Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Syntheses
2.3. X-ray Crystalography Study
2.4. Preparation and Photoactivity Study of Composites
2.5. The Computational Details
3. Results
3.1. Structures of (Ti4O2(OiBu)10(O2CR’)2) Clusters
3.2. Analysis of Vibrational Spectra
3.3. UV-Vis Absorption Spectra, Band Gap Determination and DOS
3.4. Photoluminescent Properties
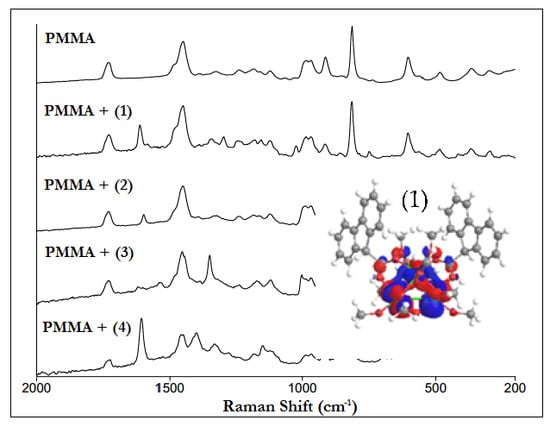
3.5. Preparation and Characteristic of Polymer Composites (PMMA + TOCs)
3.6. Photocatalytic Degradation of Methylene Blue (MB)
3.7. ESR Evidence of Paramagnetic Species
4. Discussion
5. Summary
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Li, N.; Matthews, P.D.; Luo, H.-K.; Wright, D.S. Novel properties and potential applications of functional ligand-modified polyoxotitanate cages. Chem. Commun. 2016, 52, 11180–11190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedict, J.B.; Freindorf, R.; Trzop, E.; Cogswell, J.; Coppens, P. Large Polyoxotitanate Clusters: Well-Defined Models for Pure-Phase TiO2 Structures and Surfaces. J. Am. Chem. Soc. 2010, 132, 13669–13671. [Google Scholar] [CrossRef] [PubMed]
- Benedict, J.B.; Coppens, P. The Crystalline Nanocluster Phase as a Medium for Structural and Spectroscopic Studies of Light Absorption of Photosensitizer Dyes on Semiconductor Surfaces. J. Am. Chem. Soc. 2010, 132, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Coppens, P.; Chen, Y.; Trzop, E. Crystallography and Properties of Polyoxotitanate Nanoclusters. Chem. Rev. 2014, 114, 9645–9661. [Google Scholar] [CrossRef] [PubMed]
- Ohlmaier-Delgadillo, F.; Castillo-Ortega, M.M.; Ramirez-Bon, R.; Armenta-Villegas, L.; Rodriguez-Félix, D.E.; Santacruz-Ortega, H.; del Castillo-Castro, T.; Santos-Sauceda, I. Photocatalytic properties of PMMA-TiO2 class I and class II hybrid nanofibers obtained by electrospinning. J. Appl. Polym. Sci. 2016, 133, 44334. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Schubert, U. Cluster-Based Inorganic-Organic Hybrid Materials. Chem. Soc. Rev. 2011, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Gross, S. Oxocluster-Reinforced Organic-Inorganic Hybrid Materials: Effect of Transition Metal Oxoclusters on Structural and Functional Properties. J. Mater. Chem. 2011, 21, 15853–15861. [Google Scholar] [CrossRef]
- Carraro, M.; Gross, S. Hybrid Materials Based on the Embedding of Organically Modified Transition Metal Oxoclusters or Polyoxometalates into Polymers for Functional Applications: A Review. Materials 2014, 7, 3956–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanam, N.; Chintakrinda, K.; Fang, W.-H.; Kang, Y.; Zhang, L.; Zhang, J. Azole Functionalized Polyoxo-Titanium Clusters with Sunlight-Driven Dye Degradation Applications: Synthesis, Structure, and Photocatalytic Studies. Inorg. Chem. 2016, 55, 10294–10301. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Toya, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-Light-Promoted Photocatalytic Hydrogen Production by Using an Amino-Functionalized Ti(IV) Metal-Organic Framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Rozes, L.; Sanchez, C. Titanium oxo-clusters: Precursors for a Lego-like construction of nanostructured hybrid materials. Chem. Soc. Rev. 2011, 40, 1006–1030. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Radtke, A.; Muzioł, T.; Richert, M.; Chojnacki, J. The conversion of multinuclear μ-oxo titanium(IV) species in the reaction of Ti(OiBu)4 with branched organic acids; results of structural and spectroscopic studies. Dalton Trans. 2012, 41, 8261–8269. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Piszczek, P.; Muzioł, T.; Wojtczak, A. The Structural Conversion of Multinuclear Titanium(IV) μ-Oxo-complexes. Inorg. Chem. 2014, 53, 10803–10810. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-H.; Zhang, L.; Zhang, J. Synthetic strategies, diverse structures and tunable properties of polyoxo-titanium clusters. Chem. Soc. Rev. 2018, 47, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U. Chemical modification of titanium alkoxides for sol-gel processing. J. Mater. Chem. 2005, 15, 3701–3715. [Google Scholar] [CrossRef]
- Liu, J.-X.; Gao, M.-Y.; Fang, W.-H.; Zhang, L.; Zhang, J. Bandgap Engineering of Titanium–Oxo Clusters: Labile Surface Sites Used for Ligand Substitution and Metal Incorporation. Angew. Chem. Int. Ed. 2016, 55, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sarkar, D.; Kim, Y.; Park, M.H.; Yoon, M.; Kim, Y.; Kim, M. Synthesis of functionalized titanium-carboxylate molecular clusters and their catalytic activity. J. Ind. Eng. Chem. 2017, 53, 171–176. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, Y.-F.; Chen, Z.-H.; Lui, F.-H.; Zhao, L.; Su, Z.-M. Synthesis, structure, and photocatalytic hydrogen of three environmentally friendly titanium oxo-clusters. Inorg. Chem. Commun. 2014, 40, 22–25. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Luo, W.; Wang, Y.-H.; Pu, Y.-Y.; Zhang, X.; You, L.-S.; Zhu, Q.-Y.; Dai, J. Titanium–oxo–Clusters with Dicarboxylates: Single-Crystal Structure and Photochromic Effect. Inorg. Chem. 2012, 51, 8982–8988. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Ge, G. Two Titanium-oxo-Clusters with Malonate and Succinate Ligands: Single-Crystal Structures and Catalytic Property. J. Clust. Chem. 2016, 27, 635–643. [Google Scholar] [CrossRef]
- Janek, M.; Muzioł, T.; Piszczek, P. The structure and photocatalytic activity of the tetranuclear titanium(IV) oxo-complex with 4-aminobenzoate ligands. Polyhedron 2018, 141, 110–1178. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, J.; Frasconi, M.; Li, X.; Cao, D.; Zhu, Z.; Schneebeli, S.T.; Schatz, G.C.; Stoddart, J.F. A Square-Planar Tetracoordinate Oxygen-Containing Ti4O17 Cluster Stabilized by Two 1,1’-Ferrocenedicarboxylato Ligands. Angew. Chem. Int. Ed. 2014, 51, 9193–9197. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis RED and CrysAlis CCD; Oxford Diffraction Ltd.: Abingdon, Oxfordshire, England, 2000.
