Graphene-Modulated Removal Performance of Nitrogen and Phosphorus Pollutants in a Sequencing Batch Chlorella Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Source of Graphene
2.1.2. Source of Chlorella
2.1.3. Simulated Domestic Wastewater
2.2. Measurement Methods
2.3. Experimental Methods
2.4. Oxidative Stress Experiment
3. Results and Discussions
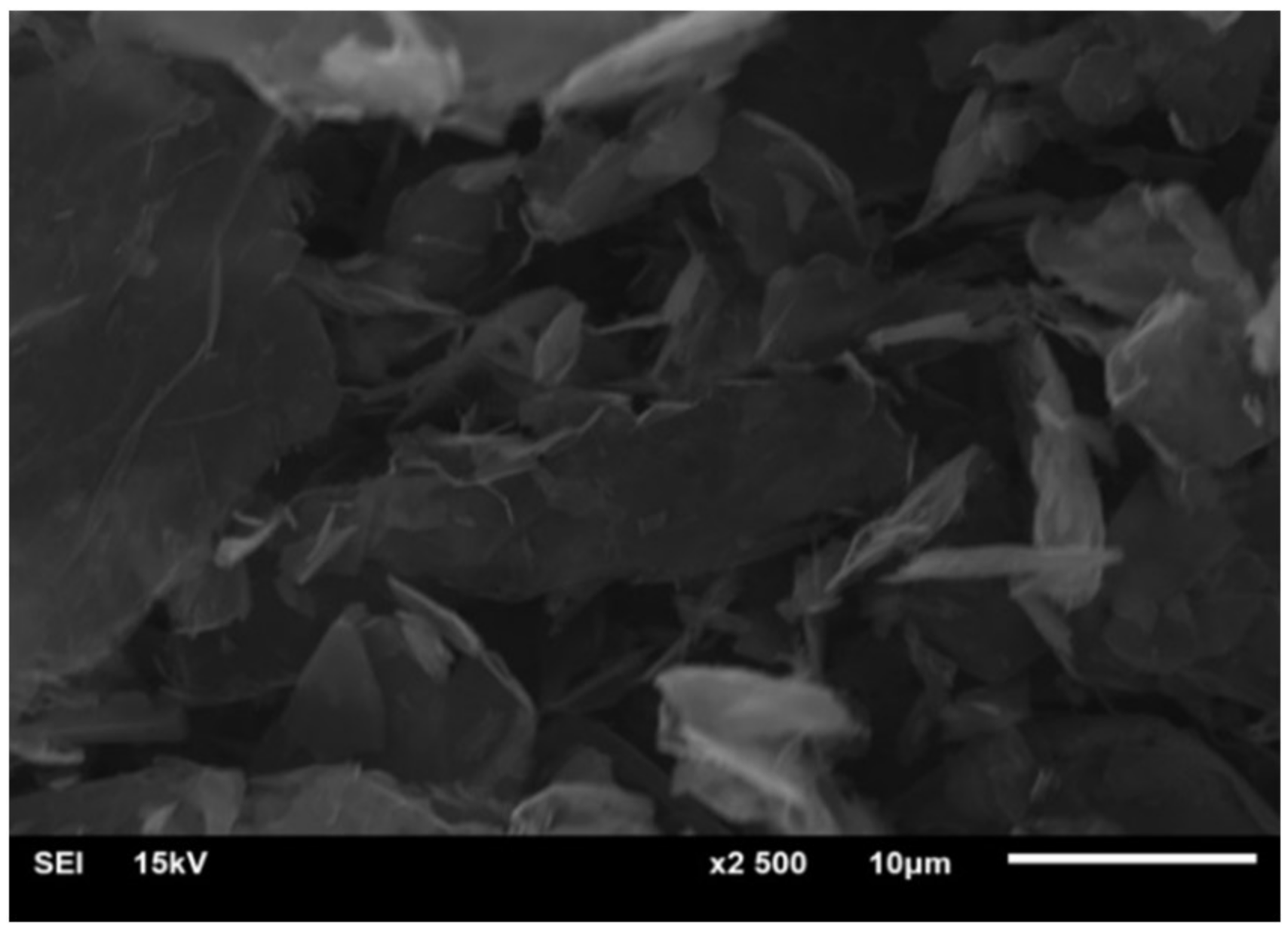
3.1. Graphene Characterization
3.2. Effect of Graphene Content and Reaction Time on Removal Efficiency of Pollutants in Reactor
3.2.1. Treatment Effect of TN in Reactors T1–T7
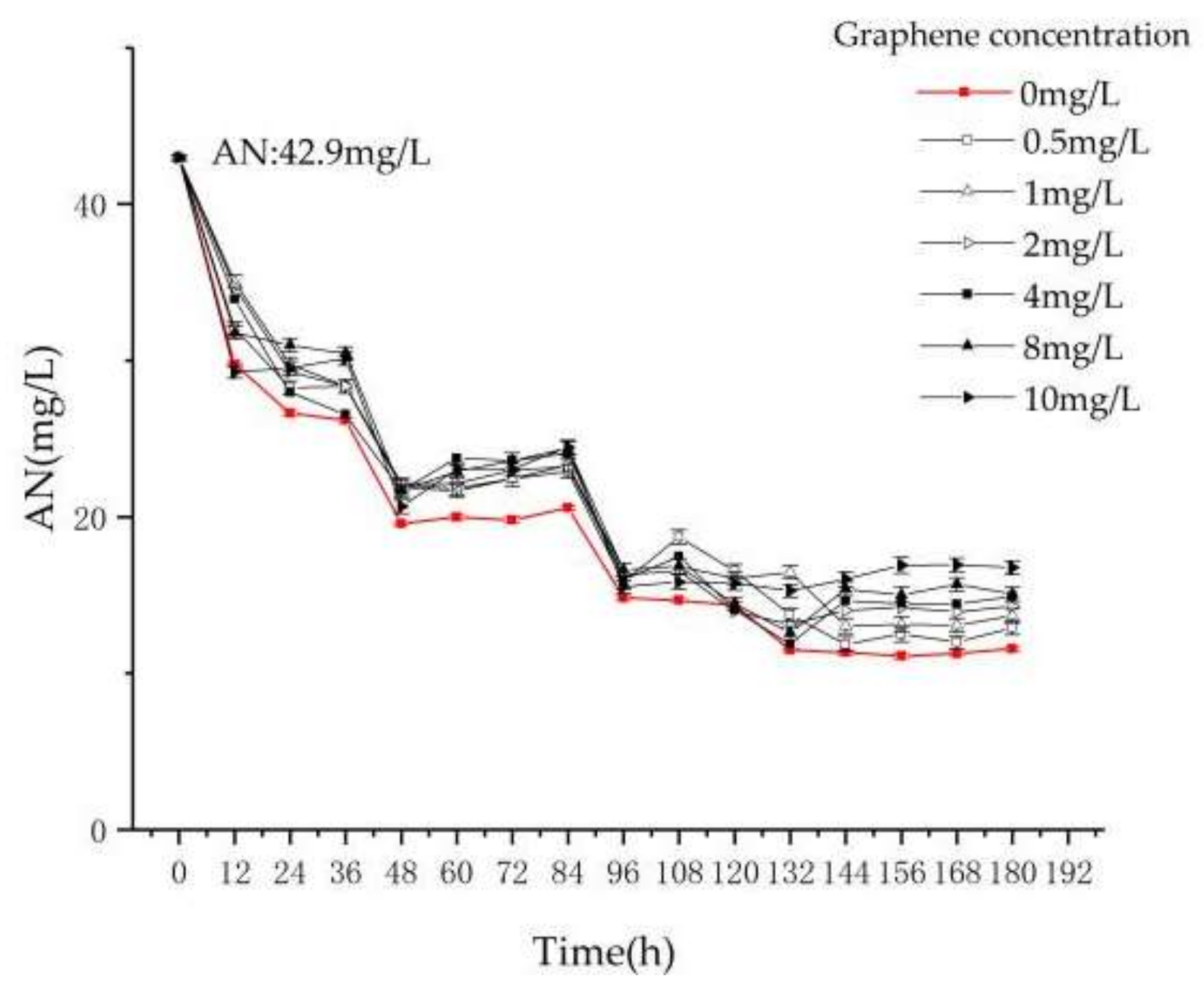
3.2.2. Treatment Effect of AN in Reactors T1–T7
3.2.3. Treatment Effect of TP in Reactors T1–T7
