Photoactive ZnO Materials for Solar Light-Induced CuxO-ZnO Catalyst Preparation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Materials
2.1.1. Polyol Method
2.1.2. Precipitation with Na2CO3
2.1.3. Precipitation with NH2CO2NH4
2.2. Photon-Assisted Preparation of Cu-ZnO Catalysts
2.2.1. Cu(acac)2 in THF-H2O Solvent
2.2.2. Cu(NO3)2 in H2O Solvent
2.3. Characterization Techniques
2.4. Evaluation of the Photocatalytic Efficiency
3. Results
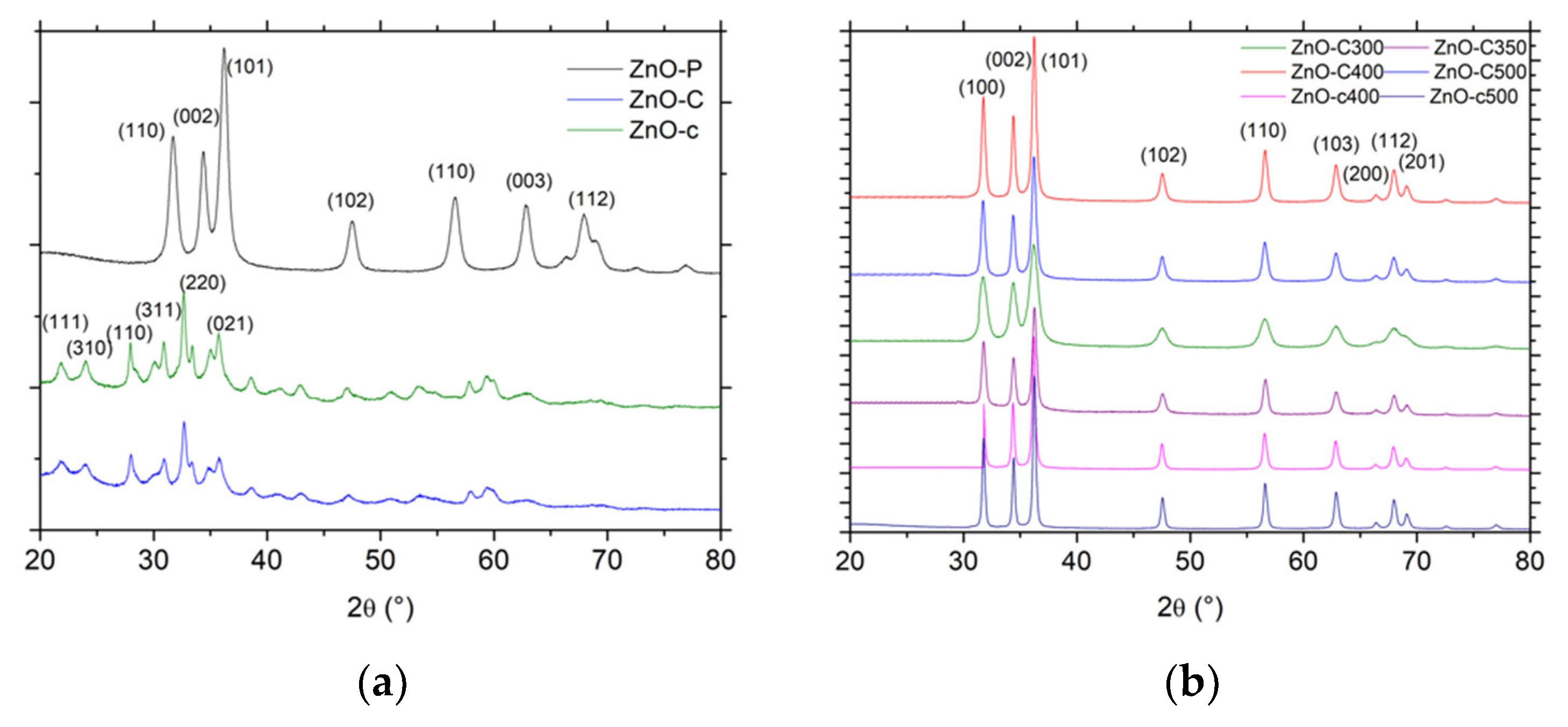
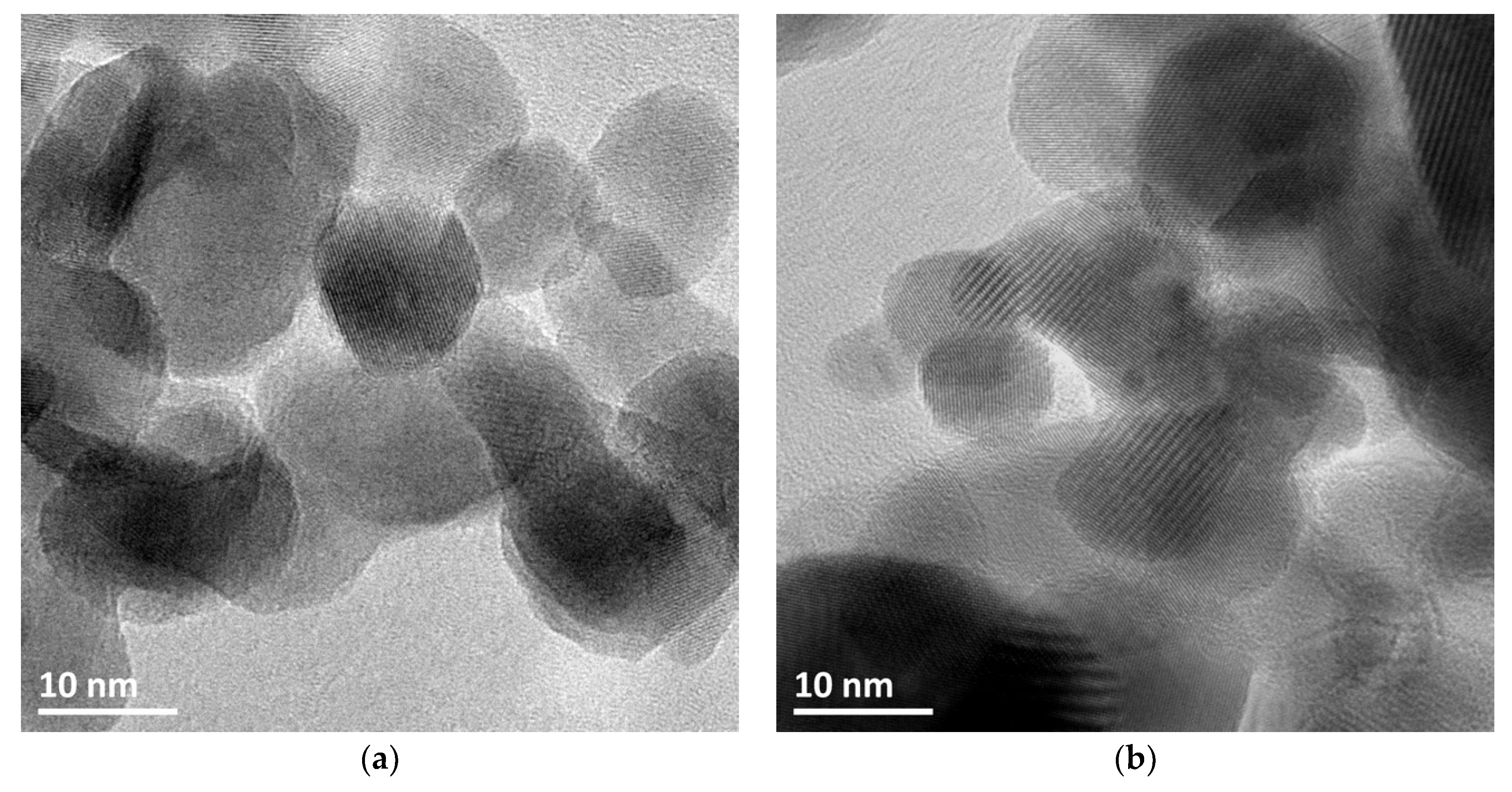
3.1. Characterization of ZnO Materials
3.2. Photocatalytic Activity of ZnO
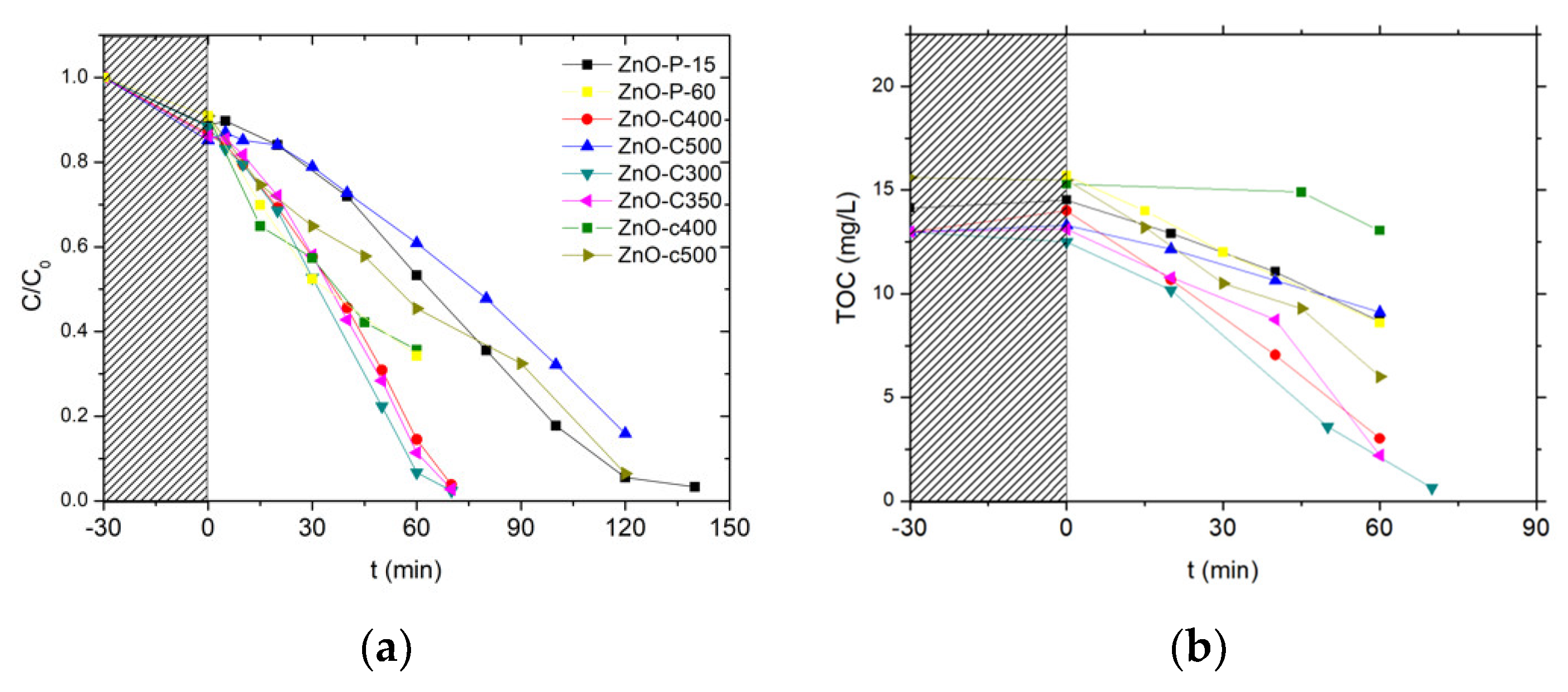
3.2.1. Influence of a Photocatalytic Pre-Cleaning Step
3.2.2. Photocatalytic Activity of ZnO Materials
3.3. Preparation of Cu-ZnO Catalysts by the Photon-Assisted Synthesis Method
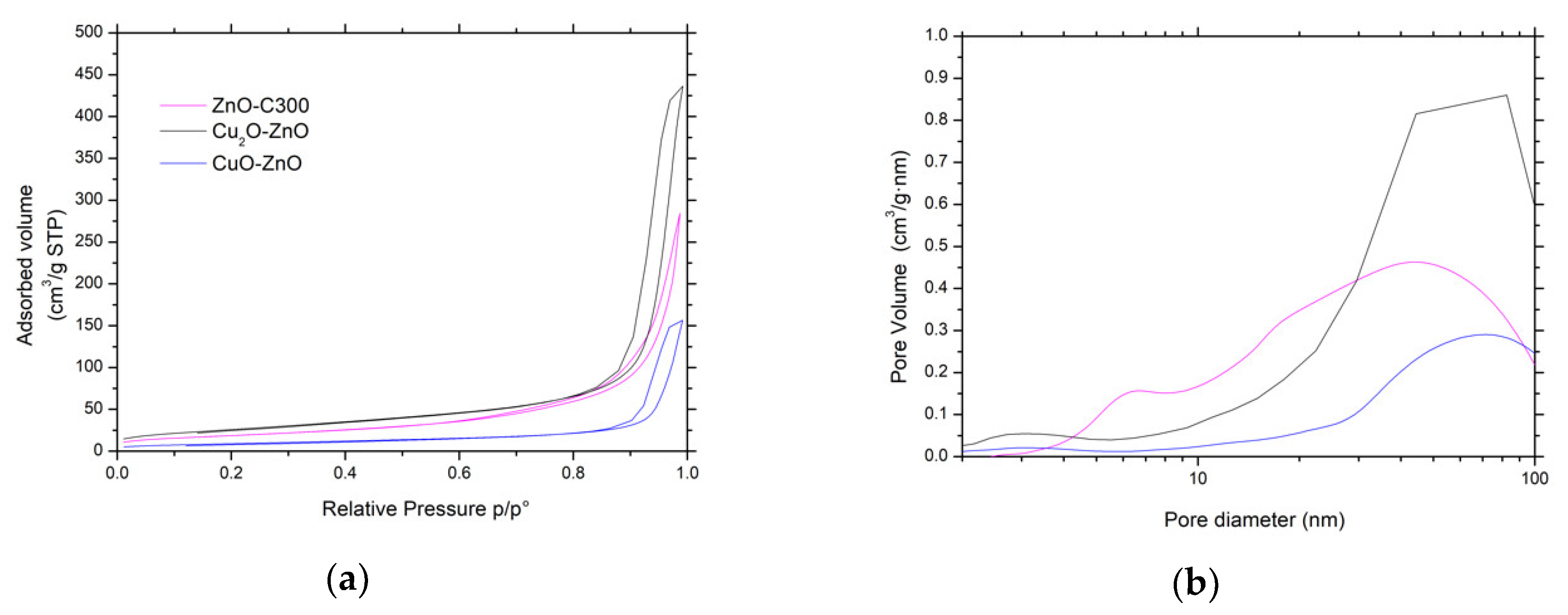
3.4. Characterization of Cu-ZnO Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Segets, D.; Gradl, J.; Taylor, R.K.; Vassilev, V.; Peukert, W. Analysis of optical absorbance spectra for the determination of ZnO nanoparticle size distribution, solubility, and surface energy. ACS Nano 2009, 3, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Matoussi, A. Electrical conduction and dielectric studies of ZnO pellets. Phys. B Condens. Matter 2012, 407, 3441–3447. [Google Scholar] [CrossRef]
