Effects of F-Doping on the Electrochemical Performance of Na2Ti3O7 as an Anode for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Preparation and Characterization
2.1. The Preparation of Na2Ti3O7 and F-Doping Na2Ti3O7 Samples
2.2. Phase Analysis and Morphology Characterization
2.3. Electrochemical Test
3. Results and Discussion
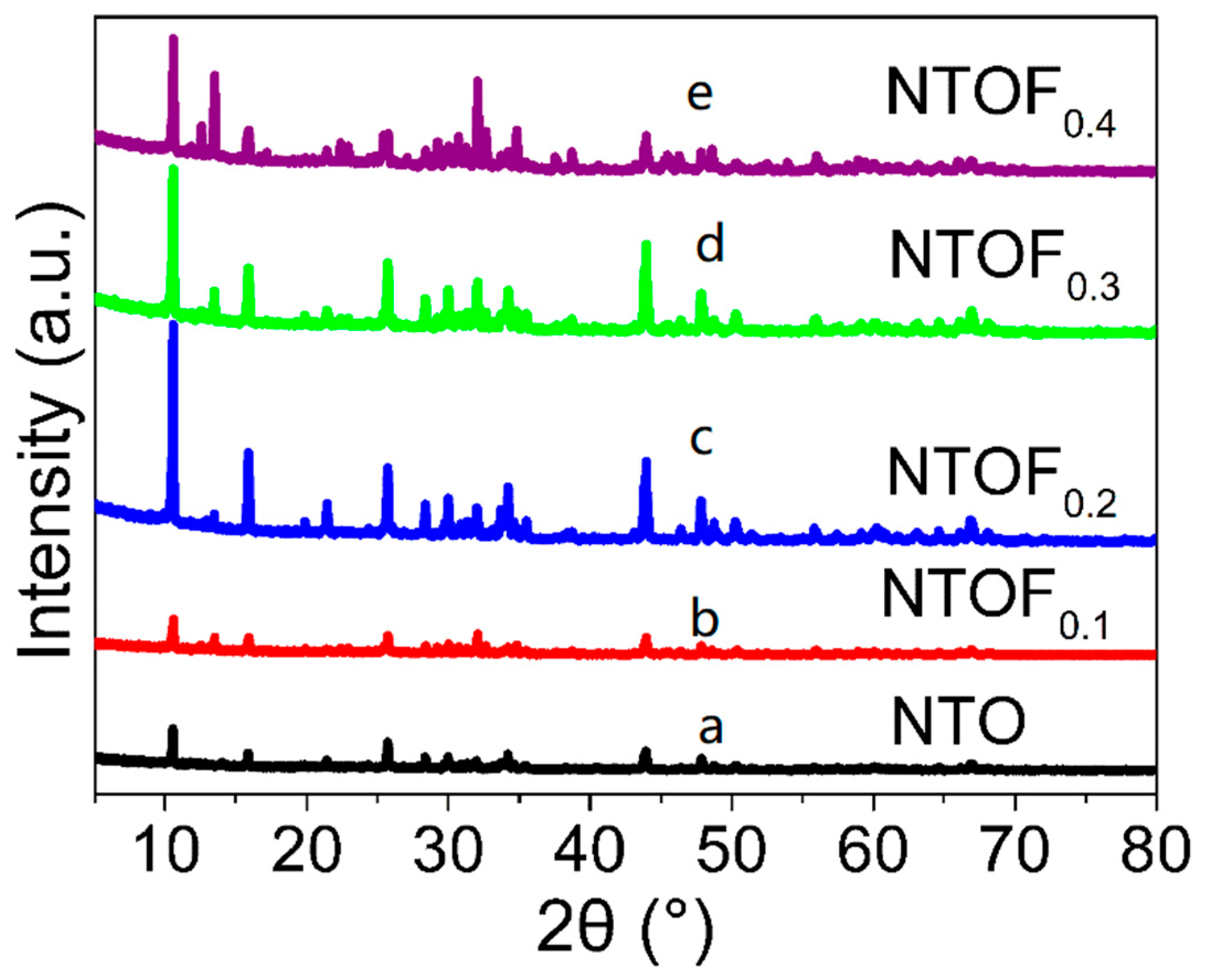
3.1. Structural and Composition Characterization
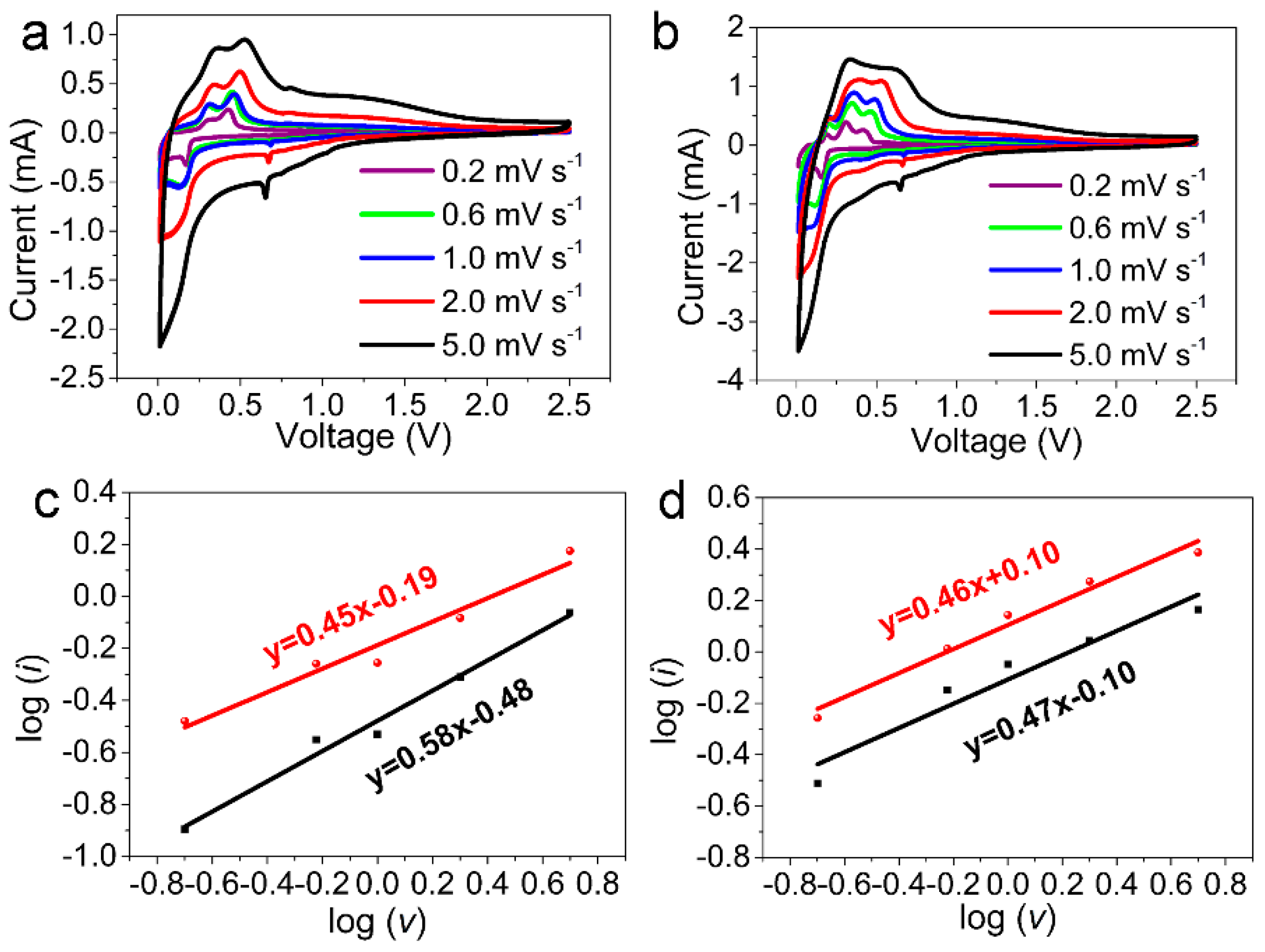
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. Engl. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Lee, K.T.; Ramesh, T.N.; Nan, F.; Botton, G.; Nazar, L.F. Topochemical Synthesis of Sodium Metal Phosphate Olivines for Sodium-Ion Batteries. Chem. Mater. 2011, 23, 3593–3600. [Google Scholar] [CrossRef]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.-M. Towards High Power High Energy Aqueous Sodium-Ion Batteries: The NaTi2(PO4)3/Na0.44MnO2 System. Adv. Eng. Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- Hou, H.D.; Gan, B.H.; Gong, Y.D.; Chen, N.; Sun, C.W. P2-Type Na0.66Ni0.23Mg0.1Mn0.67O2 as a High Performance Cathode for Sodium-Ion Battery. Inorg. Chem. 2016, 55, 9033–9037. [Google Scholar] [CrossRef]
- Hou, H.D.; Xu, Q.K.; Pang, Y.K.; Li, L.; Wang, J.L.; Zhang, C.; Sun, C.W. Efficient Storing Energy Harvested by Triboelectric Nanogenerators Using a Safe and Durable All-Solid-State Sodium-Ion Battery. Adv. Sci. 2017, 4, 1700072. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.H.; Wang, Y.S.; Sun, C.W.; Alonso, J.A.; Fernández-Díaz, M.T.; Chen, L.Q. Experimental Visualization of the Diffusion Pathway of Sodium Ions in the Na3[Ti2P2O10F] Anode for Sodium-Ion Battery. Sci. Rep. 2014, 4, 7231. [Google Scholar] [CrossRef]
- Ma, Z.H.; Sun, C.W.; Lv, Y.C.; Wang, Y.S.; Kim, Y.; Chen, L.Q. A New Oxyfluorinated Titanium Phosphate Anode for a High-Energy Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2015, 7, 1270–1274. [Google Scholar] [CrossRef]