3.3. Oxidative Stress Experiment
3.3.1. Result of MDA Measurements
3.3.2. Results of SOD Determination
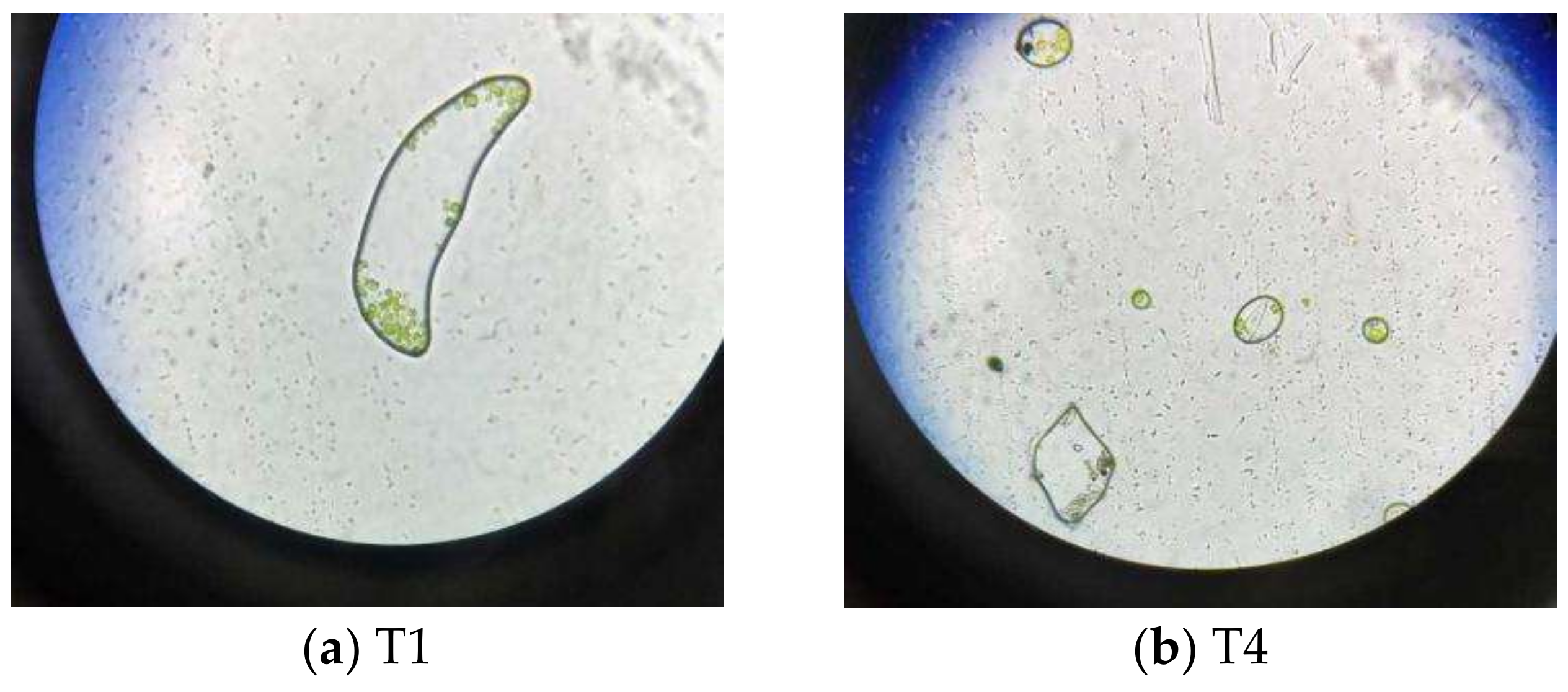
3.3.3. Observation by Optical Microscope
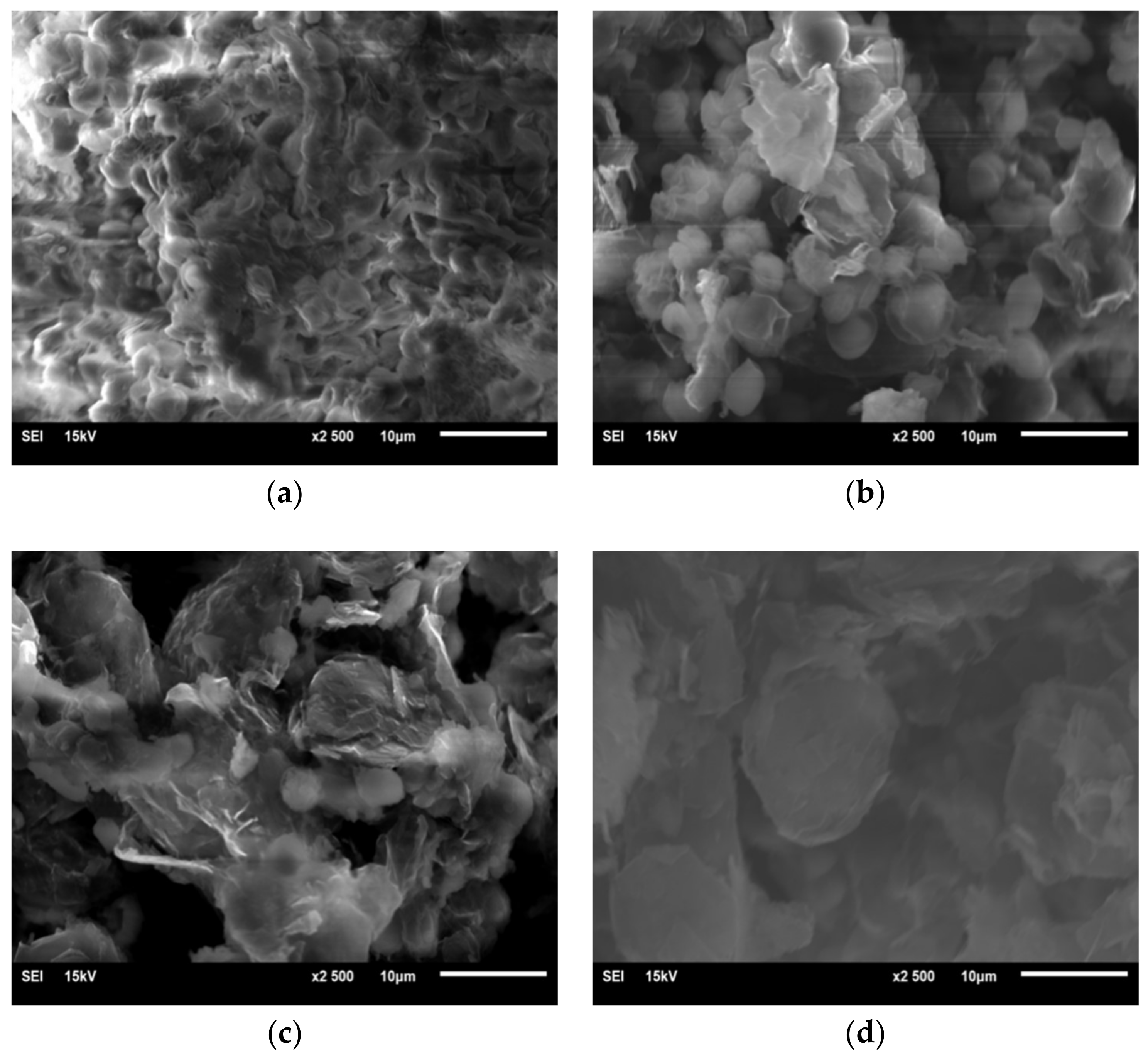
3.3.4. SEM Measurements
4. Conclusions
- (1)
- In the experiment of pollutant removal, the removal rates of TN and AN of reactors T1–T7 decrease with the increase of graphene concentration, and the removal efficiency of TN in reactor T2–T7 is lower than T1 by 4%, 8.8%, 10.2%, 12.1%, 16.8% and 20.9%, respectively. The removal rate of AN is lower by 10.2%, 16.5%, 20.2%, 21.2%, 24% and 28.4%, respectively. The removal rate of TP is lower by 9.2%, 13.3%, 19.6%, 31%, 37.6% and 38.3%, respectively.