- Bacaksiz, E.; Parlak, M.; Tomakin, M.; Özçelik, A.; Karakız, M.; Altunbaş, M. The effects of zinc nitrate, zinc acetate and zinc chloride precursors on investigation of structural and optical properties of ZnO thin films. J. Alloys Compd. 2008, 466, 447–450. [Google Scholar] [CrossRef]
- Wang, Z.L. Splendid one-dimensional nanostructures of zinc oxide: A new nanomaterial family for nanotechnology. ACS Nano 2008, 2, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, J.; Fang, B.; Lu, P.; Deng, S.; Wang, H. Synthesis and characterization of multipod, flower-like, and shuttle-like ZnO frameworks in ionic liquids. Mater. Lett. 2005, 59, 1405–1408. [Google Scholar] [CrossRef]
- Sudha, D.; Sivakumar, P. Review on the photocatalytic activity of various composite catalysts. Chem. Eng. Process. Process Intensif. 2015, 97, 112–133. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Valence-band ordering in ZnO. Phys. Rev. B 1999, 60, 2340–2344. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bagnall, D.M.; Koh, H.; Park, K.; Hiraga, K.; Zhu, Z.; Yao, T. Plasma assisted molecular beam epitaxy of ZnO on c -plane sapphire: Growth and characterization. J. Appl. Phys. 1998, 84, 3912–3918. [Google Scholar] [CrossRef]
- Udom, I.; Ram, M.K.; Stefanakos, E.K.; Hepp, A.F.; Goswami, D.Y. One dimensional-ZnO nanostructures: Synthesis, properties and environmental applications. Mater. Sci. Semicond. Process. 2013, 16, 2070–2083. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.A.; Torres-Ricárdez, R.; García-Mendoza, M.F.; Ramírez-Morales, E.; Rojas-Blanco, L.; Díaz-Flores, L.L.; Sepúlveda-Palacios, G.E.; Paraguay-Delgado, F.; Pérez-Hernández, G. Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal. Today 2018. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Duconge, J.; Arango, J.C.; Morant, C.; Pinilla, S.; Soto-Vázquez, L.; Resto, E.; Márquez, F. Hydrogen production via water splitting using different Au@ZnO catalysts under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 385–394. [Google Scholar] [CrossRef]
- Fkiri, A.; Santacruz, M.R.; Mezni, A.; Smiri, L.S.; Keller, V.; Keller, N. One-pot synthesis of lightly doped Zn1−xCuxO and Au–Zn1−xCuxO with solar light photocatalytic activity in liquid phase. Environ. Sci. Pollut. Res. 2017, 24, 15622–15633. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, M.; Shan, G.; Li, Y.; Huang, B.; Yang, G. Biocompatible ZnO/Au nanocomposites for ultrasensitive DNA detection using resonance Raman scattering. J. Phys. Chem. B 2008, 112, 6484–6489. [Google Scholar] [CrossRef] [PubMed]
- Udawatte, N.; Lee, M.; Kim, J.; Lee, D. Well-defined Au/ZnO nanoparticle composites exhibiting enhanced photocatalytic activities. ACS Appl. Mater. Interfaces 2011, 3, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, E.; Chan, F.Y.F.; Lu, Z.; Chen, R. Fabrication of ordered flower-like ZnO nanostructures by a microwave and ultrasonic combined technique and their enhanced photocatalytic