- Krug, M.; Weiss, M.S.; Heinemann, U.; Mueller, U. XDSAPP: A graphical user interface for the convenient processing of diffraction data using XDS. J. Appl. Crystallogr. 2012, 45, 568–572. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Cryst. D 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Diamond; Release 2.1e; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalman, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Boyle, T.J.; Alam, T.M.; Tafoya, C.J.; Scott, B.L. Formic Acid Modified Ti(OCHMe2)4. Syntheses, Characterization, and X-ray Structures of Ti4(μ4-O)(μ-O)(OFc)2(μ-OR)4(OR)6 and Ti6(μ3-O)6(OFc)6(OR)6 (OFc = O2CH; OR = OCHMe2). Inorg. Chem. 1998, 37, 5588–5594. [Google Scholar] [CrossRef] [PubMed]
- Eslava, S.; Hengesbach, F.; McPartlin, M.; Wright, D.S. Heterometallic cobalt(II)–titanium(IV) oxo cages; key building blocks for hybrid materials. Chem. Commun. 2010, 46, 4701–4703. [Google Scholar] [CrossRef] [PubMed]
- Eslava, S.; McPartlin, M.; Thomson, R.I.; Rawson, J.M.; Wright, D.S. Single-Source Materials for Metal-Doped Titanium Oxide: Syntheses, Structures, and Properties of a Series of Heterometallic Transition-Metal Titanium Oxo Cages. Inorg. Chem. 2010, 49, 11532–11540. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, W.W.; Hecht, H.G. Reflectance Spectroscopy; Interscience Publishers: New York, NY, USA, 1966. [Google Scholar]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI using diffuse reflectance spectroscopy. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.J.; Scuseria, G.E. Predicting Band Gaps with Hybrid Density Functionals. J. Phys. Chem. Lett. 2016, 7, 4165–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suriye, K.; Lobo-Lapidus, R.J.; Yeagle, G.J.; Praserthdam, P.; Britt, D.; Gates, B.C. Probing Defect Sites on TiO2 with [Re3(CO)12H3]: Spectroscopic Characterization of the Surface Species. Chem. Eur. J. 2008, 14, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Lu, X.-W.; Qi, M.; Su, H.C.; Zhao, X.-W.; Zhu, Q.-Y.; Dai, J. Titanium–Oxo Cluster with 9-Anthracenecarboxylate Antennae: A Fluorescent and Photocurrent Transfer Material. Inorg. Chem. 2014, 53, 7233–7240. [Google Scholar] [CrossRef] [PubMed]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Fe´rey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.B.; Li, J.L.; Yang, B.; Yu, Y. Ti3+ in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. J. Nanomater. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Prakash, A.M.; Kurshev, K.L. Electron Spin Resonance and Electron Spin Echo Modulation Evidence for the Isomorphous Substitution of Ti in TAPO-5 Molecular Sieve. J. Phys. Chem. B 1997, 101, 9794–9799. [Google Scholar] [CrossRef]
- Agarwal, N.; Nayak, P.K.; Periasamy, N. Synthesis, photoluminescence and electrochemical properties of 2,7-diarylfluorene derivatives. J. Chem. Sci. 2008, 120, 355–362. [Google Scholar] [CrossRef]
- Soeberger III, R.C.; Young, K.J.; Tang, J.; Allen, L.J.; Crabtree, R.H.; Brudvig, G.W.; Coppens, P.; Batista, V.S.; Benedict, J.B. Interfacial Electron Transfer into Functionalized Crystalline Polyoxotitanate Nanoclusters. J. Am. Chem. Soc. 2012, 134, 8911–8917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negre, C.F.A.; Young, K.J.; Oviedo, M.B.; Allen, L.J.; Sánchez, C.G.; Jarzembska, K.N.; Benedict, J.B.; Crabtree, R.H.; Coppens, P.; Brudvig, G.W.; et al. Photoelectrochemical Hole Injection Revealed in Polyoxotitanate Nanocrystals Functionalized with Organic Adsorbates. J. Am. Chem. Soc. 2014, 136, 16420–16429. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Förster, R.; Hellmig, M.; Huschmann, F.U.; Kastner, A.; Malecki, P.; Pühringer, S.; Röwer, M.; Sparta, K.; Steffien, M.; et al. The macromolecular crystallography beamlines at BESSY II of the Helmholtz-Zentrum Berlin: Current status and perspectives. Eur. Phys. J. Plus 2015, 130, 141–150. [Google Scholar] [CrossRef]







