Novel Thermosensitive Core–Shell Surface Molecularly Imprinted Polymers Based on SiO2 for the Selective Adsorption of Sulfamethazine
Abstract
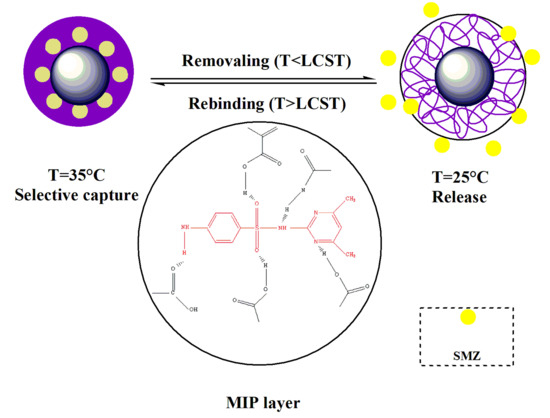
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Preparation of SiO2 Nanoparticles
2.3. Synthesis of SiO2@MPS
2.4. Preparation of Imprinted and Non-Imprinted Polymers
2.5. SMZ Adsorption Experiments
3. Results and Discussion
3.1. Preparation of Molecularly-Imprinted Nanoparticles
3.2. Characterization of Imprinted Nano-Microspheres
3.3. Temprature Sensitivity Properties of MIPs
3.4. Adsorption Experiments
3.4.1. Adsorption Kinetics Experiments
3.4.2. Adsorption Isotherms
3.4.3. Rebinding Specificity
3.4.4. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sadeghi, S.; Motaharian, A. Voltammetric sensor based on carbon paste electrode modified with molecular imprinted polymer for determination of sulfadiazine in milk and human serum. Mater. Sci. Eng. C 2013, 33, 4884–4891. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.S.; Wu, M.S.; Zuo, H.G.; Jiang, C.; Jin, S.F.; Lu, Y.C.; Yang, H. Core-shell magnetic molecularly imprinted polymers as sorbent for sulfonylurea herbicide residues. J. Agric. Food. Chem. 2015, 63, 3634–3645. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in nature environment. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta, South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Braham, R.; Black, W.D.; Claxton, J.; Yee, A.J. A Rapid Assay for Detecting Sulfonamides in Tissues of Slaughtered Animals. J. Food Prot. 2001, 64, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Zhang, Y.; Fang, G.; Zheng, W.; Wang, S. Development of an enzyme-linked immunosorbent assay for seven sulfonamide residues and investigation of matrix effects from different food samples. J. Agric. Food Chem. 2007, 55, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S. Review on enzyme-linked immunosorbent assays for sulfonamide residues in edible animal products. J. Immunol. Methods 2009, 350, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chitescu, C.L.; Nicolau, A.I.; Csuma, A.; Moisoiu, C. Simultaneous analysis of four sulfonamides in chicken muscle tissue by HPLC. Food Addit. Contam. A 2011, 28, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Reeves, V.B. Confirmation of multiple sulfonamide residues in bovine milk by gas chromatography-positive chemical ionization mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 723, 127–137. [Google Scholar] [CrossRef]
- Blasco, C.; Pico, Y.; Andreu, V. Analytical method for simultaneous determination of pesticideand veterinary drug residues in milk by CE-MS. Electrophoresis 2009, 30, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Ozkorucuklu, S.P.; Ozcan, L.; Sahin, Y.; Alsancak, G. Electroanalytical Determination of Some Sulfonamides on Overoxidized Polypyrrole Electrodes. Aust. J. Chem. 2011, 64, 965–972. [Google Scholar] [CrossRef]
- Gao, B.; An, F.; Zhu, Y. Novel surface ionic imprinting materials prepared via couple grafting of polymer and ionic imprinting on surfaces of silica gel particles. Polymer 2007, 48, 2288–2297. [Google Scholar] [CrossRef]
- Wulff, G. Molecular Imprinting in Cross-Linked Materials with the Aid of Molecular. Angew. Chem. Int. Ed. Engl. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Rao, T.P.; Daniel, S.; Gladis, J.M. Tailored materials for preconcentration or separation of metals by ion-imprinted polymers for solid-phase extraction (IIP-SPE). Trends Analyt. Chem. 2004, 23, 28–35. [Google Scholar]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Iemma, F.; Picci, N. Molecularly imprinted polymers in drug delivery: state of art and future perspectives. Expert. Opin. Drug Deliv. 2011, 8, 1379. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Xing, Z.; Zhu, A.; Zhao, Z.; Xu, G.; Li, C.; Zhou, H. Molecularly imprinted polymers based electrochemical sensor for bovine hemoglobin recognition. Sens. Actuators B Chem. 2012, 168, 395–401. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Chen, Y.; Li, W.Y.; Zhang, Y.K. Molecularly imprinted polymer anchored on the surface of denatured bovine serum albumin modified CdTe quantum dots as fluorescent artificial receptor for recognition of target protein. Biosens. Bioelectron. 