Cold Bonding Method for Metallic Powder Coatings
Abstract
:1. Introduction
2. Experimental
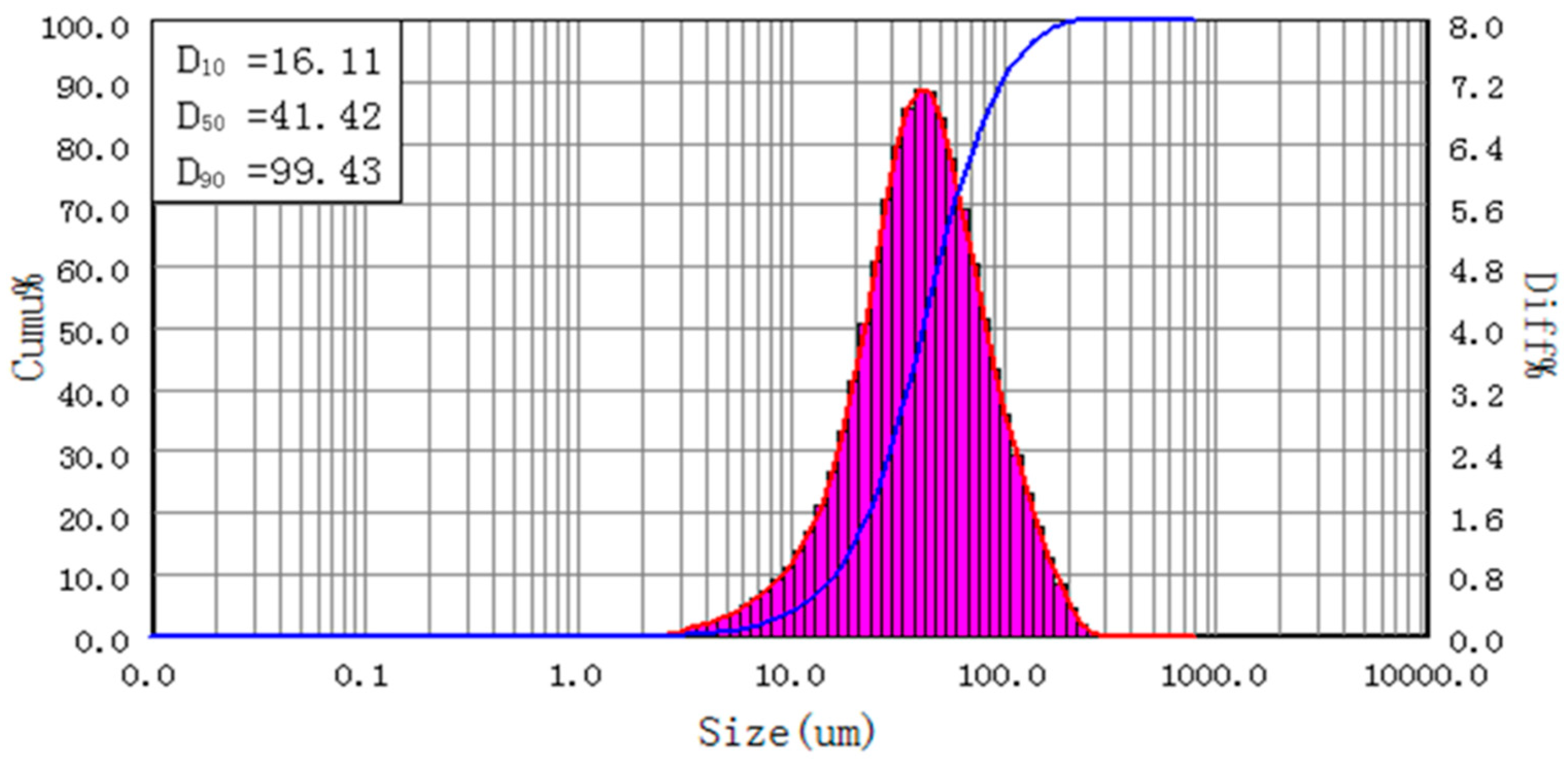
2.1. Materials
2.2. Equipment
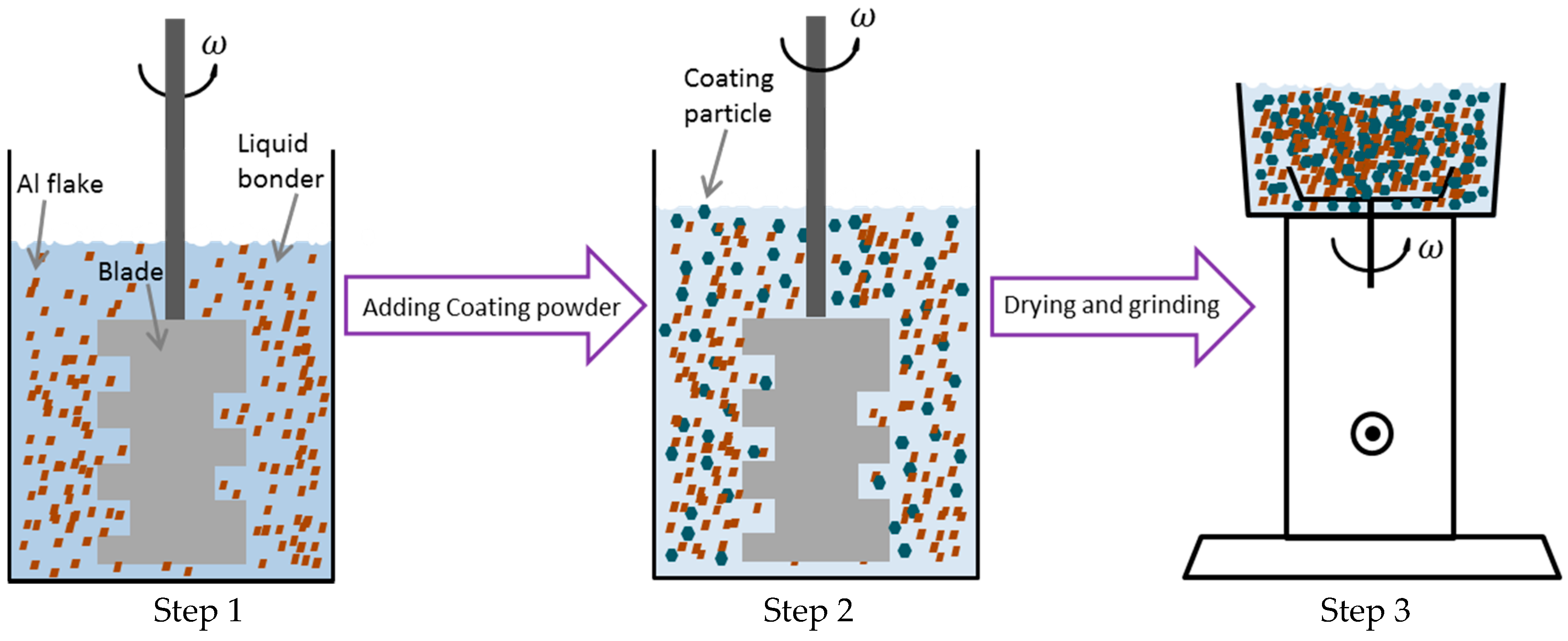
2.3. Bonding Method and Characterization of Metallic Powder Coating
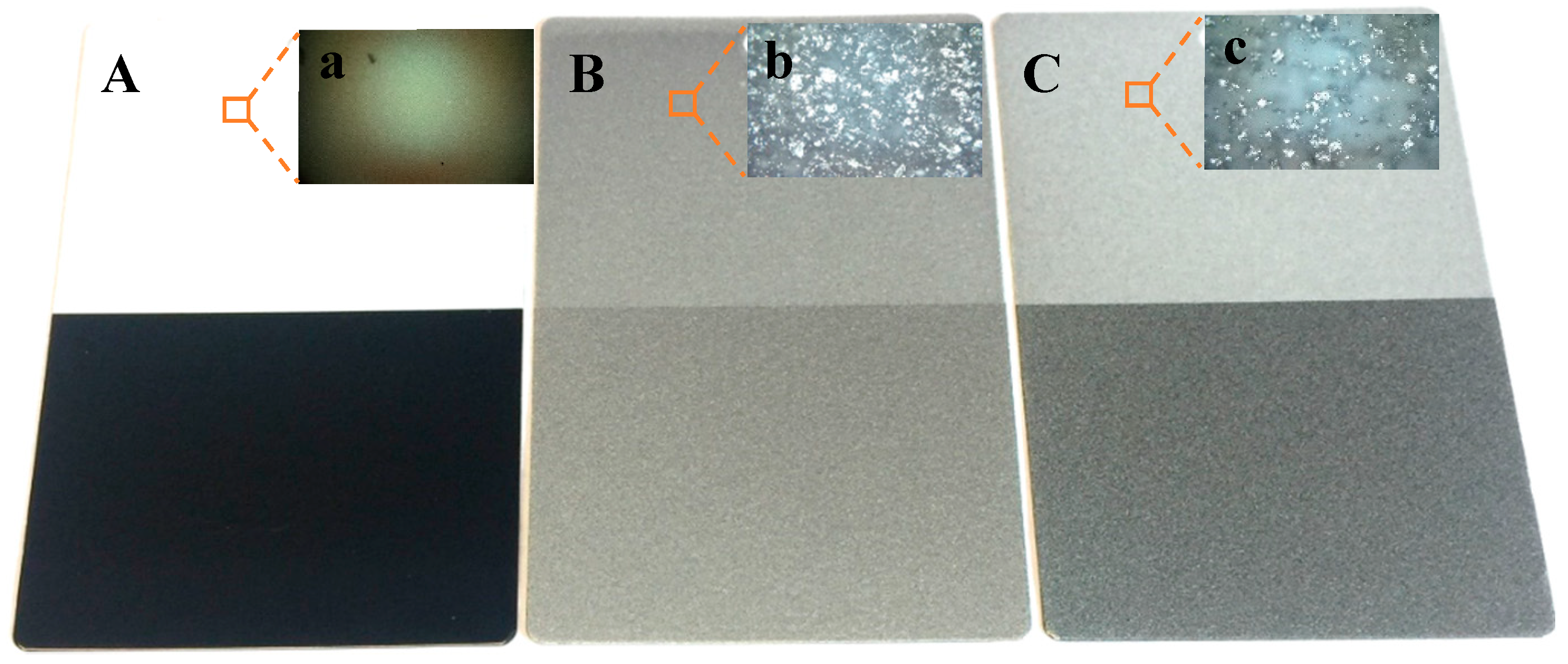
3. Results and Discussion
3.1. Control Tests
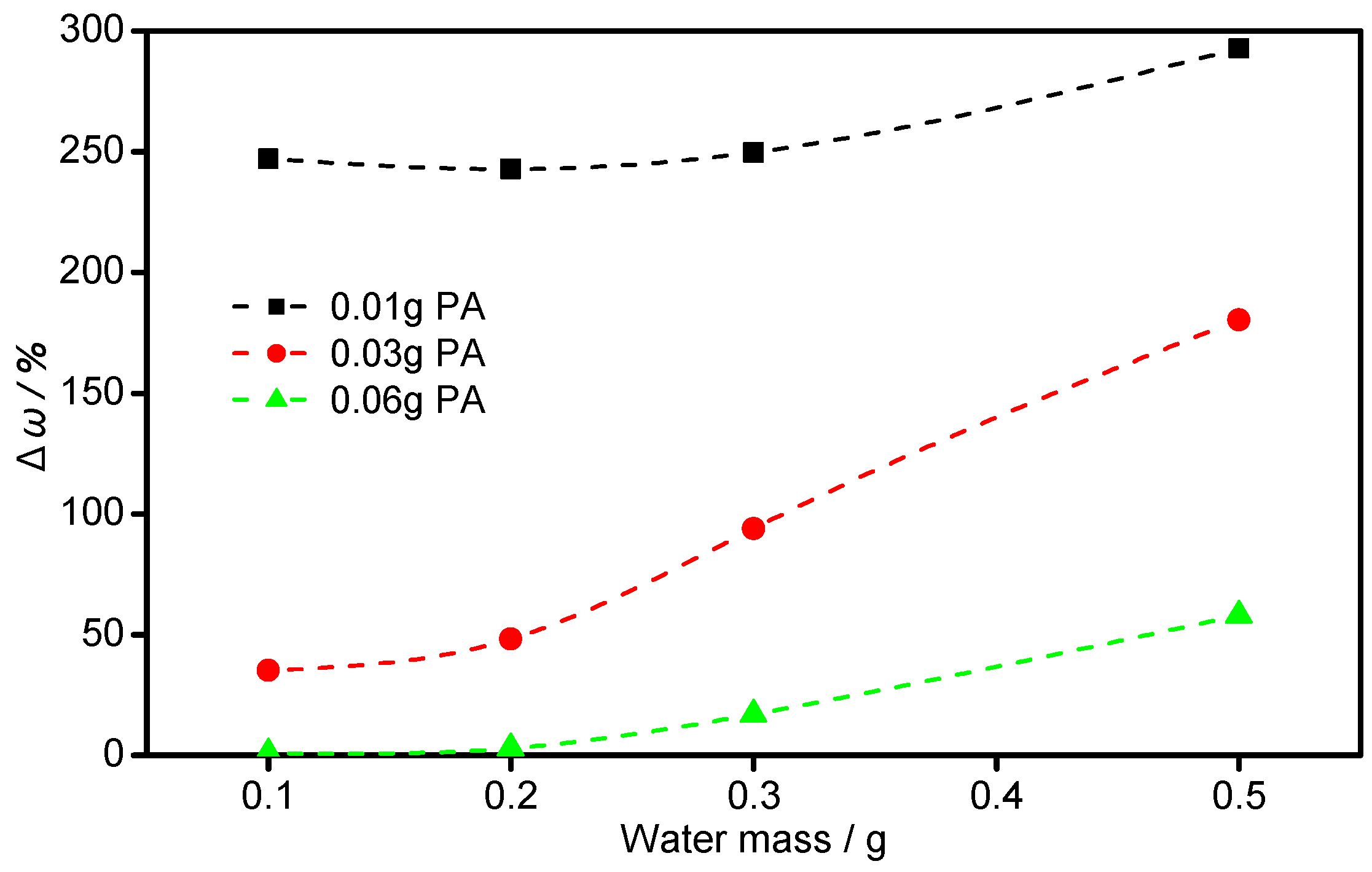
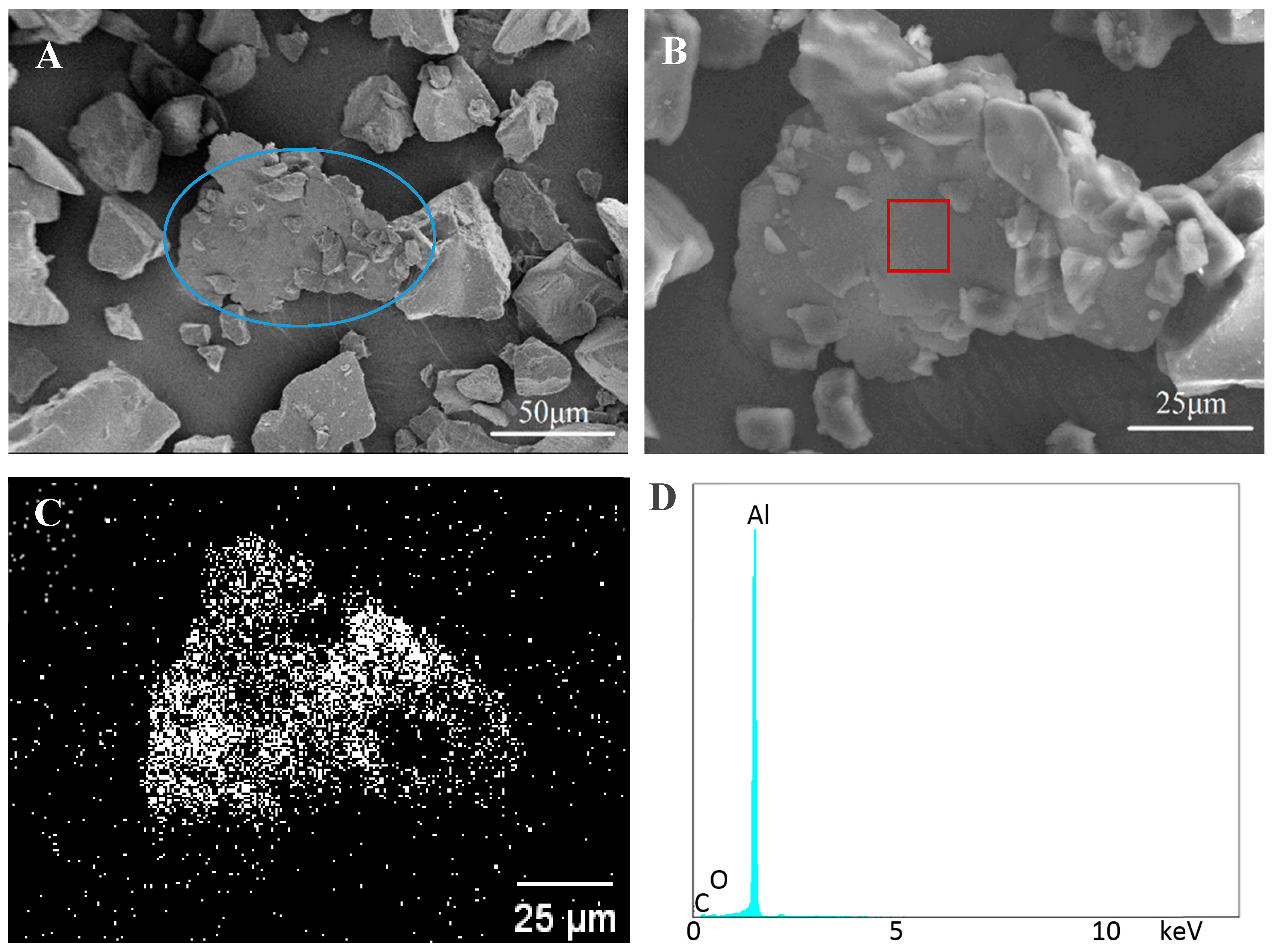
3.2. Bonded by PAA
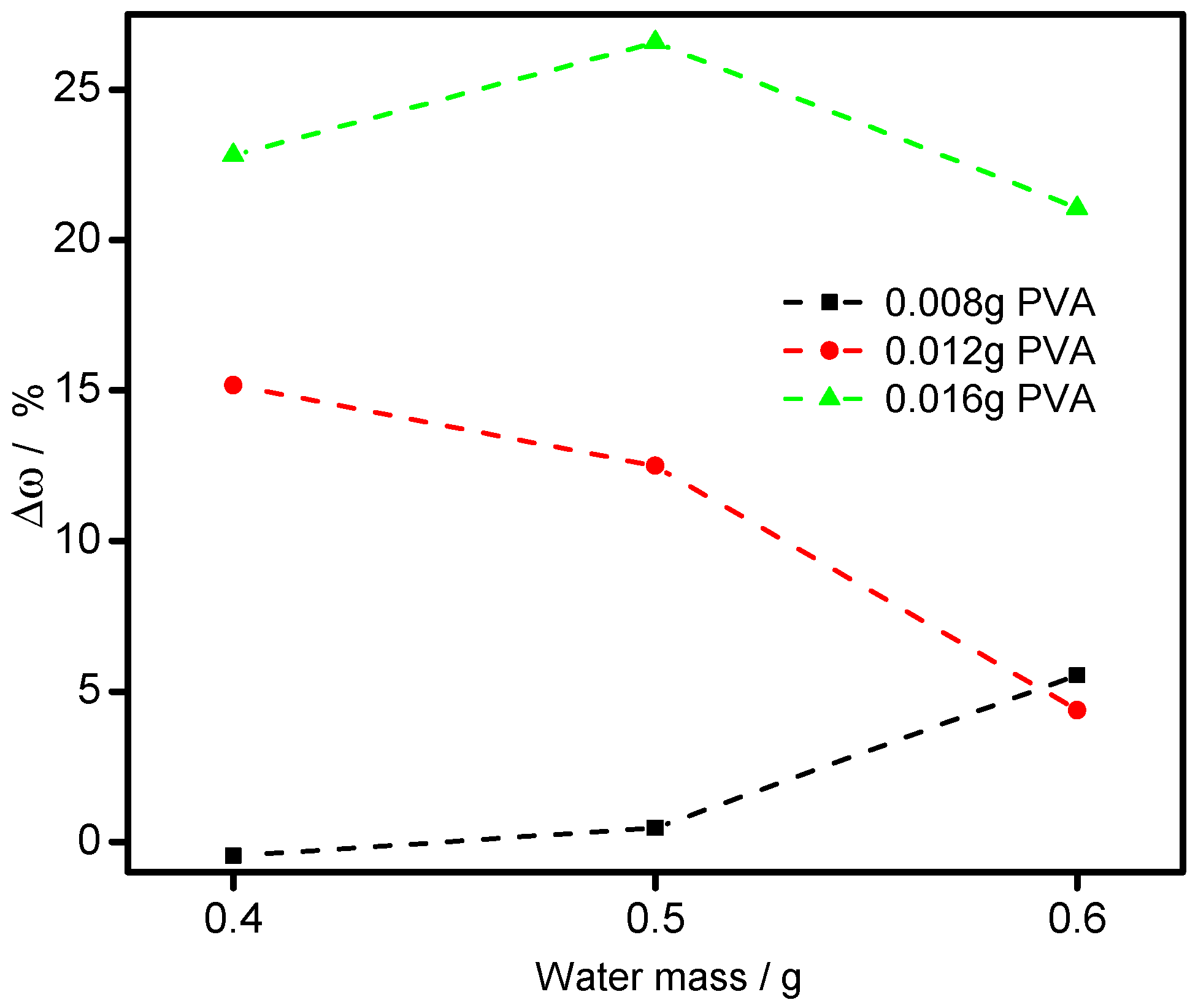
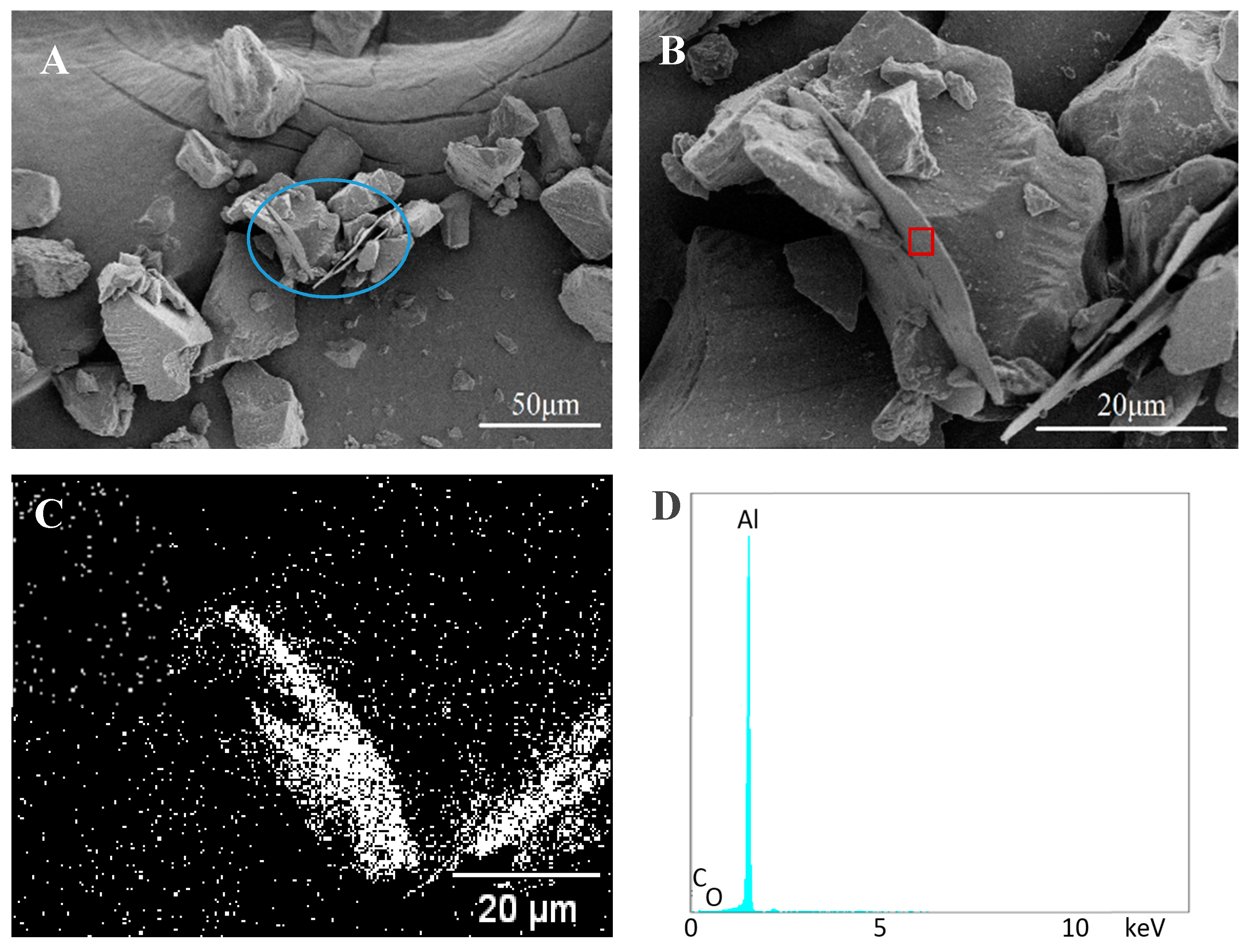
3.3. Bonded by PVA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diab, M.; Pang, X.; Jahed, H. The effect of pure aluminum cold spray coating on corrosion and corrosion fatigue of magnesium (3% Al-1% Zn) extrusion. Surf. Coat. Tech. 2017, 309, 423–435. [Google Scholar] [CrossRef]
- Nine, M.J.; Kabiri, S.; Tung, T.T.; Tran, D.N.; Losic, D. Electrostatic powder coatings of pristine graphene: A new approach for coating of granular and fibril substrates. Appl. Surf. Sci. 2018, 441, 187–193. [Google Scholar] [CrossRef]
