteogenic Markers
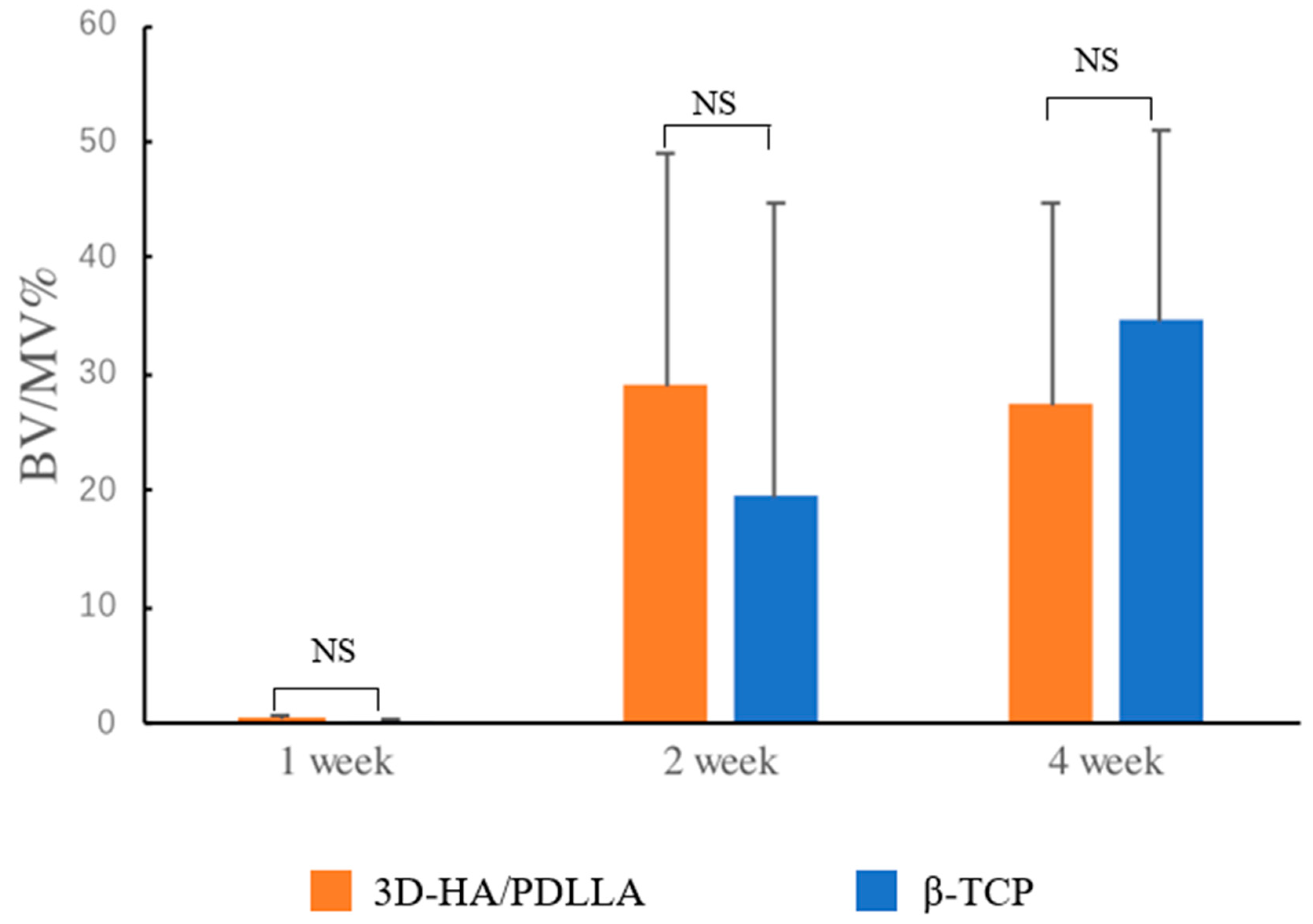
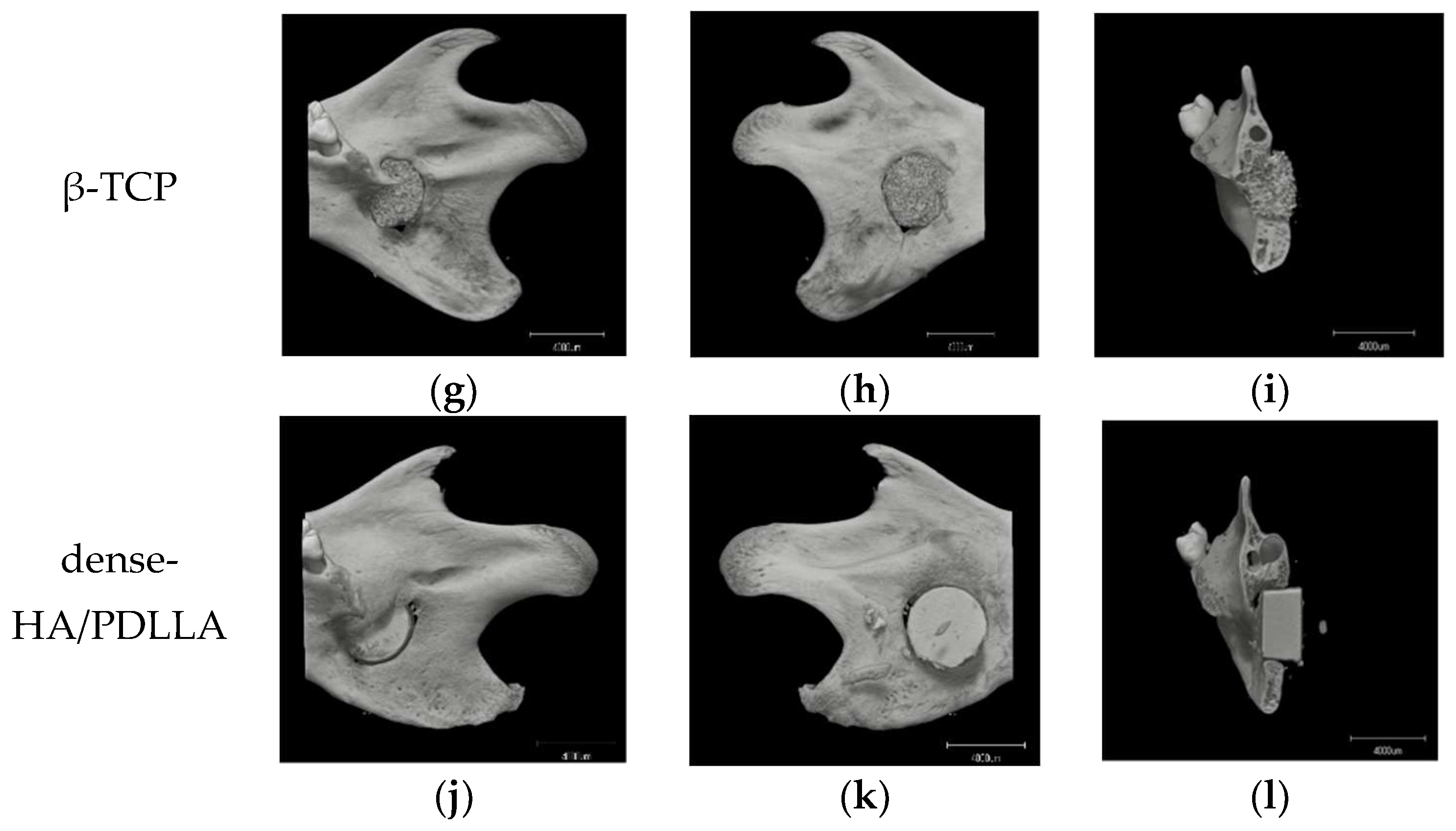
3.3. In Vivo Experiments, Histomorphology, and Micro-CT Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Ayoub, A. Bone tissue engineering in the maxillofacial region: The state-of-the-art practice and future prospects. Regen. Reconstr. Restor. 2016, 1, 8–14. [Google Scholar] [CrossRef]
- Ayoub, A.; Al-Fotawei, R. Biomaterials in the reconstruction of the oral and maxillofacial region. Biomater. Oral Craniomaxillofac. Appl. 2015, 17, 101–114. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Katase, N.; Shibata, A.; Takahashi, Y.; Furuki, Y. Clinical evaluation of an unsintered hydroxyapatite/poly-l-Lactide osteoconductive composite device for the internal fixation of maxillofacial Fractures. J. Craniofac. Surg. 2016, 27, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, A.; Sánchez-Garcés, M.Á.; Gay-Escoda, C. Materials and prognostic factors of bone regeneration in periapical surgery: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e419–e425. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Tamura, J.; Neo, M.; Goto, K.; Shikinami, Y.; Saito, M.; Kita, M.; Nakamura, T. In vivo evaluation of a porous hydroxyapatite/poly-dl-lactide composite for use as a bone substitute. J. Biomed. Mater. Res. Part A 2005, 75, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Heise, U.; Osborn, J.F.; Duwe, F. Hydroxyapatite ceramic as a bone substitute. Int. Orthop. 1990, 14, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.B. Bone as a ceramic composite material. Mater. Sci. Forum 1999, 293, 5–16. [Google Scholar] [CrossRef]
- Helen, W.; Merry, C.L.; Blaker, J.J.; Gough, J.E. Three-dimensional culture of annulus fibrosus cells within PDLLA/Bioglass® composite foam scaffolds: Assessment of cell attachment, proliferation and extracellular matrix production. Biomaterials 2007, 28, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Tamura, J.; Neo, M.; Fujibayashi, S.; Goto, K.; Shikinami, Y.; Okazaki, K.; Nakamura, T. In vivo evaluation of porous hydroxyapatite/poly-DL-lactide composite for bone substitutes and scaffolds. Key Eng. Mater. 2006, 309, 1311–1314. [Google Scholar] [CrossRef]
- Shin, M.K.; Jang, Y.H.; Yoo, H.J.; Kang, D.W.; Park, M.H.; Kim, M.K.; Song, J.H.; Kim, S.D.; Min, G.; You, H.K.; et al. N-Formyl-Methionyl-Leucyl-Phenylalanine (FMLP) promotes osteoblast differentiation via the N-Formyl peptide receptor 1-mediated signaling pathway in human mesenchymal stem cells from bone marrow. J. Biol. Chem. 2011, 286, 17133–17143. [Google Scholar] [CrossRef] [PubMed]