| Parameters | (1) | (2) | (3) |
|---|---|---|---|
| Empirical formula | C68H108O16Ti4 | C54H98Cl2O16Ti4 | C54H98N2O20Ti4 |
| Formula weight (g/mol) | 1373.14 | 1265.82 | 1286.94 |
| Temperature (K) | 100(2) | 100(2) | 293(2) |
| Wavelength (Å) | 0.89429 | 0.89429 | 0.71073 |
| Space group | Monoclinic, P 1 21 1 | Tetragonal, P 4 1 | Triclinic, P 1 |
| Unit cell dimensions (Å) and angles (°) | a = 12.447(3) b = 21.693(4) c = 14.071(3) α = γ = 90 β = 105.19(3) | a = 17.827(3) c = 41.475(8) α = β = γ = 90 | a = 17.9713(9) b = 18.0686(7) c = 22.7006(9) α = 105.742(4) β = 98.362(4) γ = 92.631(4) |
| Volume (Å3) | 3666.61(140) | 13180.83(500) | 6991.1(5) |
| Z, Calculated density (Mg/m3) | 2, 1.244 | 8, 1.276 | 4, 1.223 |
| Final R indices (I > 2sigma(I)) | R1 a = 0.0444, wR2 b = 0.1182 | R1 a = 0.0680, wR2 b = 0.1851 | R1 a = 0.0860, wR2 b = 0.2304 |
| Absolute structure parameter | 0.041(3) | 0.387(19) | N/A |
| Parameter | (1) | (2) | (3) | |
|---|---|---|---|---|
| Distances (Å) | ||||
| Ti–Ti | Ti1–Ti3 | 3.1690(11) | 3.2016(16) | 3.2060(12) |
| Ti1–Ti2 | 2.9427(12) | 2.9488(16) | 2.9521(13) | |
| Ti1–Ti4 | 3.1446(12) | 3.1456(15) | 3.1508(12) | |
| Ti2–Ti3 | 3.1566(11) | 3.1532(17) | 3.1617(13) | |
| Ti2–Ti4 | 3.1738(13) | 2.1859(17) | 3.2009(12) | |
| Ti3–Ti4 | 4.0386(16) | 3.9255(16) | 3.9510(13) | |
| Ti–(µ4–O) | Ti1–O2 | 2.0457(30) | 2.0599(39) | 2.059(3) |
| Ti2–O2 | 2.0357(28) | 2.0622(41) | 2.052(3) | |
| Ti3–O2 | 2.0820(27) | 2.0603(38) | 2.074(3) | |
| Ti4–O2 | 2.0896(27) | 2.0264(38) | 2.042(3) | |
| Ti–(µ2–O) | Ti1–O3 | 1.8326(37) | 1.8643(40) | 1.839(3) |
| Ti3–O3 | 1.8499(41) | 1.8182(39) | 1.826(3) | |
| Ti–(µ2–OR) | Ti1–O11 | 1.9609(27) | 1.9515(37) | 1.944(3) |
| Ti1–O1 | 2.0042(28) | 2.0133(38) | 2.017(3) | |
| Ti2–O21 | 1.9979(27) | 2.0095(38) | 2.020(3) | |
| Ti2–O31 | 1.9633(28) | 1.9858(38) | 1.969(3) | |
| Ti3–O1 | 1.9896(36) | 2.0041(39) | 1.993(3) | |
| Ti3–O31 | 2.0981(29) | 2.0698(45) | 2.089(3) | |
| Ti4–O11 | 2.0944(32) | 2.1355(43) | 2.114(3) | |
| Ti4–O21 | 1.9985(30) | 2.0182(44) | 2.004(3) | |
| Ti–O (carb) | Ti4–O111 | 2.027(3) | 2.051(4) | 2.068(4) |
| Ti1–O112 | 2.195(3) | 2.135(4) | 2.145(3) | |
| Ti2–O131 | 2.190(3) | 2.127(5) | 2.142(4) | |
| Ti3–O132 | 2.028(3) | 2.073(5) | 2.056(4) | |
| O–C (carb) | O111–C112 | 1.270(5) | 1.272(8) | 1.267(6) |
| O112–C112 | 1.245(5) | 1.244(8) | 1.240(6) | |
| O131–C132 | 1.245(6) | 1.259(9) | 1.260(7) | |
| O132–C132 | 1.257(6) | 1.255(9) | 1.245(6) | |
| Angles (°) | ||||
| Ti–(µ4–O)–Ti | Ti3–O2–Ti2 | 100.10(12) | 99.80(17) | 100.05(12) |
| Ti3–O2–Ti1 | 100.31(13) | 102.02(17) | 101.76(13) | |
| Ti2–O2–Ti1 | 92.28(12) | 91.36(17) | 91.79(12) | |
| Ti3–O2–Ti4 | 150.98(16) | 147.7(2) | 147.43(15) | |
| Ti2–O2–Ti4 | 100.58(13) | 102.34(18) | 102.83(14) | |