activity. Mater. Lett. 2011, 65, 3440–3443. [Google Scholar] [CrossRef]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y. Au-ZnO hybrid nanopyramids and their photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lee, G.; Anandan, S.; Wu, J.J. Synthesis of ZnO and Au tethered ZnO pyramid-like microflower for photocatalytic degradation of orange II. Mater. Sci. Eng. B 2012, 177, 190–196. [Google Scholar] [CrossRef]
- Sun, Q.; Men, Y.; Wang, J.; Chai, S.; Song, Q. Support effect of Ag/ZnO catalysts for partial oxidation of methanol. Inorg. Chem. Commun. 2018, 92, 51–54. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; Molenbroek, A.M.; Topsøe, N.Y.; Topsøe, H.; Clausen, B.S. In situ investigations of structural changes in Cu/ZnO catalysts. J. Catal. 2000, 194, 452–460. [Google Scholar] [CrossRef]
- Ichikawa, M. Catalysis by supported metal crystallites from carbonyl clusters. I. Catalytic methanol synthesis under mild conditions over supported rhodium, platinum, and iridium crystallites prepared from Rh, Pt, and Ir carbonyl cluster compounds deposited on ZnO and MgO. Bull. Chem. Soc. Jpn. 1978, 51, 2268–2272. [Google Scholar] [CrossRef]
- Cubeiro, M.L.; Fierro, J.L.G. Selective production of hydrogen by partial oxidation of methanol over ZnO-supported palladium catalysts. J. Catal. 1998, 179, 150–162. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Du, W.; Shi, D.; Ning, W.; Lu, X.; Hou, Z. Metal-organic framework derived Cu/ZnO catalysts for continuous hydrogenolysis of glycerol. Appl. Catal. B Environ. 2017, 203, 146–153. [Google Scholar] [CrossRef]