2012, 31, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Tan, T.; Svec, F. Molecular imprinting of proteins in polymers attached to the surface of nanomaterials for selective recognition of biomacromolecules. Biotechnol. Adv. 2013, 31, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Li, W.; Lei, X.; Fan, X.; Tian, L.; Zhang, H.; Zhang, Q. Preparation and characterization of bovine serum albumin surface-imprinted thermosensitive magnetic polymer microsphere and its application for protein recognition. Biosens. Bioelectron. 2014, 51, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, T.; Xu, W.; Huang, W.; Wang, N.; Yang, W. Synthesis and characterization of fluorescence molecularly imprinted polymers as sensor for highly sensitive detection of dibutyl phthalate from tap water samples. Sens. Actuators B-Chem. 2017, 240, 1114–1122. [Google Scholar] [CrossRef]
- Huang, W.; Xu, P.; Yang, W.; Xu, W. Thermosensitive molecularly imprinted polymers based on magnetic nanoparticles for the recognition of sulfamethazine. RSC Adv. 2016, 6, 74734–74741. [Google Scholar] [CrossRef]
- Huang, W.; Kong, Y.; Yang, W.; Ni, X.; Wang, N.; Lu, Y.; Xu, W. Preparation and characterization of novel thermosensitive magnetic molecularly imprinted polymers for selective recognition of norfloxacin. J. Polym. Res. 2016, 23, 94. [Google Scholar] [CrossRef]
- Jing, T.; Du, H.; Dai, Q.; Xia, H.; Niu, J.; Hao, Q.; Mei, S.; Zhou, Y. Magnetic molecularly imprinted nanoparticles for recognition of lysozyme. Biosens. Bioelectron. 2010, 26, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Wang, Y.; Jia, X.; Nie, C. Bovine serum albumin recognition via thermosensitive molecular imprinted macroporous hydrogels prepared at two different temperatures. Anal. Chim. Acta 2012, 723, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Michał, R.; Maciej, P.; Konrad, G.; Jacek, R.; Grzegorz, S.; Maryna, K.; Daniel, P.; Grzegorz, C. Dynamic water contact angle during initial phases of droplet impingement. Colloids Surf. Physicochem. Eng. Asp. 2016, 508, 57–69. [Google Scholar]
- Bai, S.; Zhang, H.; Sun, J.; Han, J.; Guo, Y. Preparation and pH-responsive performance of silane-modified poly(methylacrylic acid). J. Appl. Polym. Sci. 2014, 131, 40403. [Google Scholar] [CrossRef]
- Ma, R.; Sun, Y. Magnetic molecularly imprinted polymer for the selective extraction of quercetagetin from Calendula officinalis extract. Talanta 2015, 134, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Schirhagl, R. Bioapplications for molecularly imprinted polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]











| Composition (mmol) | MIP1 | MIP2 | MIP3 |
|---|---|---|---|
| SMZ | 1 | 1 | 1 |
| MAA | 2 | 2 | 2 |
| PNIPAM | 3 | 3 | 3 |
| EGDMA | 15 | 10 | 5 |
| MBA | 0 | 5 | 10 |
| Kinetic Models | Parameters | MIPs | NIPs |
|---|---|---|---|
| Pseudo-first-order | qe,cal (mg·g−1) | 9.715 | 4.255 |
| k1 (min−1) | 0.0043 | 0.0314 | |
| R2 | 0.8163 | 0.9392 | |
| Pseudo-second-order | qe,cal (mg·g−1) | 8.172 | 5.120 |
| k2 (min−1) | 0.0363 | 0.0067 | |
| R2 | 0.9297 | 0.8324 | |
| Experimental data | qe,exp (mg·g−1) | 8.091 | 4.124 |
| Isotherm Models | Parameters | MIPs | NIPs |
|---|---|---|---|
| Langmuir | kL (L·mg−1) | 0.9661 | 1.0322 |
| qmL (mg·g−1) | 10.862 | 5.578 | |
| R2 | 0.9914 | 0.8968 | |
| Freundlich | kF | 15.6891 | 8.0438 |
| nF | 1.9863 | 2.0468 | |
| R2 | 0.9072 | 0.9888 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Qing, Y.; Wang, N.; Lu, Y.; Liu, T.; Liu, T.; Yang, W.; Li, S. Novel Thermosensitive Core–Shell Surface Molecularly Imprinted Polymers Based on SiO2 for the Selective Adsorption of Sulfamethazine. Materials 2018, 11, 2067. https://doi.org/10.3390/ma11112067
Huang W, Qing Y, Wang N, Lu Y, Liu T, Liu T, Yang W, Li S. Novel Thermosensitive Core–Shell Surface Molecularly Imprinted Polymers Based on SiO2 for the Selective Adsorption of Sulfamethazine. Materials. 2018; 11(11):2067. https://doi.org/10.3390/ma11112067
Chicago/Turabian StyleHuang, Weihong, Yujie Qing, Ningwei Wang, Yi Lu, Tianshu Liu, Tao Liu, Wenming Yang, and Songjun Li. 2018. "Novel Thermosensitive Core–Shell Surface Molecularly Imprinted Polymers Based on SiO2 for the Selective Adsorption of Sulfamethazine" Materials 11, no. 11: 2067. https://doi.org/10.3390/ma11112067