- Trad, K.; Carlier, D.; Croguennec, L.; Wattiaux, A.; Ben Amara, M.; Delmas, C. NaMnFe2(PO4)3 Alluaudite Phase: Synthesis, Structure, and Electrochemical Properties As Positive Electrode in Lithium and Sodium Batteries. Chem. Mater. 2010, 22, 5554–5562. [Google Scholar] [CrossRef]
- Raju, V.; Rains, J.; Gates, C.; Luo, W.; Wang, X.; Stickle, W.F.; Stucky, G.D.; Ji, X. Superior cathode of sodium-ion batteries: orthorhombic V2O5 nanoparticles generated in nanoporous carbon by ambient hydrolysis deposition. Nano Lett. 2014, 14, 4119–4124. [Google Scholar] [CrossRef]
- Alcántara, R.; Jaraba, M.; Lavela, P.; Tirado, J.L. NiCo2O4 Spinel: First Report on a Transition Metal Oxide for the Negative Electrode of Sodium-Ion Batteries. Chem. Mater. 2002, 14, 2847–2848. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, L.; Pan, H.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, L. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem. Commun. 2012, 14, 86–89. [Google Scholar] [CrossRef]
- Sun, Q.; Ren, Q.-Q.; Li, H.; Fu, Z.-W. High capacity Sb2O4 thin film electrodes for rechargeable sodium battery. Electrochem. Commun. 2011, 13, 1462–1464. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, Y.; Wang, Q. Layered Na2V6O16 nanobelts as promising cathode and symmetric electrode for Na-ion batteries with high capacity. J. Alloys Compd. 2016, 688, 55–60. [Google Scholar] [CrossRef]
- Yamada, Y.; Doi, T.; Tanaka, I.; Okada, S.; Yamaki, J.-I. Liquid-phase synthesis of highly dispersed NaFeF3 particles and their electrochemical properties for sodium-ion batteries. J. Power Sources 2011, 196, 4837–4841. [Google Scholar] [CrossRef]
- Pan, H.; Lu, X.; Yu, X.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L. Sodium Storage and Transport Properties in Layered Na2Ti3O7 for Room-Temperature Sodium-Ion Batteries. Adv. Eng. Mater. 2013, 3, 1186–1194. [Google Scholar] [CrossRef]
- Rudola, A.; Saravanan, K.; Mason, C.W.; Balaya, P. Na2Ti3O7: an intercalation based anode for sodium-ion battery applications. J. Mater. Chem. A 2013, 1, 2653–2662. [Google Scholar] [CrossRef]