- González, S.; Cáceres, F.; Fox, V.; Souto, R.M. Resistance of metallic substrates protected by an organic coating containing aluminum powder. Prog. Org. Coat. 2003, 46, 317–323. [Google Scholar] [CrossRef]
- Dupuis, A.; Ho, T.H.; Fahs, A.; Lafabrier, A.; Louarn, G.; Bacharouche, J.; Chailan, J.F. Improving adhesion of powder coating on PEEK composite: Influence of atmospheric plasma parameters. Appl. Surf. Sci. 2015, 357, 1196–1204. [Google Scholar] [CrossRef]
- Wan, H.; Song, D.; Li, X.; Zhang, D.; Gao, J.; Du, C. Effect of Zinc Phosphate on the corrosion behavior of waterborne acrylic coating/metal interface. Materials 2017, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand Hadavand, B.; Ataeefard, M.; Fakharizadeh Bafghi, H. Preparation of modified nano ZnO/polyester/TGIC powder coating nanocomposite and evaluation of its antibacterial activity. Compos. Part. B Eng. 2015, 82, 190–195. [Google Scholar] [CrossRef]
- Sun, S.; Ding, H.; Hou, X. Preparation of CaCO3-TiO2 composite particles and their pigment properties. Materials 2018, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pow, E.H.; Tsoi, J.K.H.; Matinlinna, J.P. Evaluation of four surface coating treatments for resin to zirconia bonding. J. Mech. Behav. Biomed. 2014, 32, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liang, Y.; Hou, X.; Zhang, J.; Ding, H.; Sun, S.; Cao, H. Mechanical grinding preparation and characterization of TiO2-coated wollastonite composite pigments. Materials 2018, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.A.; Kasiriha, S.M. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Porter, S.C.; Woznicki, E.J. Dry Edible Film Coating Composition, Method and Coating Form. U.S. Patent 4,543,370, 24 September 1985. [Google Scholar]
- Honda, H.; Kimura, M.; Honda, F.; Matsuno, T.; Koishi, M. Preparation of monolayer particle coated powder by the dry impact blending process utilizing mechanochemical treatment. Colloid. Surf. A 1994, 82, 117–128. [Google Scholar] [CrossRef]