- Alba-Rubio, A.C.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Martín-Alonso, D.; Jiménez-López, A.; Maireles-Torres, P. Heterogeneous transesterification processes by using CaO supported on zinc oxide as basic catalysts. Catal. Today 2010, 149, 281–287. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, X.; Zheng, H.; Ding, G.; Zhu, Y.; Li, Y. Efficient synthesis of 2,5-dihydroxymethylfuran and 2,5-dimethylfuran from 5-hydroxymethylfurfural using mineral-derived Cu catalysts as versatile catalysts. Catal. Sci. Technol. 2015, 5, 4208–4217. [Google Scholar] [CrossRef]
- Llorca, J.; de la Piscina, P.R.; Dalmon, J.; Sales, J.; Homs, N. CO-free hydrogen from steam-reforming of bioethanol over ZnO-supported cobalt catalysts: Effect of the metallic precursor. Appl. Catal. B Environ. 2003, 43, 355–369. [Google Scholar] [CrossRef]
- Homs, N.; Llorca, J.; de la Piscina, P.R. Low-temperature steam-reforming of ethanol over ZnO-supported Ni and Cu catalysts: The effect of nickel and copper addition to ZnO-supported cobalt-based catalysts. Catal. Today 2006, 116, 361–366. [Google Scholar] [CrossRef]
- Ammari, F.; Lamotte, J.; Touroude, R. An emergent catalytic material: Pt/ZnO catalyst for selective hydrogenation of crotonaldehyde. J. Catal. 2004, 221, 32–42. [Google Scholar] [CrossRef]
