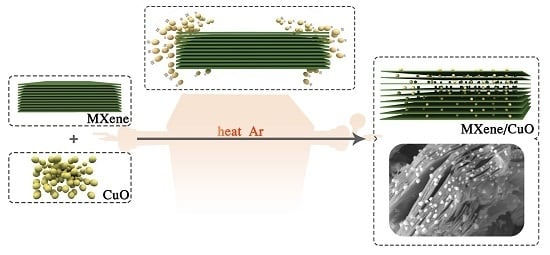
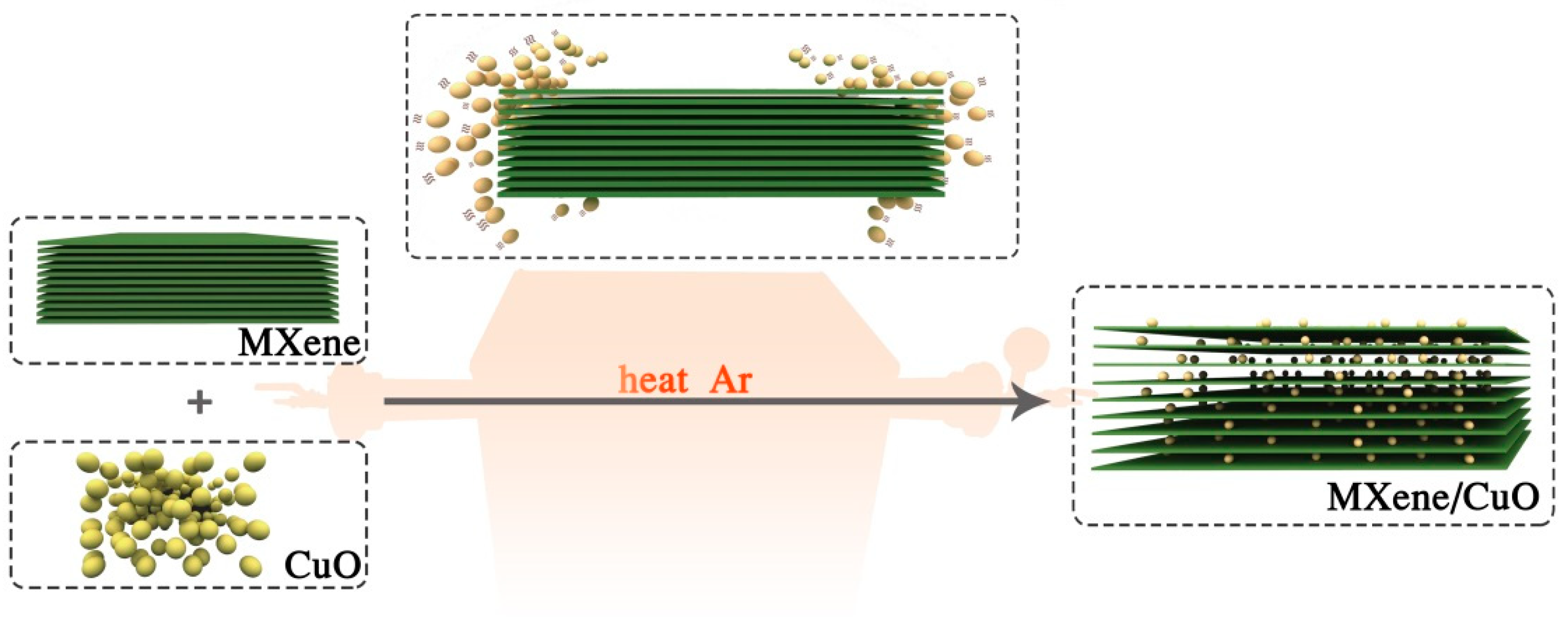
A Facile Method to Construct MXene/CuO Nanocomposite with Enhanced Catalytic Activity of CuO on Thermal Decomposition of Ammonium Perchlorate
Abstract
:1. Introduction
2. Materials and Methods
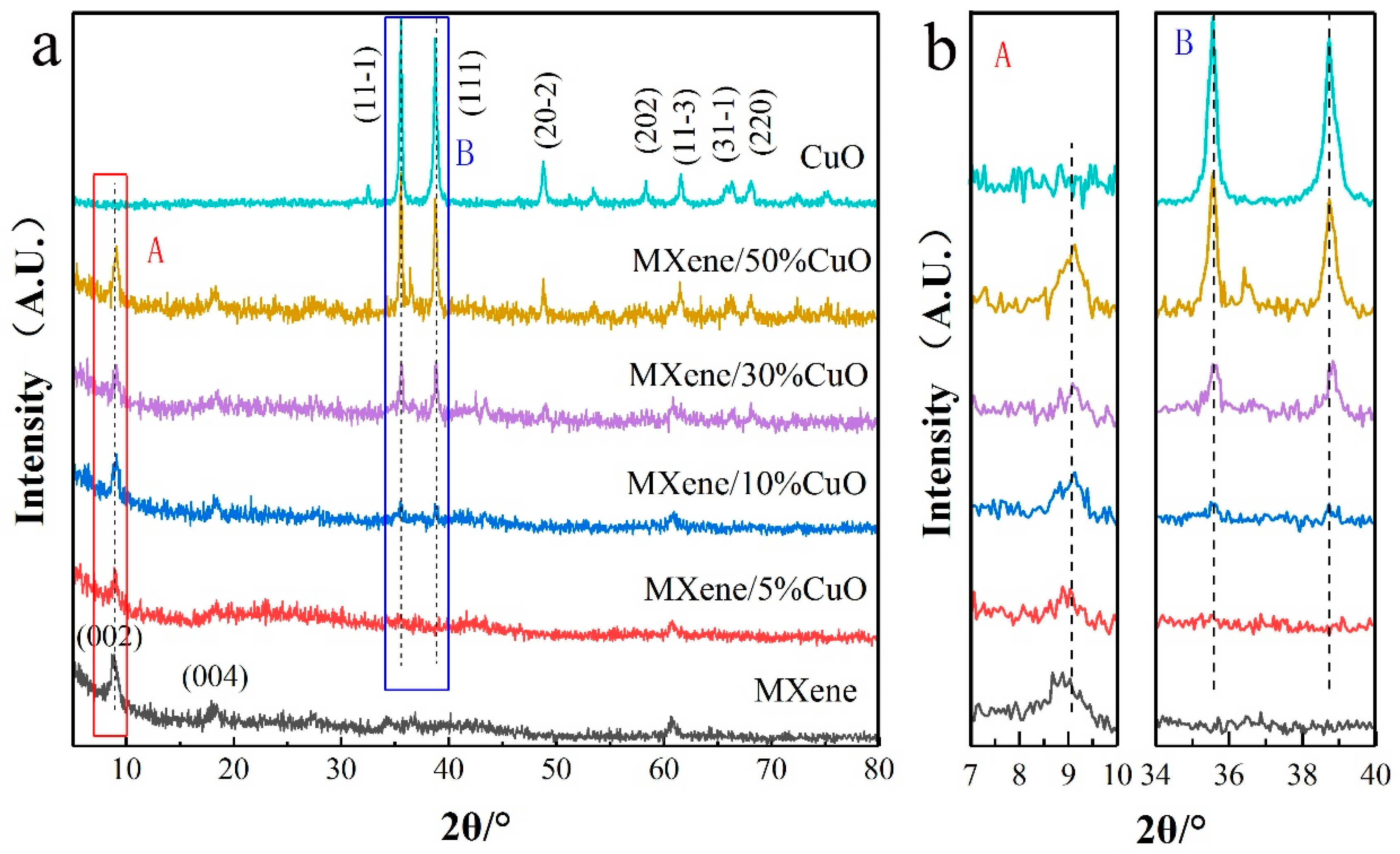
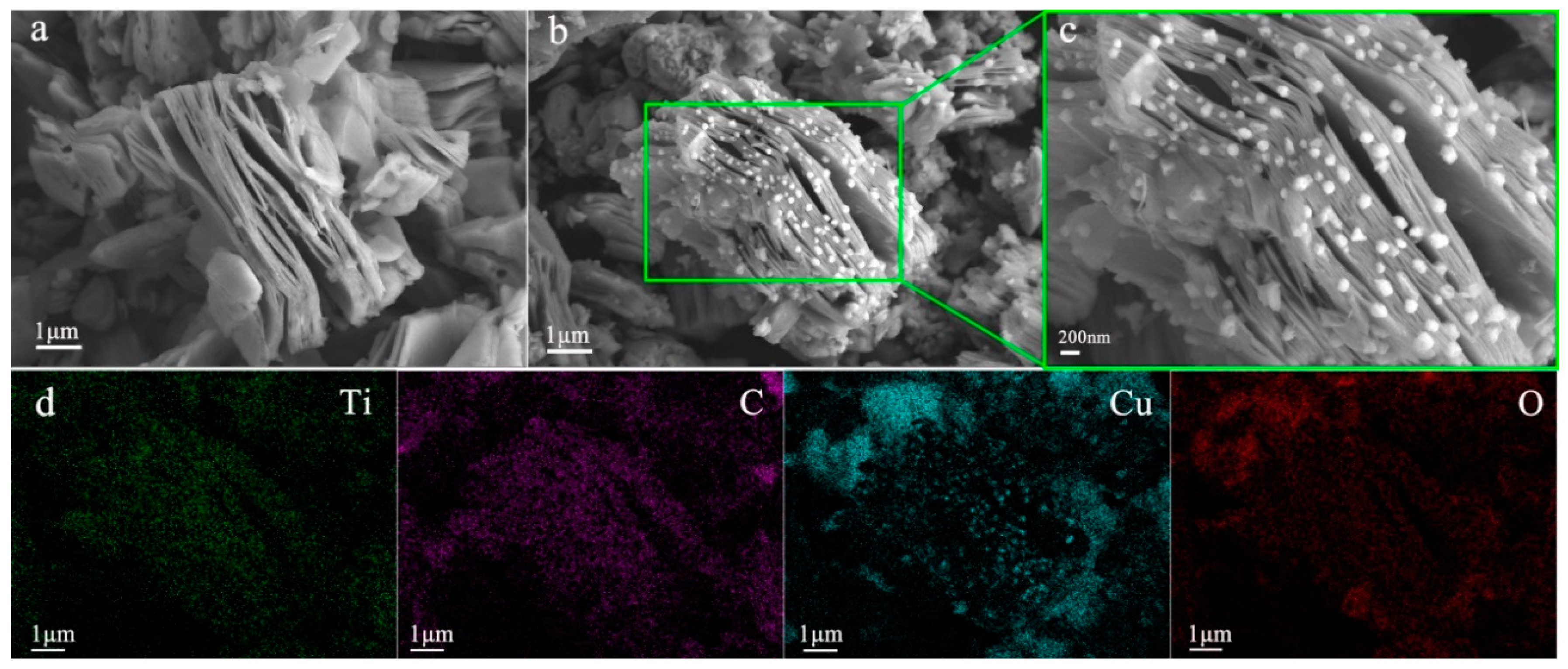
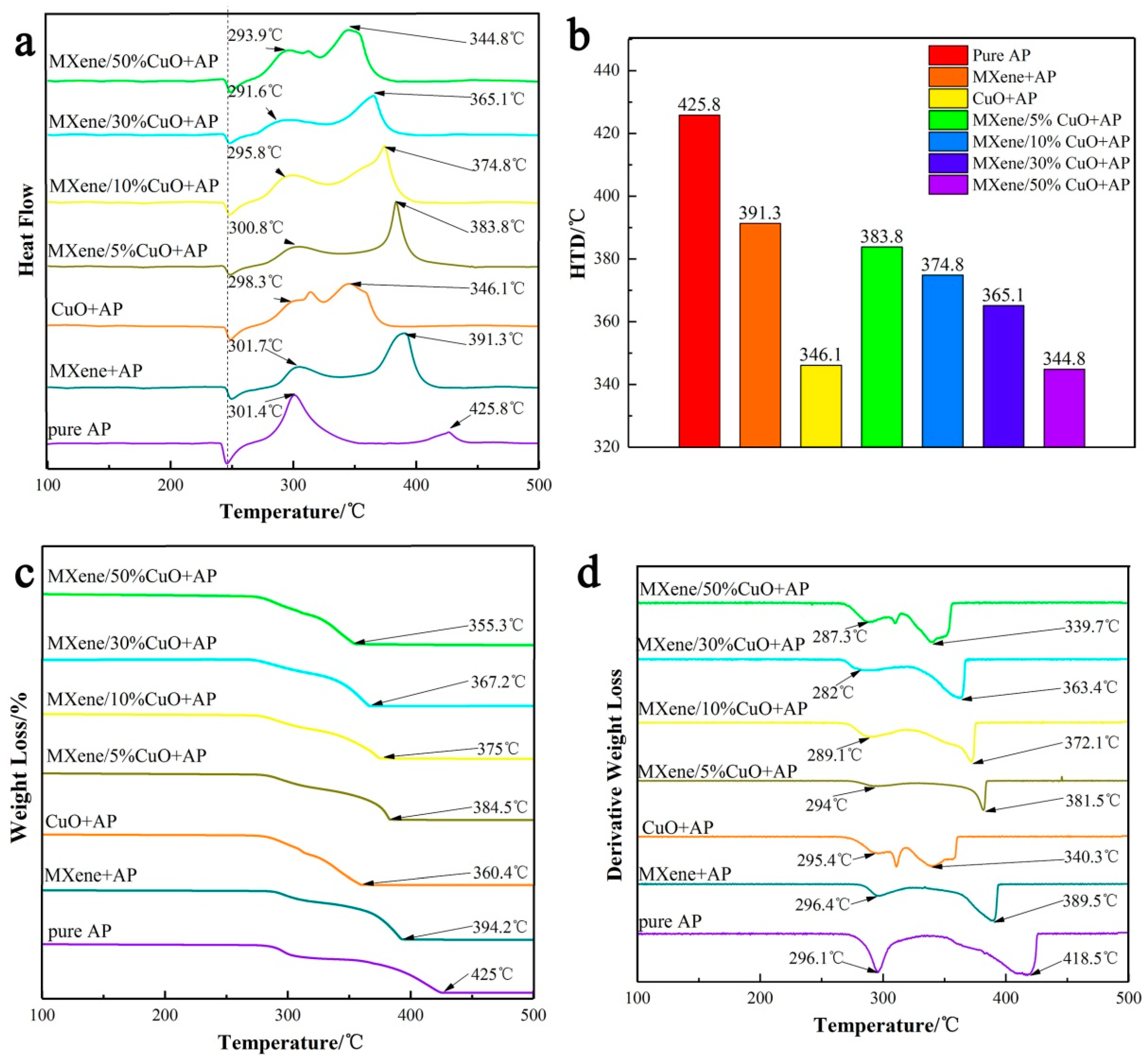
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective etching of silicon from Ti3SiC2 (MAX) To obtain 2D titanium carbide (MXene). Angew. Chem. Int. Ed. 2018, 57, 5444–5448. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Zhang, C.; Beidaghi, M.; Naguib, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Dyatkin, B.; Cook, K.M.; Kim, S.J.; Eng, B.; Xiao, X.; et al. Synthesis and charge storage properties of hierarchical niobium pentoxide/carbon/niobium carbide (MXene) hybrid materials. Chem. Mater. 2016, 28, 3937–3943. [Google Scholar] [CrossRef]
- Li, X.C.; Qian, Y.H.; Liu, T.; Cao, F.T.; Zang, Z.; Sun, X.L.; Sun, S.M.; Niu, Q.H.; Wu, J.F. Enhanced lithium and electron diffusion of LiFePO4 cathode with two-dimensional Ti3C2 MXene nanosheets. J. Mater. Sci. 2018, 53, 11078–11090. [Google Scholar] [CrossRef]