- Himes, G.R. Dry Blending and Molding Process. U.S. Patent 3,985,702, 12 October 1976. [Google Scholar]
- Sims, R.A.; Mazumder, M.K.; Liu, X.; Chok, W.; Mountain, J.R.; Wankum, D.L.; Chasser, T. Electrostatic effects on first pass transfer efficiency in the application of powder coatings. IEEE Trans. Ind. Appl. 2001, 37, 1610–1617. [Google Scholar] [CrossRef]
- Fu, J.; Krantz, M.; Zhang, H.; Zhu, J.; Kuo, H.; Wang, Y.M.; Lis, K. Investigation of the recyclability of powder coatings. Powder Technol. 2011, 211, 38–45. [Google Scholar] [CrossRef]
- Ye, Q.; Domnick, J. On the simulation of space charge in electrostatic powder coating with a corona spray gun. Powder Technol. 2003, 135, 250–260. [Google Scholar] [CrossRef]
- Liberto, N.P. Understanding powder coating equipment and application. Metal Finish 2011, 109, 27–32. [Google Scholar] [CrossRef]
- Ye, Q.; Steigleder, T.; Cheibe, A.; Domnick, J. Numerical simulation of the electrostatic powder coating process with a corona spray gun. J. Electrostat. 2002, 54, 189–205. [Google Scholar] [CrossRef]