- Spencer, M.S. The role of zinc oxide in Cu/ZnO catalysts for methanol synthesis and the water gas shift reaction. Top. Catal. 1999, 8, 259. [Google Scholar] [CrossRef]
- Pinna, F. Supported metal catalysts preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.H.; Luo, Y.C.; Mou, C.Y.; Lin, S.D.; Cheng, H.; Chen, J.M.; Lee, J.F.; Lin, T.S. Strong metal-support interactions between gold nanoparticles and ZnO nanorods in CO oxidation. J. Am. Chem. Soc. 2012, 134, 10251. [Google Scholar] [CrossRef] [PubMed]
- Le Valant, A.; Comminges, C.; Tisseraud, C.; Canaff, C.; Pinard, L.; Pouilloux, Y. The Cu–ZnO synergy in methanol synthesis from CO2, Part 1: Origin of active site explained by experimental studies and a sphere contact quantification model on Cu + ZnO mechanical mixtures. J. Catal. 2015, 324, 41–49. [Google Scholar] [CrossRef]
- Collins, S.S.E.; Cittadini, M.; Pecharromán, C.; Martucci, A.; Mulvaney, P. Hydrogen spillover between single gold nanorods and metal oxide supports: A surface plasmon spectroscopy study. ACS Nano 2015, 9, 7846. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H.; Zainal, Z.; Hussein, M.Z. Photocatalytic treatment of 4-chlorophenol in aqueous ZnO suspensions: Intermediates, influence of dosage and inorganic anions. J. Hazard. Mater. 