- (2)
- In order to verify the damage of Chlorella by graphene, the concentration of MDA and the activity of SOD in the algal cells were measured. The results show that in reactors T1–T7, the concentration of MDA increases from 4.47 nmol/mL to 27.54 nmol/mL, and the activity of SOD increases from 1.15 μ/mg prot to 19.25 μ/mg prot. As the concentration of graphene increases, MDA and SOD increase regularly during the same period. Thus, it is concluded that the addition of graphene can cause oxidative damage to Chlorella vulgaris.
- (3)
- Using optical microscopy and SEM, it is found that graphene is adsorbed on the surface of Chlorella, and enters the interior of Chlorella. It is shown that graphene causes microscopic damage on Chlorella. Through the blood plate count method, we estimated an average Chlorella reduction of 16%. Nevertheless, it is necessary to further explore whether graphene destroys or modifies the internal structure and material of chloroplasts inside Chlorella.
- (4)
- The damage of Chlorella by graphene can inhibit Chlorella from removing pollutants in sewage, and decrease the removal efficiency of nitrogen and phosphorus pollutants in the sequencing batch Chlorella reactor. This study provides theoretical and practical support for the safe use of graphene.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, W.J.; Teng, Y. Environmental behavior of graphene and its effect on the transport and fate of pollutants in environment. J. App. Ecol. 2014, 25, 2723–2732. [Google Scholar]
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T. Fate and bioavailability of engineered nanoparticles in soils: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764. [Google Scholar] [CrossRef]
- Chen, G.; Huang, X.F.; An, L.; He, S.L.; Li, X.D.; Yang, D.H. Pilot-scale research on high rate algal pond for rural domestic sewage treatment at area around Taihu Lake. Water Wastewater Eng. 2006, 32, 37–40. [Google Scholar]
- Huang, X.F.; He, S.L.; Chen, G.; Li, X.D.; Yang, D.H.; Zhou, Q. Enhancement of nutrient removal from domestic wastewater with HRAP in rural areas. Environ. Eng. 2008, 1, 7–10. [Google Scholar]
- Li, M.; Jin, J.R.; Liu, D.Q. Research on immobilized algae cell flow bed for treatment living sewage. J. Suzhou Inst. Silk Text. Technol. 2013, 6, 6–9. [Google Scholar]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants-effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.X.; Shen, Z.Y.; Niu, J.F.; Zhuang, L.P.; He, T.D. Nanotoxicity of engineered nanomaterials in the environment. Prog. Chem. 2011, 23, 1769–1781. [Google Scholar]
- Chen, A.W.; Zeng, G.M.; Chen, G.Q.; Yi, B.; Guo, Z. Advance in research on toxicity of metal nanomaterials. Environ. Chem. 2014, 33, 568–575. [Google Scholar]
- Li, X.K.; Hu, X.G.; Zhou, Q.X. Research progress in phytotoxicity of carbon nanoparticles and its mechanisms. J. Agro-Environ. Sci. 2015, 11, 2041–2047. [Google Scholar]
- Li, Y.X.; Feng, W.; Gong, N.; Sun, Y.Q.; Xiong, D.Q. Study of inhabitation of NiO nanoparticles to Chlorella sp. Mari. Environ. Sci. 2009, 28, 151–153. [Google Scholar]