- Nan, R.Z.; Nan, Y.; Liu, Z.T. Preliminary Discussion on Powder Coatings Containing Aluminum Powder. Modern Paint Finish 2006, 8, 003. [Google Scholar]
- Bailey, J.; Worden, D.; Breton, E.; Wolf, J. Coating Metallic Substrate with Powdered Filler and Molten Metal. U.S. Patent 3,743,556, 3 July 1973. [Google Scholar]
- Breton, E.J.; Handzel, J.M.; Tennant, O.K. Process for Making Wear-Resistant Coatings. U.S. Patent 6,649,682, 18 November 2003. [Google Scholar]
- Van Steenkiste, T.; Smith, J.; Teets, R. Aluminum coatings via kinetic spray with relatively large powder particles. Surf. Coat. Technol. 2002, 154, 237–252. [Google Scholar] [CrossRef]
- Richart, D.S.; Daly, A.T. Thermosetting Resin-Based Coating Powders Containing Metal Flakes. U.S. Patent 5,187,220, 16 February 1993. [Google Scholar]
- Tuan, W.H.; Wu, H.; Yang, T. The preparation of Al2O3/Ni composites by a powder coating technique. J. Mater. Sci. 1995, 30, 855–859. [Google Scholar] [CrossRef]
- Misev, T.A. Powder Coatings: Chemistry and Technology; Wiley: New York, NY, USA, 1991; pp. 304–324. [Google Scholar]
- Beckermann, G.W.; Pickering, K.L. Engineering and evaluation of hemp fiber reinforced polypropylene composites: Micro-mechanics and strength prediction modelling. Compos. Part A Appl. Sci. 2009, 40, 210–217. [Google Scholar] [CrossRef]
- De Boer, A.; Bolhuis, G.; Lerk, C. Bonding characteristics by scanning electron microscopy of powders mixed with magnesium stearate. Powder Technol. 1978, 20, 75–82. [Google Scholar] [CrossRef]
- ASTM D5630-13—Standard Test Method for Ash Content in Plastics; ASTM Standard: New York, NY, USA, 2013.
- Mistry, J.K.; Frick, J.P.; Petersen, J. Microwave Bonding for Coating Compositions. U.S. Patent 15,463,131, 6 July 2017. [Google Scholar]
- Gunde, M.K.; Kunaver, M.; Hrovat, A.; Cvelbar, U. Bonding process efficiency and Al-flake orientation during the curing of powder coatings. Prog. Org. Coat. 2005, 54, 113–119. [Google Scholar] [CrossRef]