- Loye, A.M.; Kinser, E.R.; Bensouda, S.; Shayan, M.; Davis, R.; Wang, R.; Chen, Z.; Schwarz, U.D.; Schroers, J.; Kyriakides, T.R. Regulation of mesenchymal stem cell differentiation by nanopatterning of bulk metallic glass. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Makihira, S.; Nikawa, H.; Murata, H.; Hosokawa, R.; Hiyama, A.; Mimura, S. Impact of titanium ions on osteoblast-, osteoclast- and gingival epithelial-like cells. J. Prosthodont. Res. 2010, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Karakida, T.; Yamamoto, R.; Nagano, T.; Yamakoshi, Y.; Hayakawa, T.; Oida, S.; Gomi, K. Differentiation potential of osteoblast from cultured C2C12 cells on zirconia disk. Dent. Mater. J. 2014, 33, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Benito-Martin, A.; Mighty, J.; Chang, L.; Ghoroghi, S.; Wu, H.; Wong, M.; Guariglia, S.; Baranov, P.; Young, M.; et al. Retinal progenitor cells release extracellular vesicles containing developmental transcription factors, microRNA and membrane proteins. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okazaki, K.; Saito, M.; Okuno, M.; Hasegawa, S.; Tamura, J.; Fujibayashi, S.; Nakamura, T. Bioactive and bioresorbable cellular cubic-composite scaffolds for use in bone reconstruction. J. R. Soc. Interface 2006, 3, 805–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devin, J.E.; Attawia, M.A.; Laurencin, C.T. Three-dimensional degradable porous polymer-ceramic matrices for use in bone repair. J. Biomater. Sci. Polym. Ed. 1996, 7, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Flahiff, C.M.; Blackwell, A.S.; Hollis, J.M.; Feldman, D.S. Analysis of a biodegradable composite for bone healing. J. Biomed. Mater. Res. 1996, 32, 419–424. [Google Scholar] [CrossRef]
- Furukawa, T.; Matsusue, Y.; Yasunaga, T.; Nakagawa, Y.; Okada, Y.; Shikinami, Y.; Okuno, M.; Nakamura, T. Histomorphometric study on high-strength hydroxyapatite/poly(l-Lactide) composite rods for internal fixation of bone fractures. J. Biomed. Mater. Res. 2000, 50, 410–419. [Google Scholar] [CrossRef]