- Zhu, M.S.; Huang, Y.; Deng, Q.H.; Zhou, J.; Pei, Z.X.; Xue, Q.; Huang, Y.; Wang, Z.F.; Li, H.F.; Huang, Q.; et al. Highly flexible, freestanding supercapacitor electrode with enhanced performance obtained by hybridizing polypyrrole chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Rufford, T.E.; Chen, X.Z.; Li, N.; Lyu, M.Q.; Dai, L.M.; Wang, L.Z. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78-U171. [Google Scholar] [CrossRef]
- Zhang, Q.; Teng, J.; Zou, G.; Peng, Q.; Du, Q.; Jiao, T.; Xiang, J. Efficient phosphate sequestration for water purification by unique sandwich-like MXene/magnetic iron oxide nanocomposites. Nanoscale 2016, 8, 7085–7093. [Google Scholar] [CrossRef]
- Morales-Garcia, A.; Fernandez-Fernandez, A.; Vines, F.; Illas, F. CO2 abatement using two-dimensional MXene carbides. J. Mater. Chem. A 2018, 6, 3381–3385. [Google Scholar] [CrossRef]
- Zang, L.; Sun, W.Y.; Liu, S.; Huang, Y.K.; Yuan, H.T.; Tao, Z.L.; Wang, Y.J. Enhanced hydrogen storage properties and reversibility of LiBH4 confined in two-dimensional Ti3C2. ACS Appl. Mater. Interfaces 2018, 10, 19598–19604. [Google Scholar] [CrossRef]