- Hussain, R.; Tabassum, S.; Gilani, M.A.; Ahmed, E.; Sharif, A.; Manzoor, F.; Siddiqi, S.A. In situ synthesis of mesoporous polyvinyl alcohol/hydroxyapatite composites for better biomedical coating adhesion. Appl. Surf. Sci. 2016, 364, 117–123. [Google Scholar] [CrossRef]
- Po, R. Water-absorbent polymers: a patent survey. J. Macromol. Sci. Part C Polym. Rev. 1994, 34, 607–662. [Google Scholar] [CrossRef]

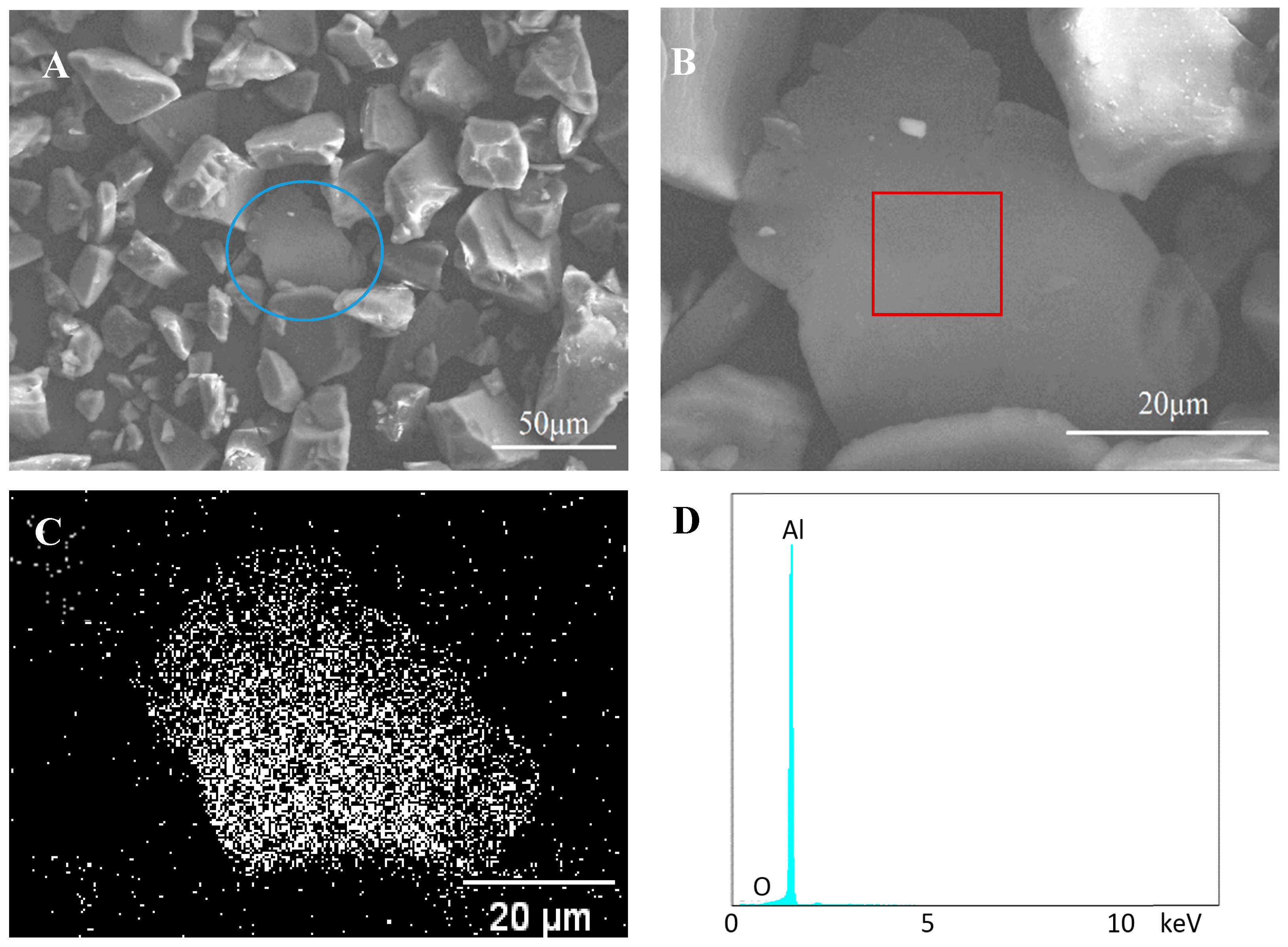

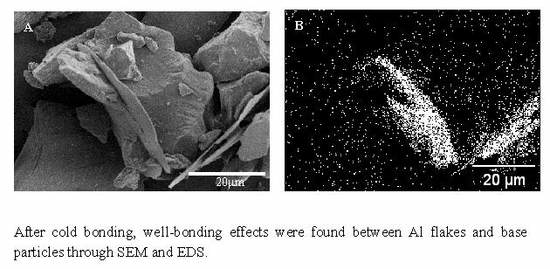
| Control Sample | |||||||
|---|---|---|---|---|---|---|---|
| ωorig/wt.% | ωdep/wt.% | ||||||
| 1 | 2 | 3 | Average | 1 | 2 | 3 | Average |
| 2.14 | 2.17 | 2.15 | 2.15 ± 0.02 | 5.05 | 5.10 | 5.08 | 5.08 ± 0.03 |
| Content of Al | ωorig/wt.% | ωdep/wt.% | ||||
|---|---|---|---|---|---|---|
| H2O/g | PAA/g | PAA/g | ||||
| 0.010 | 0.030 | 0.060 | 0.010 | 0.030 | 0.060 | |
| 0.10 | 2.19 | 2.19 | 2.23 | 7.60 | 2.96 | 2.25 |
| 0.20 | 2.10 | 2.18 | 2.04 | 7.20 | 3.23 | 2.10 |