2009, 168, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Hashemi, M.; Ghanbari, F. Degradation of 4-chlorophenol using catalyzed peroxymonosulfate with nano-MnO2/UV irradiation: Toxicity assessment and evaluation for industrial wastewater treatment. J. Clean. Prod. 2018, 195, 1389–1397. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Liu, T.; Du, S.; Qiang, Q.; Wang, Y.; Yin, S.; Sato, T. Comparison of photocatalytic reaction-induced selective corrosion with photocorrosion: Impact on morphology and stability of Ag-ZnO. Appl. Catal. B Environ. 2017, 201, 348–358. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.; Weng, B.; Xu, Y. Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891–16903. [Google Scholar] [CrossRef] [PubMed]
- Naya, S.I.; Tanaka, M.; Kimura, K.; Tada, H. Visible-light-driven copper acetylacetonate decomposition by BiVO4. Langmuir 2011, 27, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, L.; Peng, Z.; Song, Y.; Chen, W. Field emission properties of one-dimensional single CuO nanoneedle by in situ microscopy. J. Mater. Sci. 2010, 45, 3791–3796. [Google Scholar] [CrossRef]
- Kim, M.H.; Lim, B.; Lee, E.; Xia, Y. Polyol synthesis of Cu2O nanoparticles: Use of chloride to promote the formation of a cubic morphology. J. Mater. Chem. 2008, 18, 4069–4073. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]

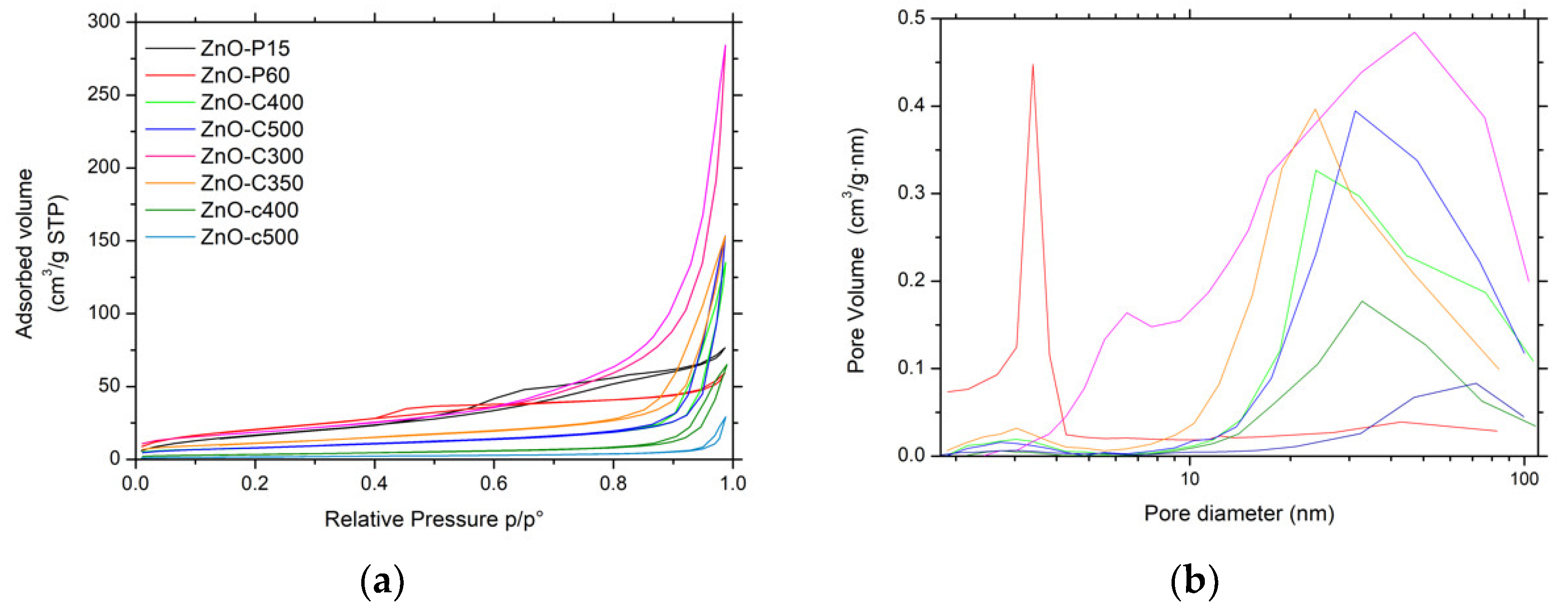







| Sample | Mean Crystallite Size (nm) a | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| ZnO-P-15 | 7 | 78 | 0.13 | 5 |
| ZnO-P-60 | 11 | 66 | 0.09 | 6 |