- Cao, S.W.; Shen, B.J.; Tong, T.; Fu, J.W.; Yu, J.G. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, P.; Sun, N.; Anasori, B.; Zhu, Q.Z.; Liu, H.; Gogotsi, Y.; Xu, B. Self-assembly of transition metal oxide nanostructures on MXene nanosheets for fast and stable lithium storage. Adv. Mater. 2018, 30, 1707334. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.Z.; Zhou, S.; Wang, Z.Y.; Zhao, J.J.; Qiu, J.S. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Xue, Q.; Pei, Z.X.; Huang, Y.; Zhu, M.S.; Tang, Z.J.; Li, H.F.; Huang, Y.; Li, N.; Zhang, H.Y.; Zhi, C.Y. Mn3O4 nanoparticles on layer-structured Ti3C2 MXene towards the oxygen reduction reaction and zinc-air batteries. J. Mater. Chem. A 2017, 5, 20818–20823. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.J.; Zhu, J.F.; Yang, H.B.; Chen, X.J.; Wang, L.; Yang, C.H. Facile synthesis SnO2 nanoparticle-modified Ti3C2 MXene nanocomposites for enhanced lithium storage application. J. Mater. Sci. 2017, 52, 3556–3565. [Google Scholar] [CrossRef]
- Guo, X.; Xie, X.; Choi, S.; Zhao, Y.; Liu, H.; Wang, C.; Chang, S.; Wang, G. Sb2O3/MXene(Ti3C2Tx) hybrid anode materials with enhanced performance for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 12445–12452. [Google Scholar] [CrossRef]