| 0.30 | 1.99 | 1.98 | 2.22 | 6.96 | 3.84 | 2.60 |
| 0.50 | 2.06 | 2.19 | 2.14 | 8.09 | 6.14 | 3.38 |
| Content of Al | ωorig/wt.% | ωdep/wt.% | ||||
|---|---|---|---|---|---|---|
| H2O/g | PVA/g | PVA/g | ||||
| 0.010 | 0.030 | 0.060 | 0.010 | 0.030 | 0.060 | |
| 0.40 | 2.12 | 1.91 | 2.28 | 2.11 | 2.20 | 2.80 |
| 0.50 | 2.17 | 2.08 | 2.07 | 2.18 | 2.34 | 2.62 |
| 0.60 | 2.17 | 1.83 | 2.11 | 2.29 | 1.91 | 2.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Fu, J.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Cold Bonding Method for Metallic Powder Coatings. Materials 2018, 11, 2086. https://doi.org/10.3390/ma11112086
Liu W, Fu J, Zhang H, Shao Y, Zhang H, Zhu J. Cold Bonding Method for Metallic Powder Coatings. Materials. 2018; 11(11):2086. https://doi.org/10.3390/ma11112086
Chicago/Turabian StyleLiu, Wei, Jing Fu, Haiping Zhang, Yuanyuan Shao, Hui Zhang, and Jesse Zhu. 2018. "Cold Bonding Method for Metallic Powder Coatings" Materials 11, no. 11: 2086. https://doi.org/10.3390/ma11112086