| ZnO-C-300 | 10 | 68 | 0.45 | 21 |
| ZnO-C-350 | 22 | 41 | 0.24 | 21 |
| ZnO-C-400 | 20 | 29 | 0.21 | 27 |
| ZnO-C-500 | 18 | 29 | 0.24 | 30 |
| ZnO-c-400 | 28 | 12 | 0.10 | 30 |
| ZnO-c-500 | 30 | 6 | 0.05 | 28 |
| Cu2O-ZnO | 13 | 95 | 0.68 | 26 |
| CuO-ZnO | 20 | 33 | 0.24 | 29 |
| Catalyst | k’TOC (mg/Lmin)/R2 | k’Phenol (min−1)/R2 |
|---|---|---|
| ZnO-P15 | 0.097/0.99 | 0.008/0.98 |
| ZnO-P60 | 0.130/0.88 | 0.011/0.94 |
| ZnO-C300 | 0.180/0.99 | 0.210/0.99 |
| ZnO-C350 | 0.173/0.90 | 0.017/0.99 |
| ZnO-C400 | 0.180/0.99 | 0.014/0.99 |
| ZnO-C500 | 0.071/0.99 | 0.007/0.97 |
| ZnO-c400 | 0.030/0.89 | 0.010/0.93 |
| ZnO-c500 | 0.153/0.98 | 0.007/0.98 |
| Run | XTOC (%) |
|---|---|
| First | 100 |
| Second | 88 |
| Third | 61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, M.; García-Muñoz, P.; Ruppert, A.M.; Keller, N. Photoactive ZnO Materials for Solar Light-Induced CuxO-ZnO Catalyst Preparation. Materials 2018, 11, 2260. https://doi.org/10.3390/ma11112260
Brzezińska M, García-Muñoz P, Ruppert AM, Keller N. Photoactive ZnO Materials for Solar Light-Induced CuxO-ZnO Catalyst Preparation. Materials. 2018; 11(11):2260. https://doi.org/10.3390/ma11112260
Chicago/Turabian StyleBrzezińska, Magdalena, Patricia García-Muñoz, Agnieszka M. Ruppert, and Nicolas Keller. 2018. "Photoactive ZnO Materials for Solar Light-Induced CuxO-ZnO Catalyst Preparation" Materials 11, no. 11: 2260. https://doi.org/10.3390/ma11112260