- Pan, L.; Wang, K.-X.; Zhu, X.-D.; Xie, X.-M.; Liu, Y.-T. Hierarchical assembly of SnO2 nanowires on MnO2 nanosheets: A novel 1/2D hybrid architecture for high-capacity, reversible lithium storage. J. Mater. Chem. A 2015, 3, 6477–6483. [Google Scholar] [CrossRef]
- Rance, G.A.; Marsh, D.H.; Bourne, S.J.; Reade, T.J.; Khlobystov, A.N. van der Waals interactions between nanotubes and nanoparticles for controlled assembly of composite nanostructures. ACS Nano 2010, 4, 4920–4928. [Google Scholar] [CrossRef]
- Xu, J.H.; Li, D.N.; Chen, Y.; Tan, L.H.; Kou, B.; Wan, F.S.; Jiang, W.; Li, F.S. Constructing sheet-On-sheet structured graphitic carbon nitride/reduced graphene oxide/layered MnO2 ternary nanocomposite with outstanding catalytic properties on thermal decomposition of ammonium perchlorate. Nanomaterials 2017, 7, 450. [Google Scholar] [CrossRef]
- Maleski, K.; Mochalin, V.N.; Gogotsi, Y. Dispersions of two-dimensional titanium carbide MXene in organic solvents. Chem. Mater. 2017, 29, 1632–1640. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-derived sodium/potassium titanate nanoribbons for high-performance sodium/potassium ion batteries with enhanced capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef]