- Väli, R.; Jänes, A.; Thomberg, T.; Lust, E. D-Glucose Derived Nanospheric Hard Carbon Electrodes for Room-Temperature Sodium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1619–A1626. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Y.; Sun, S.; Huang, Y.; Peng, J.; Luo, J.; Zhang, Q.; Qiu, Y.; Fang, C.; Han, J. Low-Cost and High-Performance Hard Carbon Anode Materials for Sodium-Ion Batteries. ACS Omega 2017, 2, 1687–1695. [Google Scholar] [CrossRef]
- Zukalová, M.; Pitňa Lásková, B.; Mocek, K.; Zukal, A.; Bouša, M.; Kavan, L. Electrochemical performance of sol-gel-made Na2Ti3O7 anode material for Na-ion batteries. J. Solid State Electrochem. 2018, 22, 2545–2552. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, K.H.; Oh, S.M.; Lee, K.T. High-capacity anode materials for sodium-ion batteries. Chemistry 2014, 20, 11980–11992. [Google Scholar] [CrossRef]
- Araújo-Filho, A.A.; Silva, F.L.R.; Righi, A.; da Silva, M.B.; Silva, B.P.; Caetano, E.W.S.; Freire, V.N. Structural, electronic and optical properties of monoclinic Na2Ti3O7 from density functional theory calculations: A comparison with XRD and optical absorption measurements. J. Solid State Chem. 2017, 250, 68–74. [Google Scholar] [CrossRef]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Eng. Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Dynarowska, M.; Kotwiński, J.; Leszczynska, M.; Marzantowicz, M.; Krok, F. Ionic conductivity and structural properties of Na2Ti3O7 anode material. Solid State Ionics 2017, 301, 35–42. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Mei, C.; Xu, J.; Wong, C.-P. Improving the sodiation performance of Na2Ti3O7 through Nb-doping. Electrochim. Acta 2017, 224, 446–451. [Google Scholar] [CrossRef]