- Pawar, R.V.; Blacksin, M.F. Traumatic sternal segment dislocation in a 19-month-old. Emerg. Radiol. 2007, 14, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Escobar Ivirico, J.L.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Biodegradable polyphosphazene-based blends for regenerative engineering. Regen. Eng. Transl. Med. 2017, 3, 51. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Nau, T.; Teuschl, A. Regeneration of the anterior cruciate ligament: Current strategies in tissue engineering. World J. Orthop. 2015, 6, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Higashi, S.; Yamamuro, T.; Nakamura, T.; Ikada, Y.; Hyon, S.H.; Jamshidi, K. Polymer-hydroxyapatite composites for biodegradable bone fillers. Biomaterials 1986, 7, 183–187. [Google Scholar] [CrossRef]
- Kanno, T.; Tatsumi, H.; Karino, M.; Yoshino, A.; Koike, T.; Ide, T.; Sekine, J. Applicability of an unsintered hydroxyapatite particles/poly-l-lactide composite sheet with tack fixation for orbital fracture reconstruction. J. Hard Tissue Biol. 2016, 25, 329–334. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Koyama, Y.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Tanaka, S.; Furuki, Y. Precision of post-traumatic orbital reconstruction using unsintered hydroxyapatite particles/poly-l-lactide composite bioactive/resorbable mesh plate with and without navigation: A retrospective study. J. Hard Tissue Biol. 2017, 26, 274–280. [Google Scholar] [CrossRef]
- Kanno, T.; Karino, M.; Yoshino, A.; Koike, T.; Ide, T.; Tatsumi, H.; Tsunematsu, K.; Yoshimatsu, H.; Sekine, J. Feasibility of single folded unsintered hydroxyapatite particles/poly-l-lactide composite sheet in combined orbital floor and medial wall fracture reconstruction. J. Hard Tissue Biol. 2017, 26, 237–244. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Kawai, H.; Shibata, A. Clinical report long-term bioresorption of bone fixation devices made from composites of unsintered hydroxyapatite particles and poly-l-lactide. J. Hard Tissue Biol. 2015, 24, 219–224. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Manabe, Y.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Furuki, Y. Biomechanical loading evaluation of unsintered hydroxyapatite/poly-l-lactide plate system in bilateral sagittal split ramus osteotomy. Materials 2017, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kanno, T.; Shibata, A.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Sakaida, K.; Furuki, Y. Use of the bioactive resorbable plate system for zygoma and zygomatic arch replacement and fixation with modified crockett’s method for maxillectomy: A technical note. Mol. Clin. Oncol. 