- Wu, S.H.; Lv, W.Q.; Lei, T.Y.; Han, Y.D.; Jian, X.; Deng, M.; Zhu, G.L.; Liu, M.Z.; Xiong, J.; Dickerson, J.H.; et al. Distinctive supercapacitive properties of copper and copper oxide nanocrystals sharing a similar colloidal synthetic route. Adv. Energy Mater. 2017, 7, 1700105. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H.N. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef]
- Bao, W.Z.; Liu, L.; Wang, C.Y.; Choi, S.; Wang, D.; Wang, G.X. Facile synthesis of crumpled nitrogen-doped MXene nanosheets as a new sulfur host for lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; Fani, S.; Rahmanian, M. Hierarchical CuCo2S4 hollow nanoneedle arrays as novel binder-free electrodes for high-performance asymmetric supercapacitors. Chem. Commun. 2016, 52, 4517–4520. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide-CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B Environ. 2016, 181, 352–362. [Google Scholar] [CrossRef]
- Li, R.Z.; Lin, Z.J.; Ba, X.; Li, Y.Y.; Ding, R.M.; Liu, J.P. Integrated copper-nickel oxide mesoporous nanowire arrays for high energy density aqueous asymmetric supercapacitors. Nanoscale Horiz. 2016, 1, 150–155. [Google Scholar] [CrossRef]