- Zhu, X.S. Study on the Ecotoxicology of Several Artificial Nanomaterials. Master’s Thesis, University of Nankai, Tianjin, China, 2007. (In Chinese). [Google Scholar]
- Xiao, H.X. Dissolution Behavior of Nano ZnO and Its Effect on Phosphorus Removal Efficiency of Chlorella Vulgaris. Master’s Thesis, University of Xiangtan, Xiangtan, China, 2007. (In Chinese). [Google Scholar]
- Wang, X.J.; Li, Z.S.; Xing, G.L.; Li, Z.N.; Yuan, H.L.; Yang, J.S. Optimization of Chlorella pyrenoidosa-15 photoheterotrophic culture and its use in wastewater treatment. Environ. Sci. 2012, 33, 2735–2740. [Google Scholar]
- Huang, K.; Liu, L.; Dong, H.L. Study on chlorella in sewage treatment. Jiangxi Chem. Ind. 2007, 1, 25–26. (In Chinese) [Google Scholar]
- Lv, F.R.; Yang, H.B.; Li, Y.M.; Zhang, X.H.; Yu, Y.; Liu, Y. Study on the N,P purification ability of chlorella under autotrophic condition. Biotechnology 2003, 13, 46–47. [Google Scholar]
- Cai, Y.F.; Wei, Q.; Guo, L.N.; Sun, H.Y.; Zhou, J.; Zhang, J.L. Nitrogen and phosphorus removing from wastewater by six species of algal biofilm. J. Guangxi Univ. (Nat. Sci. Ed.) 2013, 3, 668–672. (In Chinese) [Google Scholar]
- Huang, K. Study on the Removal of Nitrogen and Phosphorus from Sewage by Algae and Its Mechanism. Master’s Thesis, University of Nanchang, Nanchang, China, 2007. (In Chinese). [Google Scholar]
- Zhang, Y.L.; Zhu, H.Q.; Zhou, X.F.; Su, G.X. The Principle and Technology of Wastewater Microalgae Resource Treatment, 1st ed.; Science Press: Beijing, China, 2015; pp. 89–98. (In Chinese) [Google Scholar]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Li, M.L.; Jiang, Y.L. Behaviors of engineered nanoparticles in aquatic environments and impacts on marine phytoplankton. Environ. Sci. 2015, 36, 365–372. [Google Scholar]
- Zhang, N.; Jin, X.L.; Li, X.; Yue, J.J.; Wei, D.F. Progress of toxic effects of artificial nano materials on alga. J. Anhui Agric. Sci. 2011, 39, 6000–6003. [Google Scholar]
- Zhao, W. Toxic Effects of Nanoscale TiO2 on Typical Red Tide Algae. Master’s Thesis, Ocean University of China, Qingdao, China, 2007. (In Chinese). [Google Scholar]
- Yang, X.P.; Zhao, F.J. A review of uptake translocation and phytotoxicity of engineered nanoparticles in plants. Environ. Sci. 2013, 34, 4495–4502. [Google Scholar]





| T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
|---|---|---|---|---|---|---|---|
| Graphene (mg/L) | 0 | 0.5 | 1 | 2 | 4 | 8 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, G.; Xu, W.; Fang, Q.; Mou, Z.; Pan, Z. Graphene-Modulated Removal Performance of Nitrogen and Phosphorus Pollutants in a Sequencing Batch Chlorella Reactor. Materials 2018, 11, 2181. https://doi.org/10.3390/ma11112181
Xia G, Xu W, Fang Q, Mou Z, Pan Z. Graphene-Modulated Removal Performance of Nitrogen and Phosphorus Pollutants in a Sequencing Batch Chlorella Reactor. Materials. 2018; 11(11):2181. https://doi.org/10.3390/ma11112181
Chicago/Turabian StyleXia, Gonghan, Wenlai Xu, Qinglin Fang, Zishen Mou, and Zhicheng Pan. 2018. "Graphene-Modulated Removal Performance of Nitrogen and Phosphorus Pollutants in a Sequencing Batch Chlorella Reactor" Materials 11, no. 11: 2181. https://doi.org/10.3390/ma11112181