- Xu, M.W.; Xiao, P.H.; Shannon, S.; Song, J.; Henkelman, G.; Goodenough, J.B. Theoretical and Experimental Study of Vanadium-Based Fluorophosphate Cathodes for Rechargeable Batteries. Chem. Mater. 2014, 26, 3089–3097. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Campos, H.; Villarreal, J.; Alcoutlabi, M. A comparative study on the performance of binary SnO2/NiO/C and Sn/C composite nanofibers as alternative anode materials for lithium ion batteries. Electrochim. Acta 2017, 224, 608–621. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite Nanofibers as Advanced Materials for Li-ion, Li-O2 and Li-S Batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-Based Nanocomposites for Energy Storage. Adv. Eng. Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-B.; Li, B.; Liu, M.; Zhang, C.; Lv, W.; Yang, C.; Li, J.; Du, H.; Zhang, B.; Yang, Q.-H.; et al. Gassing in Li4Ti5O12-based batteries and its remedy. Sci. Rep. 2012, 2, 913. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Chen, H.; Xu, Q.; Liu, H.; Wang, Y.G.; Xia, Y. Black Phosphorus Stabilizing Na2Ti3O7/C Each Other with an Improved Electrochemical Property for Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2018, 10, 37163–37171. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhao, K.; Luo, W.; Li, S.; Zhou, L.; Mai, L. Interconnected LiCuVO4 networks with in situ Cu generation as high-performance lithium-ion battery anode. Phys. Chem. Chem. Phys. 2017, 19, 13341–13347. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Lu, L.; Gao, Y.; Zhang, Q.; Zhang, C.; Sun, C.; Chen, X. Effects of F-Doping on the Electrochemical Performance of Na2Ti3O7 as an Anode for Sodium-Ion Batteries. Materials 2018, 11, 2206. https://doi.org/10.3390/ma11112206
Chen Z, Lu L, Gao Y, Zhang Q, Zhang C, Sun C, Chen X. Effects of F-Doping on the Electrochemical Performance of Na2Ti3O7 as an Anode for Sodium-Ion Batteries. Materials. 2018; 11(11):2206. https://doi.org/10.3390/ma11112206
Chicago/Turabian StyleChen, Zehua, Liang Lu, Yu Gao, Qixiang Zhang, Chuanxiang Zhang, Chunwen Sun, and Xingying Chen. 2018. "Effects of F-Doping on the Electrochemical Performance of Na2Ti3O7 as an Anode for Sodium-Ion Batteries" Materials 11, no. 11: 2206. https://doi.org/10.3390/ma11112206