- Shah, S.A.; Habib, T.; Gao, H.; Gao, P.; Sun, W.; Green, M.J.; Radovic, M. Template-free 3D titanium carbide (Ti3C2Tx) MXene particles crumpled by capillary forces. Chem. Commun. 2017, 53, 400–403. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Lv, J.; Sang, J.; Zhu, L.; Zheng, P.; Andrew, G.L.; Tan, L. A Facile Method to Construct MXene/CuO Nanocomposite with Enhanced Catalytic Activity of CuO on Thermal Decomposition of Ammonium Perchlorate. Materials 2018, 11, 2457. https://doi.org/10.3390/ma11122457
Zhao H, Lv J, Sang J, Zhu L, Zheng P, Andrew GL, Tan L. A Facile Method to Construct MXene/CuO Nanocomposite with Enhanced Catalytic Activity of CuO on Thermal Decomposition of Ammonium Perchlorate. Materials. 2018; 11(12):2457. https://doi.org/10.3390/ma11122457
Chicago/Turabian StyleZhao, Haifeng, Jing Lv, Junshan Sang, Li Zhu, Peng Zheng, Greg. L. Andrew, and Linghua Tan. 2018. "A Facile Method to Construct MXene/CuO Nanocomposite with Enhanced Catalytic Activity of CuO on Thermal Decomposition of Ammonium Perchlorate" Materials 11, no. 12: 2457. https://doi.org/10.3390/ma11122457