2017, 7, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U.; Gresser, J.D.; Wise, D.L.; Trantolo, D.J. Bioresorbable bone graft substitutes of different osteoconductivities: A histologic evaluation of osteointegration of poly (propylene glycol-co-fumaric acid)-based cement implants in rats. J. Orthop. Res. 2000, 21, 757–764. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-l-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. 2002, 59, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, H.; Hara, Y.; Tagawa, M.; Kato, T.; Ochi, H.; Koga, D.; Okawa, A.; Asou, Y. Evaluation of the association between runt-related transcription factor 2 expression and intervertebral disk aging in dogs. Am. J. Vet. Res. 2012, 73, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of skeletal development by the Runx family of transcription factors. J. Cell. Biochem. 2005, 95, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Wirth, J.; Meyer, J.; Zabel, B.; Held, M.; Zimmer, J.; Pasantes, J.; Bricarelli, F.D.; Keutel, J.; Hustert, E.; et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 1994, 79, 1111–1120. [Google Scholar] [CrossRef]
- Foster, J.W.; Dominguez-Steglich, M.A.; Guioli, S.; Kwok, C.; Weller, P.A.; Stevanović, M.; Weissenbach, J.; Mansour, S.; Young, I.D.; Goodfellow, P.N.; et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 1994, 372, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.; Wakabayashi, M.; Hata, K.; Matsubara, T.; Honma, S.; Wakisaka, S.; Kiyonari, H.; Shioi, G.; Yamaguchi, A.; Tsumaki, N.; et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J. Biol. Chem. 2012, 287, 33179–33190. [Google Scholar] [CrossRef] [PubMed]
- Stickens, D.; Behonick, D.J.; Ortega, N.; Heyer, B.; Hartenstein, B.; Yu, Y.; Fosang, A.J.; Schorpp-kistner, M.; Angel, P.; Werb, Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004, 131, 5883–5895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akagi, H.; Ochi, H.; Soeta, S.; Kanno, N.; Yoshihara, M.; Okazaki, K.; Yogo, T.; Harada, Y.; Amasaki, H.; Hara, Y. A comparison of the process of remodeling of hydroxyapatite/poly-d/l-lactide and beta-tricalcium phosphate in a loading site. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Ogose, A.; Tokunaga, K.; Umezu, H.; Arai, K.; Kudo, N.; Hoshino, M.; Inoue, H.; Irie, H.; Kuroda, K.; et al. Osteoinduction with highly purified β-tricalcium phosphate in dog dorsal muscles and the proliferation of osteoclasts before heterotopic bone formation. Biomaterials 2006, 27, 4419–4427. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.A.; Mazor, Z.; Foitzik, C.; Prasad, H.; Rohrer, M.; Palti, A. β-tricalcium phosphate as bone substitute material: Properties and clinical applications. J. Osseointegration 2010, 2, 61–68. [Google Scholar] [CrossRef]