| Ti1–O2–Ti4 | 98.99(12) | 100.66(18) | 100.39(12) | |
| Ti–(µ2–O)–Ti | Ti1–O3–Ti2 | 106.09(15) | 106.5(2) | 107.36(16) |
| Ti–(µ2–OR)–Ti | Ti1–O1–Ti3 | 105.04(15) | 105.72(18) | 106.16(14) |
| Ti1–O11–Ti4 | 101.65(13) | 100.52(19) | 101.79(13) | |
| Ti2–O31–Ti3 | 101.97(14) | 102.04(18) | 102.35(13) | |
| Ti2–O21–Ti4 | 105.16(14) | 104.55(19) | 105.38(15) | |
| O–C–O (carb) | O111–C112–O112 | 126.0(4) | 125.5(6) | 125.5(5) |
| O131–C132–O132 | 126.6(4) | 126.1(6) | 125.8(5) | |
| Modes | (1) | (2) | (3) | |||
|---|---|---|---|---|---|---|
| IR | R | IR | R | IR | R | |
| νas(COO) | 1566 (s) | 1611 (s) 1581 (w) | 1590 (s) 1551 (m) | 1595 (s) 1552 (m) | 1595 (m) 1559 (s) | 1614 (w) 1599 (vw) 1561 (m) |
| νs(COO) | 1448 (m) | 1450 (m) | 1442 (m) | 1459 (m) | 1434 (m) | 1456 (s) |
| νas(NO2) | - | - | - | - | 1530 (m) | 1534 (m) |
| νs(NO2) | - | - | - | - | 1347 (s) | 1349 (m) |
| νa(Ti–µ–O–Ti) νs(Ti–µ–O–Ti) | 712 (w) | 700 (m) 666 (m) | - | 699 (m) 661 (m) | - | 697 (m) 653 (m) |
| νa(Ti–µ4–O–Ti) νs(Ti–µ4–O–Ti) | 636 (m) 546 (s) | 543 (vw) 414 (m) | 635 (m) 547 (m) | 548 (vw) 415 (m) | 643 (m) 546 (s) | 536 (vw) 412 (m) |
| Complex | Mode | Frequency (cm−1) |
|---|---|---|
| (Ti4O2(OMe)10(O2CC13H9)2) (1) | νa(Ti–µ–O–Ti) νs(Ti–µ–O–Ti) νa(Ti–µ4–O–Ti) νs(Ti–µ4–O–Ti) | 703 700 623 570,439 |
| (Ti4O2(OMe)10(O2CC6H4Cl)2) (2) | νa(Ti–µ–O–Ti) νs(Ti–µ–O–Ti) νa(Ti–µ4–O–Ti) νs(Ti–µ4–O–Ti) | 705 700 622 560,448 |
| (Ti4O2(OMe)10(O2CC6H4NO2)2) (3) | νa(Ti–µ–O–Ti) νs(Ti–µ–O–Ti) νa(Ti–µ4–O–Ti) νs(Ti–µ4–O–Ti) | 706 699 622 567,444 |
| Complex | Calculated Band Gap (eV) | Experimental Band Gap (eV) |
|---|---|---|
| (Ti4O2(OMe)10(O2CC13H9)2) (1) | 3.93 | 2.55 |
| (Ti4O2(OMe)10(O2CC6H4Cl)2) (2) | 4.73 | 3.59 |
| (Ti4O2(OMe)10(O2CC6H4NO2)2) (3) | 4.30 | 2.98 |
| Sample | 103 Rate Constant, h−1 | Sample | 103 Rate Constant, h−1 | 103 Rate Constant in Reference to PMMA, h−1 |
|---|---|---|---|---|
| sole MB in darkness | 0.35 ± 0.15 | (1) in light | 4.03 ± 0.32 | 1.51 ± 0.36 |
| sole MB in light | 1.52 ± 0.09 | (2) in light | 2.95 ± 0.15 | 0.43 ± 0.22 |
| PMMA in darkness | 0.26 ± 0.04 | (3) in light | 2.53 ± 0.12 | 0.01 ± 0.20 |
| PMMA in light | 2.52 ± 0.16 | (4) in light | 3.19 ± 0.22 | 0.67 ± 0.27 |
| Sample | g Parameters | Species | ||
|---|---|---|---|---|
| PMMA + | g1 | g2 | g3 | |
| (1) | 2.024 | 2.0095 | 2.0034 | O2− |
| 1.967 | 1.957 | 1.938 | Ti(III) | |
| 2.003 | - | 1.997 | O− | |
| (2) | 2.024 | 2.0095 | 2.0034 | O2− |
| (3) | 2.0185 | 2.0052 | 1.987 | O− |
| (4) | 2.0182 | 2.005 | 1.987 | O− |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janek, M.; Radtke, A.; Muzioł, T.M.; Jerzykiewicz, M.; Piszczek, P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials 2018, 11, 1661. https://doi.org/10.3390/ma11091661
Janek M, Radtke A, Muzioł TM, Jerzykiewicz M, Piszczek P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials. 2018; 11(9):1661. https://doi.org/10.3390/ma11091661
Chicago/Turabian StyleJanek, Maciej, Aleksandra Radtke, Tadeusz M. Muzioł, Maria Jerzykiewicz, and Piotr Piszczek. 2018. "Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity" Materials 11, no. 9: 1661. https://doi.org/10.3390/ma11091661