- Chazono, M.; Tanaka, T.; Komaki, H.; Fujii, K. Bone formation and bioresorption after implantation of injectable β-tricalcium phosphate granules–hyaluronate complex in rabbit bone defects. J. Biomed. Mater. Res. Part A 2004, 70, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Preparation, characterization, and in vitro degradation of bioresorbable and bioactive composites based on Bioglass®-filled polylactide foams. J. Biomed. Mater. Res. Part A 2003, 66, 335–346. [Google Scholar] [CrossRef] [PubMed]


| Target Gene (NCBI Accession Number) | Sequence (5′–3′) | Amplicon Size (bp) |
|---|---|---|
| Runx2 [12,13] (NM_001278478.1) | F: CCAGATGGGACTGTGGTTACC R: ACTTGGTGCAGAGTTCAGGG | 381bp |
| Sp7/Osterix [14,15] (NM_001300837.1) | F: CTGGGGAAAGGAGGCACAAAGAAG R: GGGTTAAGGGGAGCAAAGTCAGAT | 200bp |
| Gapdh [16] (NM_001289745.2) | F: ACCACAGTCCATGCCATCAC R: TCCACCACCCTGTTGCTGTA | 452bp |
| NCBI, National Center for Biotechnology Information; F: forward primer; R: reverse primer. Annealing temperature was set at 50 °C for all primers, according to the manufacture’s protocol. | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Kanno, T.; Tatsumi, H.; Miyamoto, K.; Sha, J.; Hideshima, K.; Matsuzaki, Y. Feasibility of a Three-Dimensional Porous Uncalcined and Unsintered Hydroxyapatite/poly-d/l-lactide Composite as a Regenerative Biomaterial in Maxillofacial Surgery. Materials 2018, 11, 2047. https://doi.org/10.3390/ma11102047
Bai Y, Kanno T, Tatsumi H, Miyamoto K, Sha J, Hideshima K, Matsuzaki Y. Feasibility of a Three-Dimensional Porous Uncalcined and Unsintered Hydroxyapatite/poly-d/l-lactide Composite as a Regenerative Biomaterial in Maxillofacial Surgery. Materials. 2018; 11(10):2047. https://doi.org/10.3390/ma11102047
Chicago/Turabian StyleBai, Yunpeng, Takahiro Kanno, Hiroto Tatsumi, Kenichi Miyamoto, Jingjing Sha, Katsumi Hideshima, and Yumi Matsuzaki. 2018. "Feasibility of a Three-Dimensional Porous Uncalcined and Unsintered Hydroxyapatite/poly-d/l-lactide Composite as a Regenerative Biomaterial in Maxillofacial Surgery" Materials 11, no. 10: 2047. https://doi.org/10.3390/ma11102047





